Abstract
We explored the relationship between sleep disturbances and mild cognitive impairment (MCI) in community-dwelling seniors. Recent evidence suggests that sleep habits are differentially compromised in different subtypes of MCI, but the relationship between sleep disruption and MCI remains poorly understood. We gathered daily objective measures of sleep disturbance from 45 seniors, including 16 with MCI (mean age 86.9 ± 4.3 years), over a six month period. We also collected self-report measures of sleep disturbance. Although there were no differences between groups in any of our self-report measures, we found that amnestic MCI (aMCI) volunteers had less disturbed sleep than both non-amnestic MCI (naMCI) and cognitively intact volunteers, as measured objectively by movement in bed at night (F2,1078=4.30, p=0.05), wake after sleep onset (F2,1078=41.6, p<0.001), and times up at night (F2,1078=26.7, p<0.001). The groups did not differ in total sleep time. In addition, the aMCI group had less day-to-day variability in these measures than the intact and naMCI volunteers. In general, the naMCI volunteers showed a level of disturbed sleep that was intermediate to that of aMCI and intact volunteers. These differences in sleep disruption between aMCI and naMCI may be related to differences in the pathology underlying these MCI subtypes.
Keywords: MCI (Mild Cognitive Impairment), Assessment of cognitive disorders/dementia, Sleep Habits, Cohort studies
INTRODUCTION
Disrupted sleep, including nighttime awakenings, difficulty falling asleep, and early awakening, are common in the elderly1-4. Estimates of the prevalence of sleep disturbances in the elderly range from 21-54%. One of the most important functional aspects of sleep in the elderly is a strong association between poor sleep and cognitive impairment. There is ample evidence of sleep disturbance in Alzheimer’s disease compared to normal elderly5, including increased daytime sleepiness6, 7, longer duration of nighttime sleep8, 9, poor sleep efficiency10, and more frequent awakening at night5. There is also increasing evidence that sleep disturbances play an important role in mild cognitive impairment (MCI) in seniors9, 11-15, and some studies have suggested that disruptive nighttime behaviors are the most common clinically significant neuropsychiatric symptom in patients with MCI14, 15.
Mild cognitive impairment (MCI) is used to describe a syndrome of cognitive impairment in the absence of functional impairment. However, MCI is a heterogeneous construct, with the long-term prognosis for patients varying depending on their MCI subtype. Amnestic MCI (aMCI) refers to those patients with a primary complaint of declining memory, whereas patients with non-amnestic MCI (naMCI) have no complaint of memory deficits but show impairment in one or more other cognitive domains. Recently, evidence from screening questions about sleep disturbances has suggested that the frequency16, 17 and severity18 of nighttime disturbances may be greater in non-amnestic than in amnestic MCI. This is consistent with recent actigraphy studies examining the relationship between sleep disturbances and performance on tests of executive function. For example, Naismith used actigraphy to follow 15 seniors with naMCI using actigraphy over 14 days and looked at the correlation between the number and duration of arousals (wake after sleep onset: WASO) and scores on executive function tests12. They found a negative correlation between WASO and sorting and attention tasks, and a positive correlation to response inhibition tasks. Similarly, Blackwell and colleagues used actigraphy to assess sleep measures in cognitively healthy seniors in the MrOS study over a five night period, and found that WASO greater than 90 minutes was associated with poorer performance on a test of executive function (Trails B)19. In another study, they found a correlation between less time spent in REM sleep and performance on Trails B20. In contrast, Westerberg looked at the relationship between wrist-worn actigraphy measures of sleep over 14 nights in ten amnestic MCI and ten controls to two memory tasks completed each day. They found that the night-to-night variability in sleep latency, WASO, and total sleep time were correlated with performance on the next-day memory tests21. It could be expected that WASO might be increased in naMCI patients as compared to controls and to aMCI patients; however, such a study has not been done.
