Abstract
Body mass index (BMI) and waist circumference (WC) are two common anthropometric measures of obesity in clinical and public health practice. Consensus, however, remains elusive regarding their utility for predicting cardiovascular disease risk in multiethnic populations. We address this gap in the literature by analyzing cross-sectional data from the first round of the Los Angeles County Health and Nutrition Examination Survey, 2011. We characterized the relationships between BMI, WC, waist-to-hip ratios, waist-to-height ratios, and chronic disease extent, as confirmed by the presence of hypertension, diabetes, and/or two or more other chronic conditions as defined by a composite indicator ‘comorbidity’. To account for race/ethnicity, age, gender, and cigarette smoking frequency, adjusted odds ratios (aOR) were generated and reported for each of the regression analyses. Whereas being overweight was associated with hypertension alone (aOR 2.10; 95% CI 1.12–3.94), obesity was associated with hypertension (aOR 5.04; 95% CI 2.80–9.06) as well as diabetes (aOR 5.28; 95% CI 2.25–12.3) and comorbidity (aOR 3.69; 95% CI 2.02–6.77). In whites and African-Americans, BMI and WC were positively related to diabetes, hypertension and comorbidity. In Hispanics, BMI and WC were also positively related to diabetes and comorbidity, but only the former measure was associated with hypertension (p<0.050). In Asians, BMI was not a significant predictor of diabetes, hypertension and/or comorbidity. Collectively, the findings suggest that BMI is not universally informative and waist circumference and its derivatives may represent a viable, more racially/ethnically appropriate alternative for use with selected minority groups.
Keywords: anthropometry, race/ethnicity, obesity, diabetes, hypertension, health disparities
Introduction
Body mass index (BMI) is a commonly used anthropometric proxy for obesity in epidemiologic studies, given the ease of using height and weight, which can be self-reported by participants during telephone or in-person interviews. Body composition categories have been established by the World Health Organization (WHO), using BMI cut-off points – underweight, normal weight, overweight, and obese. BMI, however, is not an accurate measure of body composition since the weight contribution of muscle, fat and bone cannot be fully disentangled. BMI also does not provide information on the distribution of weight across the body, which is often of greater interest in evaluating cardiovascular disease (CVD) risk. Previously published studies have focused on sex differences in anthropometric measures as risk factors for CVD [1, 2]. The risk profiles for men and women are influenced by percentages of visceral versus subcutaneous fat deposits, reflecting the importance of visceral adipose tissue in the etiology of CVD. As a result, measures of visceral adiposity such as waist circumference (WC) and waist-to-hip ratio (WHR) have emerged as the preferred anthropometric measures for ascertaining risk across sex [3–6]. Use of waist-to-height ratio (WHtR) by researchers has also increased recently, particularly in stratifying risk by sex and in intra-category stratification of normal and overweight BMI [7].
The utility of an anthropometric measure as a predictor of CVD and CVD risk factors is dependent on race as well as sex. Several published studies have examined the appropriateness of specific anthropometric measures as risk factors for CVD in different racial groups [8–17]. Although relatively meticulous in their analysis, these studies were largely conducted outside of the United States (U.S.) and in geographic regions where race, diet, and environmental exposures are homogeneous within the country in which the study originated. Only a handful of studies of anthropometry, race, and CVD have been conducted in the U.S. in racially heterogeneous populations that shared dietary and environmental exposures [18]. Zhu et al., for example, used cross-sectional NHANES III data and found WC to be a more sensitive indicator of CVD in a multiethnic sample of whites, African-Americans, and Mexican-Americans [19]. Another study by Lutsey and colleagues examined data from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort and observed that race modifies the effect of BMI and WC on incident diabetes [20].
In this cross-sectional study, we used primary data from the 2011 Los Angeles County Health and Nutritional Examination Survey (LA HANES) to examine the association of anthropometric measures and CVD risk factors in a multiethnic population in Los Angeles, California. We hypothesize that race/ethnicity will modify the utility of anthropometric measures as predictors of prevalent diabetes, hypertension and/or other chronic disease comorbidities.
Methods
Study Population
The first round of the LA HANES is a population-based, cross-sectional survey of adult residents of Los Angeles County. LA HANES participants were sampled from participants in the 2010–11 Los Angeles County Health Survey (LACHS) (n=4926) and from a random probability sample of visitors to public health clinics in Los Angeles County (n=1393).
