Abstract
Background
Tumor necrosis factor (TNF) levels are associated with risk for heart failure (HF). The soluble TNF type-1 (sTNF-R1) and type-2 (sTNF-R2) receptors are elevated in patients with manifest HF, but whether they are associated with risk for incident HF is unclear.
Methods and Results
Using Cox proportional hazard models, we examined the association between baseline levels of sTNF-R1 and sTNF-R2 with incident HF risk among 1285 participants of the Health, Aging, and Body Composition Study (age 74.0±2.9 years; 51.4% women; 41.1% black). At baseline, median (interquartile range) of TNF, sTNF-R1, and sTNF R2 levels were 3.14 (2.42-4.06) pg/ml, 1.46 (1.25-1.76) ng/ml, and 3.43 (2.95-4.02) ng/ml, respectively. During a median follow-up of 11.4 (6.9, 11.7) years, 233 (18.1%) participants developed HF. In models controlling for other HF risk factors, TNF (hazard ratio [HR], 1.28; 95% confidence interval [CI], 1.02-1.61 per log2 increase), and sTNF-R1 (HR, 1.68; 95%CI, 1.15-2.46 per log2 increase), but not sTNF-R2 (HR, 1.15; 95%CI, 0.80-1.63 per log2 increase), were associated with a higher risk for HF. These associations were consistent across whites and blacks (TNF, sTNF-R1, sTNF-R2, interaction P=0.531, 0.091 and 0.795, respectively), and in both genders (TNF, sTNF-R1, sTNF-R2, interaction P=0.491, 0.672 and 0.999, respectively). TNF-R1 was associated with a higher risk for HF with preserved versus reduced ejection fraction (HR, 1.81; 95%CI, 1.03, 3.18; P=0.038 for preserved vs. HR, 0.90; 95%CI, 0.56, 1.44; P=0.667 for reduced ejection fraction, interaction P=0.05).
Conclusions
In older adults, elevated levels of sTNF-R1 are associated with an increased risk for incident HF. However, addition of TNF-R1 to the previously validated Health ABC HF risk model did not demonstrate material improvement in net discrimination or reclassification.
Keywords: heart failure, tumor necrosis factor, inflammation
While the incidence of cardiovascular diseases increase with age, the predictive value of traditional risk factors diminishes in older individuals, suggesting the potential important role of alternate mechanisms and markers influencing risk in the elderly.1-3 Inflammatory markers, including tumor necrosis factor (TNF) and its soluble receptors, TNF receptor type 1 (sTNF-R1) and TNF receptor type 2 (sTNF-R2), are elevated in patients with manifest heart failure (HF).4-6 Cytokines, e.g. TNF, are soluble polypeptides acting as immune regulators, and affect the inflammatory cascade and myocardial function.7, 8 Previous studies have suggested an association between circulating levels of inflammatory cytokines and risk of HF.9-13 Circulating cytokine receptors may also play an important role in the inflammatory process. Stimuli that cause cytokine levels to rise may induce shedding of soluble receptors in an attempt to dampen the inflammatory response. Thus, elevated levels of soluble receptors may represent a more prolonged or severe underlying inflammation.14, 15 Soluble cytokine receptors may provide more reliable markers of chronic inflammation as they have a longer half-life and tend to have more consistent serum levels than cytokines themselves.16-19 To date, the independent association of these receptors with risk for HF has not been rigorously evaluated.20 In this study, we aimed to assess the association of baseline sTNF-R1 and R2 levels and incident HF risk in older adults.
Methods
Study Population
The study population included participants in the Health, Aging, and Body Composition (Health ABC) Study, a population-based cohort of 3,075 participants who were age 70 to 79 years at inception and recruited from April 1997 to June 1998 from areas surrounding Pittsburgh, Pennsylvania, and Memphis, Tennessee. To be eligible, study participants had to, (1) report no difficulty in walking ¼ mile, climbing 10 stairs without resting, or performing basic activities of daily living; (2) be free of life-threatening illness; and (3) have no intention of moving within 3 years. Participants had telephone contacts every 6 months and clinical visits every year. Clinical diseases at baseline were ascertained using algorithms similar to the Cardiovascular Health Study.21 The institutional review boards approved the protocol. These results represent the outcomes during 11.4 years of follow-up on the 1285 random participant samples that were evaluated for sTNF-R1 and R2 levels as part of an ancillary study.