Polysomnography (PSG) studies have also revealed more subtle changes in sleep measures in aMCI patients. While most studies find no difference between aMCI and healthy controls on typical measures of sleep disruption, such as WASO and total sleep time (TST), the use of electroencephalography and surface electromyography in PSG has shown an increase in the number of periodic leg movement arousals23, 24 and slow wave sleep arousals22 in aMCI. Interestingly, the latter study also showed that aMCI APoE4 carriers had less REM sleep and fewer slow wave sleep arousals than non-ApoE4 carriers, leading the investigators to hypothesize that increased wake duration caused by SWS fragmentation in aMCI patients may contribute to the production of toxic amyloid. Similar studies have not been done in naMCI patients.
The objective of the present study was two-fold. First, we wanted to determine whether sleep patterns assessed using objective measures differ between aMCI and naMCI volunteers. We hypothesized that we would see more disrupted sleep in MCI volunteers as compared to controls, and in naMCI volunteers as compared to aMCI volunteers. Second, we want to further examine the question of night-to-night variability in sleep measures across these groups. Based on Westerberg’s findings, we expected to see increased variability in our aMCI cohort, but also wanted to know if this variability was typical of naMCI volunteers as well. Thus, using in-home sensors to collect objective sleep measures over an extended period of time26, we explored the relationship between sleep disturbances and MCI in community-dwelling seniors.
METHODS
Participants
Forty-five ambulatory community-dwelling elderly volunteers (mean age 86.9 ± 4.3 years; 40 female) currently being monitored in their homes as part of an Oregon Center for Aging and Technology (ORCATECH) longitudinal study27 were included in this analysis. All volunteers were recruited from the Portland, Oregon metropolitan area and provided written informed consent before participating in study activities. The protocol was approved by the OHSU Institutional Review Board (IRB #2353). Inclusion criteria were a score less than 5 on the short version of the Geriatric Depression Scale28 (not depressed), a Mini-Mental State Examination29 score of more than 23, and a Clinical Dementia Rating scale30 score ≤ 0.5 (not demented). In addition, all MCI participants had to have had a diagnosis of aMCI or naMCI in two or more consecutive annual visits to be included (see Independent Variables, below). Medical illnesses with the potential to limit physical participation (e.g., wheelchair bound) or likely to lead to untimely death over the monitoring period (such as certain cancers) were exclusions for the original study; for the current analysis, participants for whom more than six weeks of monitoring data were missing (due to travel, or to sensor outages) during the 26-week monitoring period were also excluded. All volunteers included in the current analysis lived alone.
Procedures
Volunteers were clinically assessed in their home at baseline upon their enrollment in the study, at six months (by telephone), and during annual in-home visits with research personnel who administered standardized health and function questionnaires and physical and neurological examinations. In addition, volunteers completed weekly questionnaires concerning medication changes, falls, injuries, health changes, emergency room visits, depression, changes to living space, vacations, and visitors. The Sleep Disturbance Symptom Questionnaire (SDSQ5) was administered on-line every six months.
In order to detect movement on a continuous basis, wireless passive infrared motion sensors (MS16A; X10.com) were placed in each room of the home (bedroom, bathroom, kitchen, living rooms and hallway-entry areas). These sensors fire when a person moves in their vicinity, creating an event-based time series identifying when and where activity is taking place in the home. In addition, wireless magnetic contact sensors (DS10A, X10.com) were placed on each door of the home to track door openings and closings, allowing us to determine when the participant left the home. Data from all sensors were sent wirelessly to a dedicated research laptop computer placed in the volunteer’s home, then time-stamped and stored in an SQL database. All data were encrypted and securely uploaded to a central database on a daily basis. The sensor data was used to derive nighttime activity measures, as described below, using algorithms that we developed previously26. Briefly, the nighttime activity measures are based on a determination of the individual’s status at any given moment in time. Each time a sensor fires, the timing and sequence of the previous fifteen sensor firings are considered and the status of the person is identified as out-of-bed, in-bed, or in-bed-asleep. Thus, the data are similar to that collected using actigraphy, but also give information about what the person does when they get up (e.g. go to the bathroom). In a recent study comparing nighttime activity in 21 seniors measured using both our system and wrist-worn actigraphy over twelve days, we found a 76% (±11%) correlation between the measures. Similar to actigraphy, estimations of sleep are necessarily based on periods of no movement for at least 20 minutes, and so the algorithms will overestimate sleep and underestimate sleep latency in cases of insomnia where the person is lying still but awake. Thus, rather than calculate sleep latency, we instead use “settling time” (see below) which more accurately reflects that the person has ceased movement after going to bed.