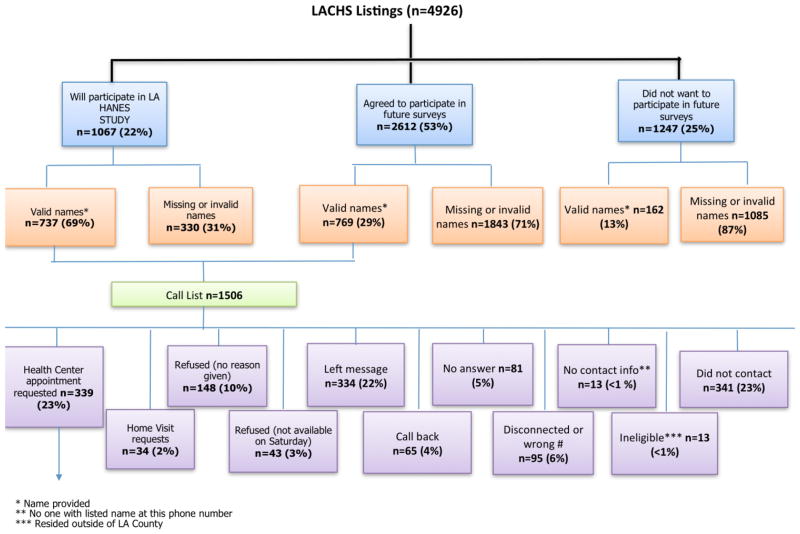
Approximately 22% and 53% of the 2010–11 LACHS participants indicated a willingness to participate in either the LA HANES (n=1067) or a future survey (n=2612), respectively, for a total of 3679 individuals (Figure 1). A call list was generated based on LACHS participants willing to participate and with valid names (n=1506). LA HANES participants were recruited by phone from the LACHS call list between February–May 2011 to either an appointment with an interviewer at a public health center (n=339) or as an in-home appointment with an interviewer (n=34). Of those clinic visitors that were probability sampled between March–April 2011, approximately 71% agreed to an appointment with an interviewer at a public health center and 726 of the 983 appointments were kept.
Figure 1.
Phone Recruitment of Participants from the 2010–11 Los Angeles County Health Survey, February–April 2011.
During the in-public health center and in-home interviews, a total of 947 participants completed the LA HANES epidemiologic survey that included questions pertaining to socio-demographic characteristics, tobacco smoking, physical activity, diet and eating behaviors, chronic conditions, and medications. Trained LA HANES staff also collected anthropometric measurements, blood pressure, and urine samples at the time of survey. Pregnant women were excluded from survey participation. All study protocols and materials were reviewed and approved by the Los Angeles County Department of Public Health Institutional Review Board (IRB#2010-12-302, approved January 2011) prior to fieldwork. Written consent was obtained from all LA HANES participants prior to data and specimen collection.
Anthropometric and Blood Pressure Measures
Height (in), weight (lbs), waist circumference (cm), hip circumference (cm), and blood pressure were measured in duplicate by trained LA HANES staff at designated public health centers or at the participant’s home between January 1–March 1, 2011. Height was measured using a stadiometer (Seca 213, seca Precision for health, United Kingdom) and weight was measured using a digital scale (Seca 876, seca Precision for health, United Kingdom). Waist and hip circumference were taken using a tape measure. Systolic and diastolic blood pressures were measured for each participant by trained staff using a manual or digital blood pressure sphyngomanometer. BMI was calculated using the standard formula: weight (lb)/[height (in)]2 × 703. BMI cut-off points for categories were determined according to WHO criteria for underweight (less than 18.5 kg/m2), normal (between 18.5–24.9 kg/m2), overweight (between 25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Blood pressure (systolic, diastolic, combined) readings were classified as normal (systolic <120 mm Hg and diastolic <80 mm Hg); suggestive of prehypertension (systolic 120–139 mm Hg or diastolic 80–89 mm Hg); or in stage 1 (systolic 140–159 mm Hg or diastolic 90–99 mm Hg) and stage 2 (systolic 160 mm Hg or diastolic 100 mm Hg) range, based on guidelines from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) [21]. Waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) were all dichotomized into two CVD risk categories: low CVD risk (WC, men <102cm, women <88cm; WHR, men <0.95, women <0.88; WHtR, <0.5) and high CVD risk (WC, men 102cm, women 88cm; WHR, men 0.95, women 0.88; WHtr, 0.5) [22].