Serum Biomarker Measurements
Blood samples were obtained in the morning, and after processing, the specimens were frozen at −70 degrees centigrade and shipped to the Health ABC Core Laboratory at the University of Vermont. Cytokines and cytokine soluble receptors were measured in duplicate by an enzyme-linked immunosorbent assay kit from R&D Systems (Minneapolis, Minnesota). The detectable limit for TNF (HSTA50 kit), sTNF-R1 (DRT100 kit) and sTNF-R2 (DRT200 kit) was 0.18 pg/ml, 3 pg/ml and 1 pg/ml, respectively. Blind duplicate analyses (n=150) for TNF showed inter-assay coefficients of variation of 15.8%.
Study outcomes
All first overnight hospitalization adjudicated to be related to HF were classified as incident HF. All participants were asked to report any hospitalizations and every 6 months were asked for information about interim events. When an event was reported, hospital records were collected and verified by the Health ABC disease adjudication committee at each site. Adjudication criteria required, in addition to a physician diagnosis of HF: 1) medical record documentation of HF symptoms and signs; 2) supporting clinical findings e.g. chest radiography or echocardiography; and 3) medical therapy for HF, including at least a diuretic and a vasodilator and/or digitalis. Incident coronary heart disease was defined as hospitalization for myocardial infarction, angina pectoris, or elective coronary revascularization, either surgical or percutaneous. Date and causes of death were taken from the death certificate.
Statistical Analysis
Data are presented as mean (standard deviation) for continuous and percentage for categorical variables. Differences between groups were assessed using the nonparametric rank sum test (Mann-Whitney) for continuous and Fisher’s exact test for categorical variables. Univariate relationship between TNF, sTNF-R1 and sTNF-R2 with HF risk was examined with Cox proportional hazards models. Because the distribution of TNF, sTNFR1, and sTNF-R2 was lognormal these were log-transformed. Log2 basis was used to facilitate interpretation; the hazard ratio per log2 increase expresses the risk associated with doubling of levels. Incident HF rates were calculated with the Kaplan-Meier method. The multivariable Cox models were controlled for the Health ABC HF Risk Model, which includes age, history of coronary heart disease, smoking, systolic blood pressure, creatinine, albumin, heart rate, fasting glucose, and left ventricular hypertrophy. We also controlled for ankle-arm index as a marker of subclinical vascular disease and time-varying incident coronary events.20 The association between TNF, sTNF-R1 and sTNF-R2 with incident HF was evaluated for effect modification with sex and race using appropriate interaction terms, and effect of these markers on risk for HF with preserved vs. reduced ejection fraction was assessed. The proportional hazards assumption was evaluated by examining the Schoenfeld residuals. A two sided p<0.05 was accepted as statistically significant. Analyses were performed with STATA 12.1 (StataCorp LP, College Station, TX).
Results
Participant Characteristics
The mean age of the study participants was 74.0±2.9 years and 51.4% were women and 41.1% were black, Table 1. At baseline, median (interquartile range, IQR) TNF, sTNF-R1, and sTNF-R2 levels were 3.14 (2.42-4.06) pg/ml, 1.46 (1.25-1.76) ng/ml, and 3.43 (2.95-4.02) ng/ml, respectively. Patients who developed HF had higher baseline sTNFR1 (1.54 ng/ml, IQR 1.32, 1.93 vs. 1.45 ng/ml, IQR 1.32, 1.73; P=<0.001) and sTNF-R2 (3.55 ng/ml, IQR 3.00, 4.17 vs. 3.41 ng/ml, IQR 2.94, 3.97; P=0.02) levels. Age, adiposity, diabetes mellitus, hypertension, peripheral vascular disease, coronary heart disease, and albumin, creatinine and high-density lipoprotein levels modestly correlated with TNF and its soluble receptors, (supplemental data).