Since the algorithms assumed that the individual was alone at night, we necessarily excluded periods of data when the volunteer had visitors, as well as periods when sensor data could not be collected due to sensor malfunction or power outages or when the subject was away from home. For each volunteer, we selected the earliest 26-week period in which there were reliable sensor data for every week and included those data in the current analysis. For each volunteer, their clinical assessment within that 26-week period was used to determine cognitive status, and the SDSQ questionnaire within that period was used for the self-report responses.
Variables
Control Variables
A number of possibly confounding factors were examined to identify possible health differences between groups. Functional status was assessed using the Functional Activities Questionnaire31. This questionnaire assesses the participant’s ability to independently perform key functional activities such as medication management, managing money, and traveling by car, bus or taxi. The presence of co-morbidities was assessed using the Cumulative Illness Rating Scale (CIRS)32. The CIRS assesses chronic illness burden, and is significantly correlated with physician’s estimates of medical burden33. Body mass index (BMI) was calculated using the participant’s weight in kilograms divided by their height (in meters) squared. This was examined because BMI is correlated with the incidence of sleep disorders. Finally, to assess potential medication impact on patterns of sleep, we recorded the number of stimulant and sedative medications taken by each volunteer.
Dependent variables
Subjective assessment of sleep quality
The Sleep Disturbance Symptom Questionnaire was used to assess self-reported quality of sleep. This questionnaire was administered every 6 months, and included 20 questions about sleep habits coded by frequency of occurrence (never, seldom, occasionally, frequently, always) on a 5-point scale (0-4) with higher scores reflecting that the problem occurred more frequently. Three of the questions were combined to create a subjective insomnia score (SIS: take more than 30 minutes to fall asleep, wake up at night for more than an hour, wake up too early), three questions were combined to create a subjective restlessness measure (SRS: have restless sleep, twitch or jerk during sleep, have restless legs at night), and three questions were combined to create a subjective daytime sleepiness score (SDS: feel drowsy during the day, take naps, do not wake up feeling well rested). Finally, one question (how many times do you get up at night: 0=zero, 1=once or twice, 2=three or four times, 3=five or more times) was used as the subjective measure of times up at night.
Objective measurements of sleep
The timing and location of the sensor firings were used to estimate a number of sleep variables that are commonly used to assess sleep. Note that as with all movement based estimates of sleep measures, including actigraphy and bed mats, variables such as total sleep time must be inferred from periods of inactivity. However, we have validated the algorithm used to derive these measures against ground truth measures of movement on the bed26. The variables we examined for this study were wake after sleep onset (WASO: time spent awake after initial sleep onset until the last wakening in the morning), total sleep time (TST: wake time subtracted from total time in bed), settling time (ST: time from getting into bed until the start of the first 20 minute period of no movement), times up at night (UP: when the participant actually got out of bed), and total movement in bed at night (MIB: number of bedroom sensor firings while the participant was in bed, a measure of restlessness). As noted, the objective measures used in this study were collected on a daily basis for a 26-week period. Because episodic activity outliers may skew the data (for example, up more frequently at night due to illness, increased restlessness due to unusual levels of daytime activity), the median of each measure was taken for each week, together with the interquartile range to assess variability within each week. These measures provide more robust estimates in the presence of outliers than do mean and variance. Thus, for each objective measure we obtained 26 weekly summaries of central tendency and variability.