Statistical Analysis
LA HANES participants who self-reported a physician-diagnosed chronic disease (diabetes, asthma, chronic obstructive pulmonary disease [COPD], heart disease, arthritis, liver disease, hypertension, kidney disease, and/or cancer) were included in the analysis as prevalent chronic disease cases (n=368). Participants who self-reported absences of physician-diagnosed chronic disease were included in the analysis as non-cases (n=569). Participants who did not provide an answer for the questions on physician-diagnosed disease were excluded from the analysis (n=10). A composite indicator, ‘comorbidity’, was created and defined as self-reported presence of at least two physician-diagnosed chronic diseases, excluding diabetes and hypertension. An analysis of variance (ANOVA) was performed to test the hypothesis that anthropometric variables (continuous measures for BMI, WC, WHR, WHtR) do not vary across racial/ethnic groups. Multivariable logistic regression analyses were conducted to examine the potential association between categories of anthropometric measurements (BMI, WC, WHR, WHtR) and chronic disease. Adjusted odds ratios (aOR), which account for race/ethnicity (white, African-American, Hispanic/Latino, Asian/Pacific Islander), age (Centers for Disease Control & Prevention categories of 18–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, 85+ years), sex (male [men], female [women]), and cigarette smoking frequency (0 per day, <1, 1–5, 6–10, 11–20, 20+), were generated via these analyses. A chi-squared analysis was additionally performed to examine the potential association between anthropometric measures (categorical measures of BMI, WC) and case/non-case status, across racial/ethnic groups. In order to assess the potential influence of smoking status, categories of BMI and WC by sex were compared for smokers versus non-smokers. Parallel comparisons using a t-test were also carried out for BMI and WC as continuous measures. All analyses were conducted using the SAS version 9 statistical package (SAS Institute, Inc., Cary, North Carolina).
Results
Characteristics
The combined prevalent chronic disease case/non-case sample (Table 1) was largely women (58%) and either African-American (36%) or Hispanic (34%). The cases were, on average, 10 years older than non-cases (46.4 years compared to 35.1 years, respectively) and predominantly African-American. Cases also differed from non-cases across all anthropometric variables, but were similar for smoking status. Hypertension, asthma, arthritis, and diabetes were the most prevalent chronic diseases reported and 16% of the cases were classified as having met the other chronic disease comorbidity classification. The characteristics of the 937 cases and non-cases included in the study did not differ significantly from the overall LA HANES participant pool (n=947) by sex (58% female [women]), race/ethnicity (35% African-American, 34% Hispanic), or by age.
Table 1.
Characteristics of prevalent chronic disease cases and non-cases (n=937) from the 2011 Los Angeles Health and Nutrition Examination Survey (n=947).
| Prevalent Case* | % | Non-Cases | % | P** | |
|---|---|---|---|---|---|
| Recruitment Method | 368 | 569 | |||
| Total | |||||
| Phone list | 125 | 34.0 | 101 | 17.8 | <0.001 |
| Health center | 243 | 66.0 | 468 | 82.2 | |
| Sex | |||||
| Male (men) | 147 | 39.9 | 244 | 42.9 | 0.373 |
| Female (women) | 221 | 60.1 | 325 | 57.1 | |
| Age (years) | |||||
| Mean/median | 46.4/47.0 | 35.1/33.0 | <0.001 | ||
| Range | 18–87 | 18–78 | |||
| Standard deviation | 15.3 | 12.2 | |||
| Race/Ethnicity | |||||
| African-American/Black | 159 | 43.2 | 174 | 30.6 | <0.001 |
| Asian | 26 | 7.1 | 53 | 9.3 | |
| Hispanic/Latino | 94 | 25.5 | 222 | 39.0 | |
| White/Non-Hispanic | 72 | 19.6 | 84 | 14.8 | |
| Native American/Alaska Native | 1 | 0.3 | 3 | 0.5 | |
| Mixed | 15 | 4.1 | 32 | 5.6 | |
| Survey Site | |||||
| Home Visit | 12 | 3.3 | 1 | 0.2 | 0.003 |
| Health Center A | 96 | 26.1 | 137 | 24.1 | |