Table 1.
Baseline Participant Characteristics
| Characteristic | Overall | Heart failure | No heart | |
|---|---|---|---|---|
| Age, years | 73.6 (2.9) | 74.2 (2.9) | 73.4 (2.9) | <0.001 |
| Men, n (%) | 624 (48.6) | 120 (52) | 504 (48) | 0.321 |
| Blacks, n (%) | 528 (41.1) | 102 (44) | 426 (40) | 0.357 |
| Body Mass Index, kg/m2 | 27.6 (4.9) | 28.4 (5.2) | 27.4 (4.8) | 0.004 |
| Waist to thigh ratio | 1.9 (0.2) | 2.0 (0.2) | 1.9 (0.2) | 0.063 |
| Smoking, n (%) | ||||
| Current | 577 (44.9) | 115(49) | 462(44) | |
| Past | 140 (10.9) | 31(13) | 109(10) | |
| Never | 568 (44.2) | 87(37) | 481(46) | 0.055 |
| Alcohol consumption, n (%) | ||||
| Never | 654(51) | 124(53) | 530(50) | 0.524 |
| <1 drink/week | 257(20) | 44(19) | 213(20) | |
| 1–7 drinks/week | 279(21.7) | 44(19) | 235(22) | |
| >7 drinks/week | 93(7.2) | 20(9) | 73(7) | |
| Diabetes, n (%) | 205(16) | 48(21) | 157(15) | 0.033 |
| Hypertension, n (%) | 629(48.9) | 137(59) | 492(47) | 0.001 |
| Coronary Heart Disease, n (%) | 284(22.1) | 85(36) | 199(19) | <0.001 |
| Cerebrovascular disease, n (%) | 97(7.5) | 29(12) | 68(6) | 0.001 |
| Peripheral arterial disease, n (%) | 72(5.6) | 20(9) | 52(5) | 0.024 |
| Left ventricular hypertrophy, n (%) | 151(11.8) | 36(15) | 115(11) | 0.053 |
| Systolic blood pressure (mm Hg) | 135.4(20.7) | 139.1(22.7) | 134.6(20.1) | 0.002 |
| Heart rate, beats/min | 65.4(11.0) | 66.7(12.2) | 65.2(10.6) | 0.061 |
| Fasting glucose, mg/dl* | 94.0(87.0,105.0) | 96(89.0,115.0) | 94(87.0,104.0) | 0.001 |
| Albumin, g/dl | 4.0(0.3) | 3.9(0.3) | 4.0(0.3) | 0.02 |
| Creatinine, mg/dl* | 1.0(0.9,1.2) | 1(0.9,1.2) | 1(0.9,1.1) | 0.11 |
| Total cholesterol, mg/dl | 202.0(37.0) | 201.2(38.1) | 202.2(36.7) | 0.695 |
| Low-density lipoprotein, mg/dl | 121.7(33.8) | 122.1(34.1) | 121.6(33.7) | 0.832 |
| High-density lipoprotein, mg/dl | 53.1(16.5) | 50.8(15.8) | 53.6(16.6) | 0.02 |
| Triglycerides, mg/dl* | 119.0(89.0,166.0) | 126(89.0,169.0) | 118(89.0,165.0) | 0.741 |
| Beta-blockers, n (%) | 162(12.6) | 44(19) | 118(11) | 0.002 |
| Angiotensin-converting enzyme inhibitors, n (%) | 174(13.5) | 47(20) | 127(12) | 0.001 |
| Calcium channel blockers, n (%) | 301(23.4) | 80(34) | 221(21) | <0.001 |
| Statins, n (%) | 163(12.7) | 34(15) | 129(12) | 0.342 |
| Steroids, n (%) | 29(2.3) | 5(2) | 24(2) | 0.895 |
| Nonsteroidal anti-inflammatory agents, n (%) | 712(55.4) | 150(64) | 562(53) | 0.002 |
Value expressed as median (interquartile range) because of highly skewed distributions. All other values expressed as mean (standard deviation) unless otherwise stated.