Independent variables
MCI status
MCI status was determined using operationalized Petersen criteria34. Volunteers were classified as having no MCI (intact), amnestic MCI (aMCI) or non-amnestic MCI (naMCI) based on the evaluation closest to the middle of the 26-week objective sleep recording. Amnestic MCI is characterized by a memory deficit 1.5 standard deviations or more below the age-adjusted and education-adjusted norms, a subjective memory complaint usually corroborated by an informant, and essentially preserved general cognitive function and functional activities. Non-amnestic MCI is characterized by compromised cognitive function in other domains such as language, attention, or executive function but not in memory, without dementia or functional impairment.
Data Analysis
We used an overall MANOVA and then applied univariate ANOVA for significant results to compare the clinical status (control variables) of the volunteers across MCI groups. Similarly, the composite subjective measures were compared across groups using a MANOVA. Individual subjective measures were analyzed using a Kruskal-Wallis test to determine if any specific responses differed by MCI status. Objective measures were analyzed individually using a mixed-effects ANOVA model with time (repeated measure) as the within-subject random effect and group (intact, aMCI, naMCI) as the fixed effect. Tukey’s HSD was used to control for multiple comparisons. Finally, ordinal logistic regression was used to determine if the objective measure of times up at night predicted the self-report of this measure. All analyses were done using the Matlab Statistics ToolboxTM.
RESULTS
Subject characteristics
In our sample of older adults, six volunteers (13%) were classified as aMCI and 10 (22%) were classified as naMCI. Table 1 shows the demographic features and the means across groups for the control measures. MANOVA showed no differences between groups in any of these measures, indicating that these measures were not likely the source of differences in sleep behaviors between the groups.
Table 1.
Comparison of demographic and control measures across groups. MANOVA revealed no differences between groups. Although the FAQ scores were higher for aMCI volunteers than for the other groups, these differences were not significant after adjustment for multiple comparisons. FAQ: Functional Activities Questionnaire; CIRS: Cumulative Illness Rating Scale; GDS: Geriatric Depression Scale; MMSE: Mini-Mental State Exam; BMI: Body mass index
| Intact | aMCI | naMCI | |
|---|---|---|---|
|
| |||
| Age (yrs) | 87.5 ± 4.0 | 84.8 ± 6.6 | 86.5 ± 3.4 |
| Female/Male | 26/3 | 5/1 | 9/1 |
| FAQ | 0.07 ± 0.26 | 0.67 ± 1.21 | 0.10 ± 0.32 |
| CIRS | 21.9 ± 2.42 | 20.5 ± 2.66 | 22.8 ± 2.53 |
| GDS | 0.86 ± 1.30 | 0.83 ± 0.75 | 1.60 ± 1.96 |
| MMSE | 28.3 ± 2.06 | 27.2 ± 1.48 | 28.0 ± 1.89 |
| BMI | 27.0 ± 3.91 | 26.5 ± 3.35 | 27.8 ± 4.87 |
| BMI range | 19.5 – 33.8 | 21.0 – 29.8 | 21.1 – 38.3 |
| Medications | |||
| None | 59% | 33% | 50% |
| Stimulants | 21% | 33% | 40% |
| Sedatives | 3% | 0% | 0% |
| Mixed | 17% | 33% | 10% |
Cross-sectional comparisons between healthy and MCI participants
Subjective measures
Very few of the participants reported substantial sleep disturbances (those that occurred frequently or always). Although in general the aMCI group reported less insomnia and restlessness than the other groups, there were no significant differences between groups in any of the individual self-report scores. Similarly, there were no differences in the self-report of the subjective insomnia score, subjective restlessness score, subjective daytime sleepiness score, or number of times up at night (see Table 2). On average, volunteers reported that they slept well and got up only once a night. However, 33% of volunteers reported that they never or seldom woke up feeling rested, and 27% reported that they frequently or always took naps.
Table 2.
