Abstract
Background
Macrophage infiltration to the injury site during the acute response to traumatic spinal cord injury (SCI) is not uniform. Macrophage phenotype has been characterized as either pro-inflammatory (M1) or anti-inflammatory (M2). Animal studies suggest that M1/M2 dominance at the site of injury relates to spontaneous recovery following SCI.
Objective
To investigate whether the phenotype of circulating macrophage precursors-monocytes (MOs), is altered in the acute phase of SCI and corresponds to circulating inflammatory cytokines.
Study Design
Prospective observational cohort study.
Setting
A single academic medical center in Pennsylvania, US.
Patients
A cohort of 27 complete or incomplete traumatic SCI subjects enrolled within 7 days post-SCI injury.
Methods
MO phenotype was defined within the first week post-SCI, using flow cytometry, and compared to historical uninjured controls. Concentrations of 25 cytokines/chemokines were assessed using Luminex in serial blood samples up to two weeks post-SCI. ANOVA was used to determine the correlations between the phenotypes and the cytokine profiles.
Results
Patients subsets were identified with either M1 dominant or M2 dominant circulating MOs distinct from the uninjured controls. The M1-dominant was associated with higher circulating levels of pro-inflammatory mediators IL-12p70 and IP-10, and lower levels of anti-inflammatory cytokines IL-10, IL-15 and IL-7, whereas the M2-dominant exhibited the opposite cytokine profiles with significantly higher IL-10 and IL-7.
Conclusion
In the acute phase after SCI, at comparable injury severity, subgroups of patients exhibit distinct M1/M2 MOs dominance and the phenotype is correlated with M1 or M2-specific cytokine/chemokine profiles. Though further studies are needed to determine how these observed phenotypic differences relate to functional recovery, our findings 1) provide the first evidence indicating the possible individual differences in the immune responses to the comparable traumatic SCI, with potential implications for management of acute SCI and rehabilitation; 2) may represent easily accessible biomarkers with prognostic utility.
Keywords: acute spinal cord injury, macrophage/monocyte, M1/M2 phenotype, cytokine/chemokine, biomarker
INTRODUCTION
Traumatic Spinal Cord Injury (SCI) leaves approximately 10,000 individuals impaired in the US and costs society an estimated $10 billion annually 1. It is well known that excessive inflammatory responses at the injury sites of the spinal cord would extend the initial tissue damage and cause secondary neural destruction in the first several weeks post-injury 2.
Circulating monocytes (MOs) originate from myelomonocytic stem cells in bone marrow and continually replace the tissue resident macrophages. In response to traumatic SCI, MOs/macrophages are among the earliest responsive innate immune cells that infiltrate the injury sites and persist locally, suggesting that these cells play an important role in the trauma-related inflammatory process 3, 4. Infiltration of circulating MO-derived macrophages has been traditionally viewed as detrimental, as these cells cause secondary destruction to the nervous system 5, 6. However, in line with well-known actions of macrophages in wound healing, including debris-cleaning and repair activities, a beneficial role has also been suggested for the infiltration of MOs/macrophages at SCI sites, which may contribute to neuroregeneration 7–9. The possible direct impact of macrophages on functional recovery after SCI has been demonstrated in animal models 10 and humans 11. A growing body of data suggests that MOs/macrophages are not functionally homogenous. Monocytes and macrophages consist of distinct subsets: pro-inflammatory classically activated (M1) or anti-inflammatory alternatively activated (M2), each possessing different destructive and regenerative potential. The important role of M2 alternatively activated MOs/macrophages (IL-10-producing) in neural repair has been demonstrated recently in an animal model of SCI, suggesting that M2-dominant MOs contribute to scar resolution, which is a well-known critical step that determines axonal re-growth and spinal cord repair 7, 12. The presence of distinct subsets of classically activated M1 or alternatively activated M2 macrophages has also been confirmed by transcriptome profiling in cultured human MOs/macrophages 13.
As precursors of macrophages, MOs may also be heterogeneous. Passlick and colleagues 14 were the first to report that human MOs could be divided into two main populations according to the expression pattern of the cell surface monocyte marker CD14 and CD16. The two major subpopulations were first defined according to CD16 expression: MOs expressing CD14 but not CD16 (CD14+/CD16−), and those expressing both CD16 and CD14 (CD14+/CD16+). The former have been referred to as classical MOs and the latter have been considered to be inflammatory MOs, as they are expanded in response to inflammation and infection 15. However, later analyses have suggested additional heterogeneity of the human inflammatory CD14+/CD16+ MOs, with low or high expression of CD14; the CD14low/CD16+ subset of MOs is capable of producing high levels of pro-inflammatory cytokines, such as TNFα, in contrast to only low levels of the anti-inflammatory cytokine IL-10 16, 17. On the other hand, the CD14high/CD16+ subset of MOs are capable of producing high levels of the anti-inflammatory cytokine IL-10 18. The change in population of inflammatory CD14+/CD16+ MOs in response to infection and inflammation has been well described, but has not been examined in the context of SCI.