| Health Center B | 62 | 16.8 | 123 | 21.6 | |
| Health Center C | 57 | 15.5 | 102 | 17.9 | |
| Health Center D | 80 | 21.7 | 94 | 16.5 | |
| Health Center E | 61 | 16.6 | 112 | 19.7 | |
| Current Smoker | |||||
| Yes | 85 | 23.1 | 113 | 19.9 | 0.235 |
| No | 283 | 76.9 | 456 | 80.1 | |
| Cigarettes Per Day | |||||
| Non-smoker | 283 | 76.9 | 456 | 80.1 | 0.242 |
| <1 | 11 | 3.0 | 17 | 3.0 | |
| 1 | 2 | 0.5 | 11 | 1.9 | |
| 2–5 | 22 | 6.0 | 33 | 5.8 | |
| 6–10 | 28 | 7.6 | 29 | 5.1 | |
| 11–20 | 15 | 4.1 | 18 | 3.2 | |
| >20 | 6 | 1.6 | 3 | 0.5 | |
| Chronic Conditions | |||||
| Diabetes | 82 | 22.2 | - | - | |
| Asthma | 120 | 32.6 | - | - | |
| Chronic Obstructive Pulmonary Disease | 9 | 2.44 | - | - | |
| Heart Disease | 23 | 6.25 | - | - | |
| Arthritis | 109 | 29.6 | - | - | |
| Liver Disease | 17 | 4.61 | - | - | |
| Hypertension | 184 | 50.0 | - | - | |
| Kidney Disease | 19 | 5.16 | - | - | |
| Cancer | 28 | 7.60 | - | - | |
| Comorbid | 146 | 39.6 | - | - | |
| BMI (kg/m2) | |||||
| mean (+/− standard deviation) | 29.9 (6.66) | 27.4 (5.83) | <0.001 | ||
| Waist Circumference (cm) | |||||
| mean (+/− standard deviation) | |||||
| Male (men) | 95.4 (14.2) | 91.8 (12.3) | 0.004 | ||
| Female (women) | 95.7 (14.4) | 87.0 (14.0) | <0.001 | ||
| Waist to Hip Ratio | |||||
| Male (men) | 0.906 (0.070) | 0.887 (0.062) | <0.001 | ||
| Female (women) | 0.872 (0.073) | 0.843 (0.068) | |||
| Waist to Height Ratio | |||||
| Male | 0.550 (0.079) | 0.529 (0.076) | 0.006 | ||
| Female | 0.594 (0.095) | 0.544 (0.089) | |||
Prevalent case: Self-report of physician-diagnosed diabetes, asthma, chronic obstructive pulmonary disease, heart disease, arthritis, liver disease, hypertension, kidney disease, and/or cancer.
P-value reported for χ2 test.
BMI: Body mass index (kg/m2).
Totals exclude missing values. Number of survey participants excluded from this analysis, n=10.
Anthropometry and CVD Risk Factors
Anthropometric measures varied by race/ethnicity (Table 2). African-Americans had higher BMI and WC measurements when compared to whites and Asians, and similar BMI and WC measurements when compared to Hispanics. African-Americans, whites and Asians were similar across waist-to-hip and waist-to-height ratios, with the larger waist-to-hip and waist-to-height ratios observed among Hispanics. BMI, WC, waist-to-hip ratio, and waist-to-height ratio were positively associated with prevalent CVD risk factors (Table 3). Being overweight (BMI between 25.0–29.9 kg/m2) was associated with hypertension (aOR 2.10; 95% CI 1.12–3.94). A positive association was suggested for being overweight and having diabetes (aOR 2.07; 95% CI 0.80–5.31) or comorbidity (aOR 1.66; 95% CI 0.86–3.21), but the association was not statistically significant. Obesity (BMI ≥ 30 kg/m2) was associated with diabetes (aOR 5.28; 95% CI 2.25–12.3), hypertension (aOR 5.04; 95% CI 2.80–9.06), and comorbidity (aOR 3.69; 95% CI 2.02–6.77). A high risk WC and high risk waist-to-hip ratio were positively associated with diabetes, hypertension, and comorbidity in both men and women. Having a high risk waist-to-height ratio was positively associated with all CVD risk factors.
Table 2.
Anthropometry in survey participants from the 2011 Los Angeles Health and Nutrition Examination Survey (n=947), by race/ethnicity.
| Race/Ethnicity | n | Mean (SD)
|
|||
|---|---|---|---|---|---|
| BMI, kg/m2 | WC, cm | WHR | WHtR | ||
| African American/Black | 333 | 29.31 (7.15) | 93.91 (16.36) | 0.86 (0.08) | 0.55 (0.09) |
| Asian | 80 | 26.02 (5.31) | 86.94 (13.17) | 0.87 (0.07) | 0.53 (0.07) |
| Hispanic/Latino | 324 | 30.49 (6.49) | 96.51 (14.62) | 0.90 (0.06) | 0.59 (0.09) |
| White/Non-Hispanic | 155 | 27.00 (5.84) | 91.03 (15.30) | 0.87 (0.08) | 0.53 (0.09) |
| Native American/Alaska Native | 4 | 30.72 (3.17) | 97.22 (13.06) | 0.89 (0.08) | 0.56 (0.05) |
| Mixed | 47 | 28.07 (6.25) | 90.57 (14.68) | 0.86 (0.07) | 0.54 (0.09) |
| P-value* | <0.001 | <0.001 | <0.001 | <0.001 | |
BMI: Body Mass Index (kg/m2).