Incident HF
During median follow-up of 11.4 (6.9, 11.7) years, 233 (18.1%) participants developed HF and 431 died. Of the remaining 621 patients, information was not available after the 10-year follow up visit for 30 patients; therefore, loss to follow up accounted for 4.8% of censoring. Of those who developed HF, 171 (73.3%) had ejection fraction determined and documented at the time of HF diagnosis. The median ejection fraction was 41.5%. Overall 47.6% had preserved ejection fraction (≥45%) and 52.3 had reduced ejection fraction (<45%).
Baseline TNF was associated with a significantly higher risk of HF (hazard ratio [HR], 1.52; 95%CI, 1.23, 1.89 per log2 increase; P=0.0001), as was sTNF-R1 (HR, 2.36; 95%CI, 1.71, 3.25 per log2 increase; P=<0.0001) and sTNF-R2 (HR, 1.52; 95%CI, 1.16, 2.00 per log2 increase; P=0.003). After controlling for the Health ABC HF Risk Score, TNF and sTNF-R1 remained associated with a higher risk (HR, 1.28; 95%CI, 1.02, 1.61; P=0.037 for TNF, and HR, 1.68; 95%CI, 1.15, 2.46; P=0.008 for sTNF-R1); whereas sTNF-R2 was not significant anymore (HR, 1.15; 95%CI, 0.80, 1.63; P=0.45). Additional adjustment for baseline ankle-arm index and time varying incident coronary events attenuated the association for TNF but not sTNF-R1, Table 2. Addition of TNF-R1 to the Health ABC HF model did not demonstrate material improvement in net discrimination or reclassification, Table 3.
Table 2.
Inflammatory Biomarkers and Incident Heart Failure
| Inflammatory | Unadjusted | *Model 1 | †Model 2 | |||
|---|---|---|---|---|---|---|
| Marker | HR (95% CI) | P Value |
HR (95% CI) | p Value |
HR (95% CI) | p Value |
| TNF, per log2 | 1.52 (1.23-1.89) | 0.0001 | 1.28 (1.02- 1.61) |
0.037 | 1.14 (0.89- 1.46) |
0.274 |
|
| ||||||
| sTNF-R1, per log2 | 2.36 (1.71-3.25) | <0.0001 | 1.68 (1.15- 2.46) |
0.008 | 1.51 (1.01- 2.24) |
0.042 |
|
| ||||||
| sTNF-R2, per log2 | 1.52 (1.16-2.00) | 0.003 | 1.15 (0.80- 1.63) |
0.45 | 1.12 (0.76- 1.68) |
0.583 |
HR expressed per log2 (logarithm with basis 2), equivalent to the HR per doubling of the original value of the parameter
Model 1: Adjusted for Health ABC heart failure model variables: age, history of coronary heart disease, smoking, systolic blood pressure, creatinine, albumin, heart rate, fasting glucose, and left ventricular hypertrophy
Model 2: model 1 variables plus ankle-arm index and time-varying incident coronary events.
TNF=Tumor Necrosis Factor; sTNF-R1=Tumor Necrosis Factor Receptor Type I; sTNF-R2=Tumor Necrosis Factor Receptor Type II
Table 3.