Means and standard deviations of self-reported sleep measures for each group. MANOVA revealed no differences between groups. SDS: subjective daytime sleepiness score; SIS: subjective insomnia score; SRS: subjective restlessness score. Numbers of participants in each group are shown in parentheses.
| Measure | Intact (29) | aMCI (6) | naMCI (10) | p value |
|---|---|---|---|---|
|
| ||||
| SDS | 1.80 +/- 0.15 | 1.50 +/- 0.32 | 1.97 +/- 0.25 | 0.69 |
| SIS | 1.27 +/- 0.15 | 0.76 +/- 0.32 | 1.64 +/- 0.25 | 0.21 |
| SRS | 1.02 +/- 0.14 | 0.38 +/- 0.29 | 0.70 +/- 0.23 | 0.34 |
| UPTIMES | 1.13 +/- 0.14 | 1.00 +/- 0.29 | 1.00 +/- 0.23 | 0.77 |
Objective measures
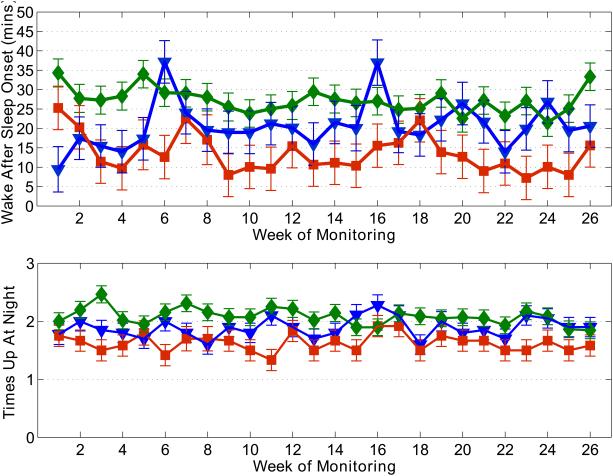
Figure 1 shows an example of the longitudinal data collected using the in-home sensors over the 26-week period, across the three groups. In general, there was marked week-to-week variability in the median weekly measures over the 26 weeks for all measures and for most volunteers. There were no significant effects of time on the objective measures, nor were there group*time interactions. Overall, the aMCI group showed significantly less sleep disturbance than the other groups: less movement in bed at night (F2,1078=4.30, p=0.05), less time awake after sleep onset (F2,1078=41.6, p<0.001), and fewer times up at night (F2,1078=26.7, p<0.001). However, their total sleep time was not different than the other groups. In contrast, the naMCI group showed greater settling time at night than the other groups (F2,1078=59.17, p<0.001).
Figure 1.
Example of the 26-week longitudinal data across groups. Group means were calculated from the median of the daily measures for the week for each volunteer. Green diamonds: intact volunteers; red squares: aMCI; blue triangles: naMCI. Top plot: wake after sleep onset; bottom plot: times up at night. Statistical significance bars calculated using the pooled variance across weeks for each group.
Similar trends were seen in the weekly inter-quartile ranges; both the WASO IQR (p<0.003) and times up at night IQR (p=0.0004) were significantly smaller for the aMCI group, indicating that they had less day-to-day variability in these measures than the intact and naMCI volunteers. In spite of this inter-subject variability, the between-subject variability was greatest for aMCI and least for the intact subjects. Table 3 summarizes the results of the analysis of the objective measures.
Table 3.
Means and standard deviations of objective sleep measures across groups. ‡p<0.05 for aMCI<naMCI; *p<0.001 for aMCI<intact, naMCI; and §p<0.001 for naMCI>intact, aMCI. All p-values after correction for multiple comparisons. Numbers of participants in each group are shown in parentheses.
| Measure | Intact (29) | aMCI (6) | naMCI (10) |
|---|---|---|---|
| ‡ Movement in Bed (MIB, in sensor firings) | 9.40 +/- 0.40 | 7.81 +/- 0.88 | 10.85 +/- 0.68 |
| * Wake After Sleep Onset (WASO, in mins) | 27.22 +/- 1.19 | 13.51 +/- 2.62 | 20.64 +/- 2.02 |
| Total Sleep Time (TST, in hours) | 8.34 +/- 0.04 | 8.50 +/- 0.09 | 8.45 +/- 0.07 |
| § Settling Time (mins) | 2.5 +/- 0.07 | 2.32 +/- 0.15 | 3.07 +/- 0.11 |
| * Times Up at night (# times) | 2.08 +/- 0.04 | 1.63 +/- 0.10 | 1.89 +/- 0.08 |
Both the average of the times up at night over the past 26 weeks and the median times up in the week immediately preceding the questionnaire were fitted using ordinal logistic regression to determine if these values predicted the self-report estimate of times up at night. Both objective measures predicted the self-report measure (previous 26 weeks: Χ2=8.15, p=0.017; previous week: Χ2=8.65, p=0.013). Sixty-five percent of volunteers got up once or twice at night. However, 47% of volunteers misreported their times up at night, with approximately equal numbers over- and under-reporting. However, there were no differences across groups in this ability to report how often they got up at night.