WC: Waist circumference.
WHR: Waist-to-hip ratio.
WHtR: Waist-to-height ratio.
SD: Standard deviation.
P-value for analysis of variance (ANOVA).
Totals exclude missing values. Number of survey participants excluded from this analysis, n=10.
Table 3.
Anthropometry and chronic disease in survey participants from the 2011 Los Angeles Health and Nutrition Examination Survey (n=937).
| Any Chronic Disease** | Diabetes | Hypertension | Comorbidity | |||||
|---|---|---|---|---|---|---|---|---|
| Ca/NC* | ORadj1 | Ca/NC | ORadj1 | Ca/NC | ORadj1 | Ca/NC | ORadj1 | |
| BMI (kg/m2) | ||||||||
| Underweight (<18.5) | 5/6 | 2.87 (0.79–10.47) | 0/11 | NAC | 0/11 | NAC | 0/11 | NAC |
| Normal (18.5–24.9) | 78/201 | 1.00 | 9/271 | 1.00 | 23/256 | 1.00 | 22/258 | 1.00 |
| Overweight (25.0–29.9) | 107/186 | 1.45 (0.96–2.17) | 17/277 | 2.07 (0.80–5.31) | 48/246 | 2.10 (1.12–3.94) | 36/261 | 1.66 (0.86–3.21) |
| Obese (≥30.0) | 176/176 | 2.53 (1.71–3.76) | 56/296 | 5.28 (2.25–12.3) | 111/242 | 5.04 (2.80–9.06) | 87/270 | 3.69 (2.02–6.77) |
| WC (cm) | ||||||||
| Low risk men (<102) | 92/191 | 1.00 | 11/273 | 1.00 | 37/247 | 1.00 | 25/261 | 1.00 |
| Low risk women (<88) | 61/167 | 0.69 (0.45–1.07) | 4/226 | 0.36 (0.09–1.35) | 21/208 | 0.60 (0.30–1.17) | 16/214 | 0.66 (0.31–1.43) |
| High risk men (≥102) | 54/53 | 2.07 (1.24–3.47) | 17/90 | 4.23 (1.80–9.97) | 36/70 | 3.55 (1.89–6.69) | 27/82 | 3.41 (1.71–6.81) |
| High risk women (≥88) | 158/158 | 2.13 (1.47–3.10) | 49/266 | 4.05 (1.97–8.31) | 89/228 | 2.52 (1.54–4.15) | 77/242 | 3.42 (1.96–5.97) |
| Waist to Hip Ratio | ||||||||
| Low risk men (<0.95) | 98/192 | 1.00 | 13/278 | 1.00 | 43/246 | 1.00 | 28/265 | 1.00 |
| Low risk women (<0.88) | 101/210 | 0.91 (0.63–1.33) | 16/296 | 1.14 (0.51–2.50) | 50/262 | 1.05 (0.63–1.76) | 34/280 | 1.14 (0.63–2.05) |
| High risk men (≥0.95) | 47/52 | 1.52 (0.88–2.62) | 15/84 | 3.14 (1.32–7.45) | 29/71 | 2.24 (1.15–4.32) | 24/77 | 2.53 (1.24–5.15) |
| High risk women (≥0.88) | 118/115 | 1.90 (1.28–2.83) | 37/196 | 3.37 (1.67–6.82) | 60/174 | 1.80 (1.08–3.01) | 59/176 | 2.92 (1.67–5.11) |
| Waist to Height Ratio | ||||||||
| Low risk (<0.5) | 69/190 | 1.00 | 4/256 | 1.00 | 17/242 | 1.00 | 8/253 | 1.00 |
| High risk (≥0.5) | 295/379 | 1.72 (1.19–2.48) | 77/598 | 4.91 (1.71–14.0) | 165/511 | 3.28 (1.82–5.92) | 137/545 | 5.88 (2.61–13.2) |
Ca/NC: case/non-case.