Risk discrimination and reclassification with sTNF-R1
| Discrimination | |
| C-index (95% CI): Health ABC risk predictors* | 0.732(0.673,0.791) |
|
| |
| Change in C index (95% CI) on adding sTNFR1 | 0.003(0.015-0.009) |
| Reclassification | |
| Participants who did not develop heart failure at 5 years | |
| Appropriately reclassified | 35(3.38%) |
| Inappropriately reclassified | 30(2.90%) |
| Participants who developed heart failure at 5 years | |
| Appropriately reclassified | 2(2.53%) |
| Inappropriately reclassified | 1(1.27%) |
| † Net reclassification index (95% CI) Events | 0.0152(0.0331, 0.0635) |
| † Net reclassification index (95% CI) Non Events | 0.0011(−0.014,0.016) |
| † Net reclassification index (95% CI) Overall | 0.0163(−0.032,0.065) |
| † Integrated discrimination index (95% CI) | 0.0034(−0.001,0.008) |
Health ABC heart failure risk model included age, smoking status, heart rate, history of coronary heart disease, systolic blood pressure, fasting glucose, serum albumin and creatinine
The reference risk prediction model included the Health ABC heart failure risk model, which was then extended to additionally include sTNFR1 as the alternative model sTNF-R1=soluble tumor necrosis factor receptor Type I
Subgroups
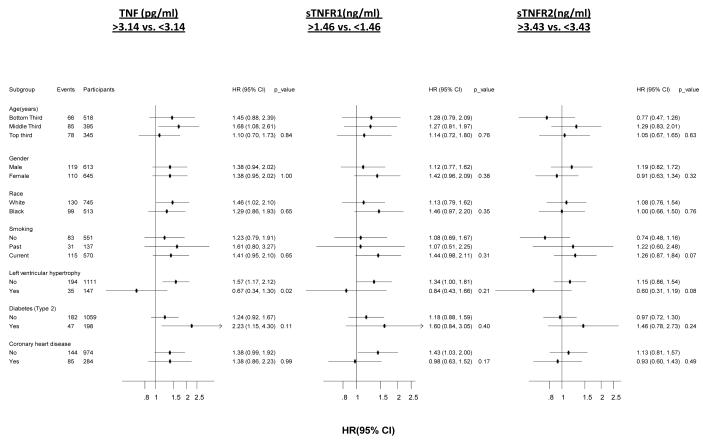
Levels of TNF, sTNF-R1 and sTNF-R2 and incident HF risk were consistent across whites vs. blacks (interaction P=0.531, 0.091 and 0.795 for TNF, sTNF-R1, sTNF-R2 respectively) and across both genders (interaction P=0.491, 0.672 and 0.999 for TNF, sTNF-R1, sTNF-R2, respectively). After adjusting for Health ABC HF Risk Model and stratified by baseline coronary heart disease, no significant differences were found between levels of sTNFR1 and sTNF-R2 above and below the median and incident HF among several subgroups including, age, gender, race, smoking status, presence of left ventricular hypertrophy, type 2 diabetes and coronary heart disease, Figure. TNF-R1 was associated with a higher risk of incident HF in patients who developed HF with preserved ejection fraction (≥45%) (HR, 1.81; 95%CI, 1.03, 3.18; P=0.038) and not those with reduced ejection fraction (<45%) (HR, 0.90; 95%CI, 0.56, 1.44; P=0.667, interaction P=0.05). Table 4.
Figure.
Comparison of association of markers of inflammation with risk of incident heart failure in the Health ABC study.
** HR [95% CI] is reported for a comparison of biomarker values below versus above the median of the biomarker distribution. TNF=Tumor Necrosis Factor; sTNF-R1=Tumor Necrosis Factor Receptor Type I; sTNF-R2=Tumor Necrosis Factor Receptor Type II Analysis is adjusted for Health ABC HF risk score variables
Table 4.