DISCUSSION
Using in-home sensors to collect ongoing objective measures of sleep and nighttime behaviors, we found that aMCI, naMCI, and cognitively intact volunteers show different patterns of sleep disturbances. In particular, amnestic MCI volunteers had less disturbed sleep than both non-amnestic MCI and cognitively intact volunteers, as measured by movement in bed at night, wake after sleep onset, and times up at night. In general, the naMCI volunteers showed a level of disturbed sleep that was intermediate to that of aMCI and intact volunteers. The one exception was movement in bed, which measured restlessness at night, and which was greater in naMCI volunteers than in aMCI volunteers. These differences were seen even though the self-report of sleep behaviors did not differ between groups. Interestingly, the total sleep times were equivalent across groups, which is consistent with the few reports of this measure in patients with mild cognitive impairment35. The relationship between sleep and MCI status is challenging to untangle given the evidence that poor sleep can lead to compromised cognitive function21, 36, 37. A recent study by Westerberg and colleagues suggested that poorer scores on next-day word and face recall were associated with less time in bed and with lower subjective sleep quality in aMCI but not in intact volunteers21. However, they did not see an influence of total sleep time or wake after sleep onset on the next-day scores, nor did they see differences in objective sleep measures derived from actigraphy between intact and aMCI volunteers.
In contrast with past studies, our findings suggest that aMCI volunteers typically experience less sleep disruption during the night than cognitively intact volunteers. Some studies using objective measures of sleep such as polysomnography (PSG)25 or actigraphy data21 found no differences between aMCI and healthy control in sleep measures such as WASO, TST, and sleep latency. Other PSG studies have reported a greater number of slow wave sleep (SWS) arousals22, shifts from non-REM sleep38 and arousals due to periodic leg movements (PLM)23, 24 in aMCI and demented patients. One possible reason for the difference in our findings is that SWS arousals and shifts from non-REM sleep, measurable only with PSG, may occur more frequently in aMCI patients. Even periodic leg movements may be small enough that our sensors do not capture them. Since actigraphy also does not capture PLMs, this would also explain why this increase has been reported in PSG but not in actigraphy studies. However, polysomnography is known to be disruptive to sleep39, with a strong “first-night effect”. Since our data are collected continuously over six months, our approach provides data about individual’s “typical” night rather than a single night in a PSG clinic. This would be consistent with Westerberg’s finding that the variability in sleep latency, WASO, and total sleep time were correlated with performance on the memory tests for aMCI patients – i.e. that these measures may be highly variable in an aMCI population21. Our measures capture differences in night-to-night sleep disruption that are not seen with PSG.
Of great interest is the recent observation in humans that suggests those with less disrupted sleep have lower CSF β-amyloid concentrations40, a CSF profile associated with AD, Although we do not have CSF β-amyloid concentrations for our participants, given the greater association of amnestic MCI with AD, it is plausible that the sleep metrics we have observed may reflect underlying amyloid production dynamics regulated in part by activity cycles associated with the development of AD.
It is notable that only about half of the volunteers were able to reliably report how often they got up at night. Accurate measures of nighttime behaviors such as times up at night are particularly important for medication studies, where reliable measures are needed to determine if a medication intended to improve for example nocturia is effective. This difficulty in self-report may be in part due to the significant night-to-night variability that is revealed by the objective measures. When reporting sleep behavior during a clinic visit, patients will undoubtedly vary in what experience they choose to emphasize. For example, they may report the most recent night’s sleep, or their general impression of the past couple of weeks.