Diabetes, asthma, chronic obstructive pulmonary disease, heart disease, arthritis, liver disease, high blood pressure, kidney disease, and/or cancer.
Adjusted for race/ethnicity (categorical), age (categorical), sex (categorical), and cigarette smoking frequency (categorical).
BMI: Body mass index (kg/m2).
WC: Waist circumference.
Totals exclude missing values. Number of survey participants excluded from this analysis, n=10.
Central Adiposity, Race/Ethnicity, and Chronic Disease
BMI and WC correlated positively with prevalent diabetes, hypertension and comorbidity in whites and African-Americans (Table 4). For Hispanics, BMI and WC both related positively with diabetes and comorbidity; however, BMI is the more prominent predictor of hypertension. Among Asians, BMI is not strongly related to prevalent diabetes, hypertension and comorbidity, but WC is for all three conditions.
Table 4.
Anthropometry and chronic disease in survey participants from the 2011 Los Angeles Health and Nutrition Examination Survey (n=937), by race/ethnicity.
| Site | Strata | Prevalent Chronic Illness | |||
|---|---|---|---|---|---|
| Any* | Diabetes* | Hypertension* | Comorbid* | ||
| White/Non-Hispanic | BMI | 0.029 | <0.001 | 0.005 | 0.013 |
| WC | 0.001 | <0.001 | 0.032 | <0.001 | |
| African-American/Black | BMI | 0.002 | 0.029 | <0.001 | <0.001 |
| WC | <0.001 | 0.001 | <0.001 | <0.001 | |
| Hispanic/Latino | BMI | 0.001 | 0.012 | 0.001 | 0.010 |
| WC | 0.004 | 0.002 | 0.071 | 0.031 | |
| Asian | BMI | 0.140 | 0.461 | 0.099 | 0.074 |
| WC | 0.033 | 0.053 | 0.008 | <0.001 | |
Any: Diabetes, asthma, chronic obstructive pulmonary disease, heart disease, arthritis, liver disease, high blood pressure, kidney disease, and/or cancer.
BMI: Body mass index (kg/m2) – underweight, normal weight, overweight, obese.
WC: Waist circumference (cm) – low risk, high risk.
P-value reported for χ2 test.
Totals exclude missing values. Number of survey participants excluded from this analysis, n=10.
Central Adiposity and Tobacco Smoking
The relationship between BMI and smoking status was not modified by sex; however, sex did modify the relationship between WC and smoking status (Table 5). There was no observed difference in the WC of women who currently smoke in comparison with women who are either former or never smokers. There was a significant difference for men, with current smokers having a waist circumference that is 3 cm, on average, larger than men who never or were former smokers (p<0.05).
Table 5.
Anthropometry and cigarette smoking behaviors in non-cases from the 2011 Los Angeles Health and Nutrition Examination Survey (n=937).
| Women - BMI | Men - BMI | Women -WC | Men - WC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2* | t-test mean (SD) | t-test* | χ2* | t-test mean (SD) | t-test * | χ2* | t-test mean (SD) | t-test * | χ2 * | t-test mean (SD) | t-test* | |
| Current Smoker | ||||||||||||
| Yes | 0.703 | 26.2 (5.97) | 0.591 | 0.224 | 27.5 (5.54) | 0.116 | 0.962 | 84.5 (14.9) | 0.619 | 0.002 | 93.0 (13.2) | 0.032 |
| No | 26.9 (5.84) | 26.9 (4.94) | 0.109 | 86.7 (13.5) | 90.4 (11.2) | |||||||
BMI: Body mass index (kg/m2) – underweight, normal weight, overweight, obese.
WC: Waist circumference (cm) – low risk, high risk.
SD: standard deviation.
P-value.
Totals exclude missing values. Number of survey participants excluded from this analysis, n=10.
Discussion
Visceral adiposity is an established risk factor for CVD; yet, few studies have examined anthropometric measures as proxies for visceral adiposity and their association with CVD risk factors in multiethnic populations. In this study, we observed positive associations between anthropometric measures and CVD risk factors in the multiethnic LA HANES population (sample). Our findings are consistent with previously published reports of positive relationships between body mass and visceral adiposity and a higher prevalence of chronic disease. Our findings suggest that WC is a more reliable predictor of diabetes across the different racial groups in Los Angeles County. We did not observe an appreciable difference across racial groups in the utility of these measures of visceral adiposity (waist circumference, waist to hip ratio) versus BMI in predicting hypertension or other chronic disease comorbidity.