Associations with heart failure and preserved vs. reduced ejection fraction
| TNF | TNFR1 | TNFR2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ejection Fraction |
HR (95% CI) |
P | P-value interactio n |
HR (95% CI) |
P | P-value interactio n |
HR (95% CI) |
P | P-value interactio n |
|
Reduced
(<45%) |
1.08(0.72- 1.64) |
0.702 | 0.90 | 0.90(0.56- 1.44) |
0.667 | 0.05 | 0.96(0.50- 1.83) |
0.891 | 0.25 |
|
Preserved
(>45%) |
1.11(0.75- 1.65) |
0.592 | 1.81(1.03- 3.18) |
0.038 | 1.72(0.87- 3.42) |
0.121 | |||
TNF=Tumor Necrosis Factor; TNFR1=Tumor Necrosis Factor Receptor Type I; TNFR2=Tumor Necrosis Factor Receptor Type II
Discussion
The traditional risk markers for cardiovascular disease have limited predictive value in the elderly. Moreover, risk prediction for incident HF is less studied than coronary diseases, especially in the elderly, and currently there are no targeted HF risk assessment and prevention recommendations, barring control of individual comorbidities like hypertension. However, considering the growing elderly population and the projected worsening of HF epidemiology,22 concentrated efforts are needed for assessment of traditional and novel risk factors for HF risk prediction, but more importantly to elucidate pathways that mediate the high risk in order to develop effective prevention interventions. In this cohort of older adults, we observed that elevated serum levels of sTNF-R1 are associated with increased risk for incident HF. sTNF-R1 had a stronger association with incident HF than TNF. This association persisted in models adjusting for established HF risk factors, baseline markers of subclinical atherosclerosis, and interim coronary events. Importantly, consistent with the cytokine analysis of the vesnarinone trial,23 these findings were consistent across sex and race.
Inflammation has long been associated with chronic HF and plays an important role in the pathogenesis of HF through direct effects on myocardial function.8 Previous studies have suggested a strong association between circulating levels of inflammatory cytokines and risk of HF.9-13 In the Framingham Heart Study,9 there was a 68% increase in risk of incident HF per tertile increment in TNF (mg/dL) among participants with no prior history of myocardial infarction or HF.9 A study from the Health ABC Study demonstrated a significant association between the inflammatory markers interleukin-6, C-reactive protein, and TNF, and heightened risk of incident HF among older persons.13 In addition, there is evidence to suggest that these inflammatory cytokines are elevated in individuals with asymptomatic left ventricular systolic and diastolic dysfunction.24 These recent studies, in addition to results from the current study, support a more direct role for inflammation in HF development than was previously thought.9, 10, 12, 13
We found that sTNF-R1 was associated with incident HF, while increased levels of sTNF-R2 were not.25 Aside from erythrocytes, sTNF-R1 is expressed in nearly all cell types, including vascular and myocardial cells.26 sTNF-R2 is found primarily in cells of the immune system but also in the heart. While the consequences of sTNF-R2 signaling are less well characterized, it is known that sTNF-R2 mediates signals that promote tissue repair and angiogenesis.27 On the other hand, pro-inflammatory and apoptotic pathways are mediated largely through sTNF-R1.25 Previous research suggests that while the relationship of sTNF-R1 and sTNF-R2 signaling is complex, it appears that sTNF-R1 aggravates, while sTNF-R2 ameliorates, chamber remodeling and hypertrophy, largely due to disparate, opposing effects on nuclear factor-kB, inflammatory activation and apoptosis.28 These differential receptor functional properties might explain why the risk associated with these receptors might vary. One study found that sTNF-R1 emerged as the strongest independent predictor, regardless of follow-up duration and independent of established markers, of HF severity.29
TNF contributes to the progression of HF through a variety of mechanisms.30 TNF is known to exert direct affects on cardiomyocyte contractility31, 32 and can influence left ventricular remodeling and hypertrophy.33-35 In the failing heart, TNF induces ß-adrenergic receptor uncoupling,36 increases reactive oxygen species formation,37 and increases inducible nitric oxide synthase synthesis resulting in high output nitric oxide formation,37 all of which contribute to contractile dysfunction. Apart from its functional effects, sustained expression of TNF at high concentrations contributes to structural alterations in the failing heart, such as cardiomyocyte hypertrophy, increased cardiomyocyte apoptosis and cardiac fibrosis. In addition to direct myocardial effects, inflammatory cytokines have been implicated in the pathogenesis of other aspects of the HF syndrome such as pulmonary edema, skeletal muscle atrophy and cachexia.38, 39 Circulating cytokine receptors play an important role in these deleterious effects of the inflammatory process. In fact, previous studies conducted in smaller cohorts suggest that circulating levels of sTNF-R1 and sTNF-R2 are more strongly correlated with severity of HF than TNF.29, 40, 41 Our study further supports these findings, demonstrating the incremental value of sTNF-R1 levels in prediction of incident HF among the elderly, while elevated levels of TNF did not add any incremental predictive value over traditional HF risk factors.