The fact that self-report measures did not differentiate the groups – even for a measure that was well correlated with its equivalent objective measure (times up at night) – underscores the value of collecting frequent in-home measurements. Over a 26-week period, all of the measures showed marked variability for most volunteers, reflecting the many influences such as life events (e.g. illness or death of friends or family) on sleep in a geriatric population. Some volunteers showed periods or bursts of increased disruption over the six-month period. We did not treat these periods differently, but this variability over the six-month period is a likely factor in the lack of an effect of time in our current models.
Although there were no differences between groups in their use of stimulants and sedatives, we did not record caffeine consumption and therefore this may varied across the groups. If so, this could account for differences in restlessness. In addition, our sample size was small, and a larger study is needed to verify these results. However, the differences between groups were quite large even in this small sample. Another limitation of this study is that it was a cohort of the oldest-old, and due to their age the participants were mostly women; thus a younger cohort may show different patterns of sleep disturbances.
Future work needs to take into account factors such as life events, seasonality, and holidays that may disrupt sleep at different time scales. Unobtrusive capture of continuous measures of sleep collected over extended periods of time provide important insights into the sleep patterns of healthy and cognitively impaired individuals, and enable the conduct of longitudinal studies. More in-depth analyses may identify specific factors resulting in acute changes in sleep patterns as well as longer-term trends and their implications for declines over time in specific neurocognitive domains, as well as the risk of developing dementia and other critical health outcomes.
ACKNOWLEDGMENTS
The authors thank Colette Duncan, Kaitlin Carter, Brittany Stone, and Jon Yeargers for their assistance with data collection. This work was supported by National Institutes of Health grants AG024978 and AG024059. Some computers used in this work were paid for by Intel Corporation.
Footnotes
Conflicts of Interest and Source of Funding: This work was supported by National Institutes of Health grants AG024978 and AG024059. Some computers used in this work were paid for by Intel Corporation. Dr. Hayes has a significant financial interest in Intel Corporation, a company that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by OHSU. Mr. Riley has no potential conflict of interest with this work. Ms. Mattek has no potential conflict of interest with this work. Dr. Pavel has no potential conflict of interest with this work. Dr. Kaye has no potential conflict of interest with this work.
REFERENCES
- 1.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995 Jul;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010 Apr;11(4):372–377. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997 Jan;45(1):1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 4.Maggi S, Langlois JA, Minicuci N, et al. Sleep complaints in community-dwelling older persons: prevalence, associated factors, and reported causes. J Am Geriatr Soc. 1998 Feb;46(2):161–168. doi: 10.1111/j.1532-5415.1998.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 5.Tractenberg RE, Singer CM, Kaye JA. Symptoms of sleep disturbance in persons with Alzheimer's disease and normal elderly. J Sleep Res. 2005 Jun;14(2):177–185. doi: 10.1111/j.1365-2869.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley DJ, Monjan AA, Masaki KH, Enright PL, Quan SF, White LR. Associations of symptoms of sleep apnea with cardiovascular disease, cognitive impairment, and mortality among older Japanese-American men. J Am Geriatr Soc. 1999 May;47(5):524–528. doi: 10.1111/j.1532-5415.1999.tb02564.x. [DOI] [PubMed] [Google Scholar]
- 7.Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. Apr;11(4):372–377. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM, Vecchierini M-F. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28(8):981–989. [PubMed] [Google Scholar]
- 9.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006 Jan-Mar;20(1):41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006 Apr;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 11.Geda YE, Smith GE, Knopman DS, et al. De novo genesis of neuropsychiatric symptoms in mild cognitive impairment (MCI) Int Psychogeriatr. 2004 Mar;16(1):51–60. doi: 10.1017/s1041610204000067. [DOI] [PubMed] [Google Scholar]