Waist circumference appears to be of greater utility in predicting CVD risk factors in Asians. Previously published studies have acknowledged that BMI categories established by the WHO are not ideal for Asian populations [23, 24]. Consistent with the evidence base, our study found that BMI categories were also not particularly useful in Asians, given the low prevalence of BMI-defined obesity in Asian populations [25–28]. In a longitudinal study of incident diabetes in a multiethnic American population, Asian participants had lower BMIs but experienced a steeper increase in risk as BMI and visceral adiposity increased as compared to other racial groups [20]. This finding supports clinical observations that Asians at high risk of CVD are often overlooked in obesity screening because of a thin-fat phenotype. Given naturally thin body frames, Asians naturally fall into this phenotype category, in part as a result of experiencing increases in visceral fat deposition, but without a corresponding increase in BMI [29]. Thus, the thin-fat phenotype is a key consideration when assessing Asians for CVD/obesity risk [26].
Limitations
Similar to other studies of this kind, there are several limitations worth noting. First, the LA HANES is a cross-sectional study using primarily self-reported variables. However, for the key CVD outcomes – blood pressure (hypertension), BMI, and WC – these study variables were objectively measured at each interview. Second, although we might expect the prevalent chronic disease cases (survey participants) to modify their diet, exercise, and smoking habits since disease diagnosis, this potential bias is likely to result in an underestimate of the association between anthropometry and CVD risk factors. This would attenuate rather than accentuate our findings toward the null. Finally, the size of the LA HANES sample limits our study in that we were unable to fully stratify on race/ethnicity, sex, and chronic disease. However, given that the LA HANES was administered a second time during 2012, there is the possibility of a future pooled analysis stratified on these variables. This more comprehensive analysis would help strengthen the external validity of the LA HANES source population.
Conclusions
Our present analysis of the first round of the LA HANES reinforces the need for more race/ethnicity appropriate measures of obesity and CVD risk. Our findings support the inclusion of waist circumference as a routine measurement in research studies and clinical practice, especially in regions with multiethnic populations. Despite the increased costs of measuring and adding waist circumference to obesity/CVD risk assessments, attaining better accuracy and population health profiles that are more comprehensive should lead to better designs and tailoring of health and public health interventions in the community. Future research are clearly needed to further delineate the utility of these anthropometric measures (WC, WHR, WHtR) in clinical and public health practice, especially with respect to health risk assessments of minority groups in which BMI is not an optimal indicator of obesity and downstream CVD risk.
Acknowledgments
The first round of the LA HANES was supported in part by a cooperative agreement from the Centers for Disease Control and Prevention (#3U58DP002485-01S1). Dr. Tarleton was supported by an NIH NCI T32 grant (CA09142, Zhang).
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views or the official positions of the Los Angeles County Department of Public Health, Loyola Marymount University or the University of California, Los Angeles.
References
- 1.Gharakhanlou R, Farzad B, Agha-Alinejad H, Steffen LM, Bayati M. Anthropometric measures as predictors of cardiovascular disease risk factors in the urban population of Iran. Arq Bras Cardiol. 2012;98(2):126–35. doi: 10.1590/s0066-782x2012005000007. [DOI] [PubMed] [Google Scholar]
- 2.Oreopoulos A, Fonarow GC, Ezekowitz JA, McAlister FA, Sharma AM, Kalantar-Zadeh K, et al. Do anthropometric indices accurately reflect directly measured body composition in men and women with chronic heart failure? Congest Heart Fail. 17(2):90–2. doi: 10.1111/j.1751-7133.2010.00204.x. [DOI] [PubMed] [Google Scholar]
- 3.Burton JO, Gray LJ, Webb DR, Davies MJ, Khunti K, Crasto W, et al. Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol Dial Transplant. 2012;27(5):1860–6. doi: 10.1093/ndt/gfr574. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Ambrosi J, Silva C, Catalan V, Rodriguez A, Galofre JC, Escalada J, et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care. 35(2):383–8. doi: 10.2337/dc11-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Zhai F, Ma G, Feskens EJ, Zhang J, Fu P, et al. Abdominal obesity and the prevalence of diabetes and intermediate hyperglycaemia in Chinese adults. Public Health Nutr. 2009;12(8):1078–84. doi: 10.1017/S1368980008003856. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai M, Miura K, Takamura T, Ota T, Ishizaki M, Morikawa Y, et al. Gender differences in the association between anthropometric indices of obesity and blood pressure in Japanese. Hypertens Res. 2006;29(2):75–80. doi: 10.1291/hypres.29.75. [DOI] [PubMed] [Google Scholar]