It is interesting to note that sTNF-R1 levels were associated with a higher risk for HF with preserved ejection fraction than reduced ejection fraction. These data should be interpreted with caution since the ejection fraction data at the time of the diagnosis of HF were not uniformly available and therefore these results are prone to selection bias and other confounding. Nevertheless, it is interesting to note that inflammation is more important predictor of incident HF with preserved ejection fraction in older adults and these data complements the recent interest in the role of systemic inflammation as a common denominator explaining the relationship between comorbidity burden and risk for HF with preserved ejection fraction. These data need further validation.42
Our study has several limitations. Diagnosis of HF was based on HF hospitalization. Therefore, the rate of incident HF in our study was likely underestimated as some participants may have developed HF while not requiring hospitalization. Furthermore, echocardiography was not performed at baseline in the Health ABC Study; therefore, participants with asymptomatic structural heart abnormalities may have been included in the analysis. However, because HF is unlikely to remain undiagnosed for several years, the observed associations cannot be ascribed merely to undetected HF at baseline.
In conclusion, we demonstrate a significant association between elevated levels of the TNF receptor, sTNF-R1, and risk of HF in older adults. These findings were consistent across sex and race based groups and persisted after controlling for HF risk factors. However, addition of TNF-R1 to the previously validated Health ABC HF risk model did not demonstrate material improvement in net discrimination or reclassification. The diagnostic and therapeutic meaning of these results need further study.
Supplementary Material
Acknowledgments
Sources of Funding This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (NIA), and contracts, N 01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant: R01-AG028050, and NINR grant R01-NR012459; and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures None.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Beckett N, Nunes M, Bulpitt C. Is it advantageous to lower cholesterol in the elderly hypertensive? Cardiovasc Drugs Ther. 2000;14:397–405. doi: 10.1023/a:1007812232328. [DOI] [PubMed] [Google Scholar]
- 3.Casiglia E, Palatini P. Cardiovascular risk factors in the elderly. J Hum Hypertens. 1998;12:575–581. doi: 10.1038/sj.jhh.1000668. [DOI] [PubMed] [Google Scholar]
- 4.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (solvd) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen KH, Lassus J, Harjola VP, Siirila-Waris K, Melin J, Punnonen KR, Nieminen MS, Laakso M, Peuhkurinen KJ. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10:396–403. doi: 10.1016/j.ejheart.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Beutler BA, Milsark IW, Cerami A. Cachectin/tumor necrosis factor: Production, distribution, and metabolic fate in vivo. J Immunol. 1985;135:3972–3977. [PubMed] [Google Scholar]
- 8.Dibbs Z, Kurrelmeyer K, Kalra D, Seta Y, Wang F, Bozkurt B, Baumgarten G, Sivasubramanian N, Mann DL. Cytokines in heart failure: Pathogenetic mechanisms and potential treatment. Proc Assoc Am Physicians. 1999;111:423–428. doi: 10.1111/paa.1999.111.5.423. [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The framingham heart study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 10.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: Results from the health abc study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 11.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (the health, aging and body composition [health abc] study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 12.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: The mesa (multi-ethnic study of atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J. Inflammatory markers and incident heart failure risk in older adults: The health abc (health, aging, and body composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aderka D, Engelmann H, Shemer-Avni Y, Hornik V, Galil A, Sarov B, Wallach D. Variation in serum levels of the soluble tnf receptors among healthy individuals. Lymphokine Cytokine Res. 1992;11:157–159. [PubMed] [Google Scholar]