- 12.Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010 Jun;23(2):123–130. doi: 10.1177/0891988710363710. [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009 Aug;21(4):654–666. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 14.Tatsch MF, Bottino CM, Azevedo D, et al. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry. 2006 May;14(5):438–445. doi: 10.1097/01.JGP.0000218218.47279.db. [DOI] [PubMed] [Google Scholar]
- 15.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. Jama. 2002 Sep 25;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 16.Ellison JM, Harper DG, Berlow Y, Zeranski L. Beyond the "C" in MCI: noncognitive symptoms in amnestic and non-amnestic mild cognitive impairment. CNS Spectr. 2008 Jan;13(1):66–72. doi: 10.1017/s1092852900016175. [DOI] [PubMed] [Google Scholar]
- 17.Rozzini L, Vicini Chilovi B, Conti M, et al. Neuropsychiatric symptoms in amnestic and nonamnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;25(1):32–36. doi: 10.1159/000111133. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg PB, Mielke MM, Appleby B, Oh E, Leoutsakos JM, Lyketsos CG. Neuropsychiatric symptoms in MCI subtypes: the importance of executive dysfunction. Int J Geriatr Psychiatry. 2011 Apr;26(4):364–372. doi: 10.1002/gps.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011 Oct;34(10):1347–1356. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011 Dec;59(12):2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westerberg CE, Lundgren EM, Florczak SM, et al. Sleep Influences the Severity of Memory Disruption in Amnestic Mild Cognitive Impairment: Results From Sleep Self-assessment and Continuous Activity Monitoring. Alzheimer Dis Assoc Disord. 2010 Jun 29; doi: 10.1097/WAD.0b013e3181e30846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hita-Yañez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE epsilon4 genotype. Curr Alzheimer Res. 2012 Mar;9(3):290–297. doi: 10.2174/156720512800107609. [DOI] [PubMed] [Google Scholar]
- 23.Chen PC, Wu D, Chen CC, Chi NF, Kang JH, Hu CJ. Rapid eye movement sleep atonia in patients with cognitive impairment. J Neurol Sci. 2011 Jun 15;305(1-2):34–37. doi: 10.1016/j.jns.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Yu JM, Tseng IJ, Yuan RY, Sheu JJ, Liu HC, Hu CJ. Low sleep efficiency in patients with cognitive impairment. Acta Neurol Taiwan. 2009 Jun;18(2):91–97. [PubMed] [Google Scholar]
- 25.Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry. 2011 Apr;19(4):374–381. doi: 10.1097/JGP.0b013e3181e9b976. [DOI] [PubMed] [Google Scholar]
- 26.Hayes TL, Riley T, Pavel M, Kaye JA. Estimation of rest-activity patterns using motion sensors. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC'10. 2010:2147–2150. doi: 10.1109/IEMBS.2010.5628022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaye JA, Maxwell SA, Mattek N, et al. Intelligent Systems for Assessing Aging Changes: Home-based, unobtrusive and continuous assessment of aging. Journal of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66B(SI):i180–i190. doi: 10.1093/geronb/gbq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997 Feb 24;157(4):449–454. [PubMed] [Google Scholar]
- 29.Folstein M, Folstein S, McHugh P. "Mini-mental state" - a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Morris J. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982 May;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 32.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995 Feb;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992 Mar;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004 Sep;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 35.Meguro K, Ueda M, Kobayashi I, et al. Sleep disturbance in elderly patients with cognitive impairment, decreased daily activity and periventricular white matter lesions. Sleep. 1995 Feb;18(2):109–114. doi: 10.1093/sleep/18.2.109. [DOI] [PubMed] [Google Scholar]
- 36.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002 Jan;17(1):73–77. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 37.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999 May 1;22(2):S354–358. [PubMed] [Google Scholar]
- 38.Spiegel R, Herzog A, Koberle S. Polygraphic sleep criteria as predictors of successful aging: an exploratory longitudinal study. Biol Psychiatry. 1999 Feb 15;45(4):435–442. doi: 10.1016/s0006-3223(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 39.Agnew HW, Jr., Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966 Jan;2(3):263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Potter R, Sigurdson W, et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol. 2011 Jan;69(1):51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]