- 7.Ashwell M, Gibson S. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: Analysis of data from the British National Diet And Nutrition Survey of adults aged 19–64 years. Obes Facts. 2009;2(2):97–103. doi: 10.1159/000203363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmaillzadeh A, Mirmiran P, Azizi F. Waist-to-hip ratio is a better screening measure for cardiovascular risk factors than other anthropometric indicators in Tehranian adult men. Int J Obes Relat Metab Disord. 2004;28(10):1325–32. doi: 10.1038/sj.ijo.0802757. [DOI] [PubMed] [Google Scholar]
- 9.Gu JJ, Rafalson L, Zhao GM, Wu HY, Zhou Y, Jiang QW, et al. Anthropometric measurements for prediction of metabolic risk among Chinese adults in Pudong new area of Shanghai. Exp Clin Endocrinol Diabetes. 119(7):387–94. doi: 10.1055/s-0031-1277141. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Kapoor S. Optimal Cut-Off Values of Anthropometric Markers to Predict Hypertension in North Indian Population. J Community Health. 2012;37(2):441–7. doi: 10.1007/s10900-011-9461-8. [DOI] [PubMed] [Google Scholar]
- 11.Islami F, Manczuk M, Vedanthan R, Vatten L, Polewczyk A, Fuster V, et al. A cross-sectional study of cardiovascular disease and associated factors. Ann Agric Environ Med. 18(2):255–9. [PubMed] [Google Scholar]
- 12.Kalichman L, Livshits G, Kobyliansky E. Indices of body composition and chronic morbidity: a cross-sectional study of a rural population in central Russia. Am J Hum Biol. 2006;18(3):350–8. doi: 10.1002/ajhb.20506. [DOI] [PubMed] [Google Scholar]
- 13.Neufeld LM, Jones-Smith JC, Garcia R, Fernald LC. Anthropometric predictors for the risk of chronic disease in non-diabetic, non-hypertensive young Mexican women. Public Health Nutr. 2008;11(2):159–67. doi: 10.1017/S136898000700002X. [DOI] [PubMed] [Google Scholar]
- 14.Trinh OT, Nguyen ND, Phongsavan P, Dibley MJ, Bauman AE. Prevalence and risk factors with overweight and obesity among Vietnamese adults: Caucasian and Asian cut-offs. Asia Pac J Clin Nutr. 2009;18(2):226–33. [PubMed] [Google Scholar]
- 15.Tybor DJ, Lichtenstein AH, Dallal GE, Daniels SR, Must A. Racial differences in central adiposity in a longitudinal cohort of black and white adolescent females. BMC Pediatr. 10:2. doi: 10.1186/1471-2431-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasudevan D, Stotts AL, Mandayam S, Omegie LA. Comparison of BMI and anthropometric measures among South Asian Indians using standard and modified criteria. Public Health Nutr. 14(5):809–16. doi: 10.1017/S1368980010003307. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Yao S, Sun G, Yu S, Sun Z, Zheng L, et al. Total and abdominal obesity among rural Chinese women and the association with hypertension. Nutrition. 28(1):46–52. doi: 10.1016/j.nut.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–53. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81(2):409–15. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 20.Lutsey PL, Pereira MA, Bertoni AG, Kandula NR, Jacobs DR., Jr Interactions between race/ethnicity and anthropometry in risk of incident diabetes: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 172(2):197–204. doi: 10.1093/aje/kwq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 22.Gwynn RC, Berger M, Garg RK, Waddell EN, Philburn R, Thorpe LE. Measures of adiposity and cardiovascular disease risk factors, New York City Health and Nutrition Examination Survey, 2004. Prev Chronic Dis. 8(3):A56. [PMC free article] [PubMed] [Google Scholar]
- 23.New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66(11):987–92. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 24.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 25.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22(12):1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 26.Gill TP. Cardiovascular risk in the Asia-Pacific region from a nutrition and metabolic point of view: abdominal obesity. Asia Pac J Clin Nutr. 2001;10(2):85–9. doi: 10.1111/j.1440-6047.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- 27.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337(8738):382–6. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 28.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9(7):381–7. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 29.Kurpad AV, Varadharajan KS, Aeberli I. The thin-fat phenotype and global metabolic disease risk. Curr Opin Clin Nutr Metab Care. 14(6):542–7. doi: 10.1097/MCO.0b013e32834b6e5e. [DOI] [PubMed] [Google Scholar]