- 15.Schroder J, Stuber F, Gallati H, Schade FU, Kremer B. Pattern of soluble tnf receptors i and ii in sepsis. Infection. 1995;23:143–148. doi: 10.1007/BF01793854. [DOI] [PubMed] [Google Scholar]
- 16.Elkind MS, Cheng J, Boden-Albala B, Rundek T, Thomas J, Chen H, Rabbani LE, Sacco RL. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke. 2002;33:31–37. doi: 10.1161/hs0102.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: Implications for clinical trials. J Am Coll Cardiol. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 18.Porsch-Oezcueruemez M, Kunz D, Kloer HU, Luley C. Evaluation of serum levels of solubilized adhesion molecules and cytokine receptors in coronary heart disease. Journal of the American College of Cardiology. 1999;34:1995–2001. doi: 10.1016/s0735-1097(99)00473-8. [DOI] [PubMed] [Google Scholar]
- 19.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (the health, aging and body composition [health abc] study) The American journal of cardiology. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: The health abc heart failure score. Circulation. Heart failure. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 22.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the united states: A policy statement from the american heart association. Circulation. Heart failure. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the vesnarinone trial (vest) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 24.Kosmala W, Derzhko R, Przewlocka-Kosmala M, Orda A, Mazurek W. Plasma levels of tnf-alpha, il-6, and il-10 and their relationship with left ventricular diastolic function in patients with stable angina pectoris and preserved left ventricular systolic performance. Coron Artery Dis. 2008;19:375–382. doi: 10.1097/MCA.0b013e3282fc617c. [DOI] [PubMed] [Google Scholar]
- 25.Kleinbongard P, Schulz R, Heusch G. Tnfalpha in myocardial ischemia/reperfusion, remodeling and heart failure. Heart failure reviews. 2011;16:49–69. doi: 10.1007/s10741-010-9180-8. [DOI] [PubMed] [Google Scholar]
- 26.Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in tnf-receptor expression. The Journal of clinical investigation. 2000;106:589–597. doi: 10.1172/JCI9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. The American journal of pathology. 2006;169:1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: Role of nuclear factor-kappab and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 30.Satoh M, Minami Y, Takahashi Y, Nakamura M. Immune modulation: Role of the inflammatory cytokine cascade in the failing human heart. Current heart failure reports. 2008;5:69–74. doi: 10.1007/s11897-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 31.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 32.Muller-Werdan U, Schumann H, Fuchs R, Reithmann C, Loppnow H, Koch S, Zimny-Arndt U, He C, Darmer D, Jungblut P, Stadler J, Holtz J, Werdan K. Tumor necrosis factor alpha (tnf alpha) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(no)-synthase (inos) or triggering serious cytotoxicity. J Mol Cell Cardiol. 1997;29:2915–2923. doi: 10.1006/jmcc.1997.0526. [DOI] [PubMed] [Google Scholar]
- 33.Bozkurt B, Kribbs SB, Clubb FJ, Jr., Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 34.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 35.Bradham WS, Moe G, Wendt KA, Scott AA, Konig A, Romanova M, Naik G, Spinale FG. Tnf-alpha and myocardial matrix metalloproteinases in heart failure: Relationship to lv remodeling. Am J Physiol Heart Circ Physiol. 2002;282:H1288–1295. doi: 10.1152/ajpheart.00526.2001. [DOI] [PubMed] [Google Scholar]
- 36.Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo tnf-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813–1820. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 38.Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 39.Hocking DC, Phillips PG, Ferro TJ, Johnson A. Mechanisms of pulmonary edema induced by tumor necrosis factor-alpha. Circ Res. 1990;67:68–77. doi: 10.1161/01.res.67.1.68. [DOI] [PubMed] [Google Scholar]
- 40.Nozaki N, Yamaguchi S, Shirakabe M, Nakamura H, Tomoike H. Soluble tumor necrosis factor receptors are elevated in relation to severity of congestive heart failure. Jpn Circ J. 1997;61:657–664. doi: 10.1253/jcj.61.657. [DOI] [PubMed] [Google Scholar]
- 41.Missov E, Campbell A, Lebel B. Cytokine inhibitors in patients with heart failure and impaired functional capacity. Jpn Circ J. 1997;61:749–754. doi: 10.1253/jcj.61.749. [DOI] [PubMed] [Google Scholar]
- 42.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.