These data suggest that the balance between the actions of M1 and M2 MOs/macrophages in the acute phase of SCI may ultimately be one determinant of further destruction or preservation and regeneration of the damaged neural system. Since assessing the dominance of the macrophages in patients with SCI would require tissue sampling of the spinal cord, MOs as circulating macrophage precursors have the potential to serve as accessible biomarkers. Moreover, understanding the balance between the actions of M1 and M2 MOs may open a new avenue of future therapeutics aimed at limiting secondary damage and promoting repair in traumatic SCI. The goal of this study, therefore, was to characterize the cellular phenotypes of peripheral blood MOs in individual patients with traumatic SCI, as well as examining the association of this cellular phenotype with plasma cytokine profiles. As a secondary goal, we also compared the MO profiles of the SCI patients to that of a historical non-injury control group. Our working hypothesis was that patients with M1-like MO predominance would have more circulating pro-inflammatory mediators, whereas the M2-like MO predominance would be associated with more anti-inflammatory mediators. Therefore, we focused the examination on the cytokines/proteins that are classically associated with MOs/macrophages
MATERIALS AND METHODS
Spinal cord injury participants
A prospective cohort of 27 acute traumatic SCI subjects including American Spinal Injury Association (ASIA) Impairment Scale (AIS) A-D was recruited in the University of Pittsburgh Medical Center (UPMC) from May 2010 to October 2011. This is a consecutive sampling of participants 18 years of age or older presenting to a level 1 trauma center with MRI confirmed diagnosis of acute traumatic spinal cord injury. The AIS and Injury Severity Score (ISS) 19, which accounts for the burden of concurrent traumatic injury, were collected within the first week post-injury for each subject. The procedures were approved by the local ethics committees. Only SCI patients with written informed consents obtained within 7 days post injury were enrolled. Exclusion criteria included pre-existing immune diseases or immune suppression treatments as well as any previous SCI or other neurological diseases which affect motor and sensory function, or non-traumatic etiology.
Blood sample collection and analyses
The first blood sample from each patient was obtained within the first week post-SCI, as well as three times per week for 2 weeks post-SCI, drawn from a venous catheter into a 5-ml EDTA-containing tube. The sample was kept on ice and MO phenotype was determined in fresh whole blood aliquots by flow cytometry. The remaining blood sample was centrifuged within 2 hours. The resulting plasma samples were stored at -80°C for later analysis of inflammatory mediators (see below).
Flow cytometry
All antibodies were purchased from BioLegend, (San Diego, CA) unless otherwise stated. 100 μl of fresh blood was incubated in the dark and at room temperature for 20 min with fluorophore-conjugated anti-human antibodies to CD14 (clone M5E2), CD16 (clone 3G8), or appropriate isotype controls (mouse IgG2a κ and mouse IgG1 κ). Following staining, erythrocytes were lysed and leukocytes were fixed using the Immunoprep Reagent kit (Beckman Coulter, Inc., Hialeah, FL). The samples were analyzed on a LSR II flow cytometer (Becton Dickinson, San Jose, CA). Data analysis was performed using FlowJo 7 (Tree Star Inc., Ashland, OR, USA).
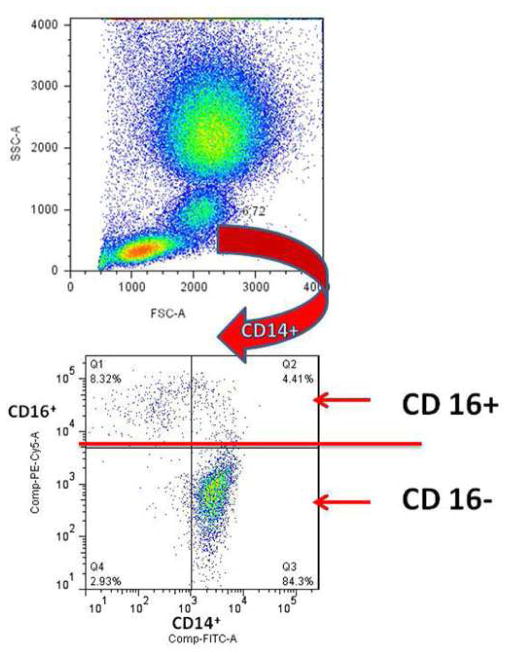
From the total leukocyte population in the peripheral blood, MOs could be identified based on their light-scatter properties: high forward-scatter (FSC-A) and intermediate side-scatter (SSC-A) (Fig. 1, Upper panel). Further analysis of the gated MOs showed different CD14/CD16 expression patterns. Accordingly, three monocyte subsets, CD14low/CD16+ (M1-like), CD14high/CD16+ (M2-like) (both subsets combined - CD14+/CD16+ MOs have been referred to as the inflammatory MO population) and CD14high / CD16− (classic) were gated according to the staining of isotype control antibodies (data not shown). This analytical approach allowed us to quantify the relative size of each monocyte subpopulation (Fig. 1, Lower panel).
Fig. 1. Identification of monocyte subsets.
MO population presented in the whole blood was identified using light scatter characteristics (Upper panel). An arrow indicates sequential gating of the relevant population in a representative scatter plot showing correlated expression of CD14 and CD16 has revealed three distinct subpopulations. First, the staining of CD16 defined the major classic MO subpopulation is CD14high / CD16− (quadrant3) and the inflammatory MO subpopulation CD14+/CD16+, which contains two CD16+ subsets with different expression levels of CD14: CD14low / CD16+ in quadrant (Q)1 and CD14high / CD16+ in Q2. The frequency of each cell subpopulation was determined and shown in the respective quadrant (Lower panel).
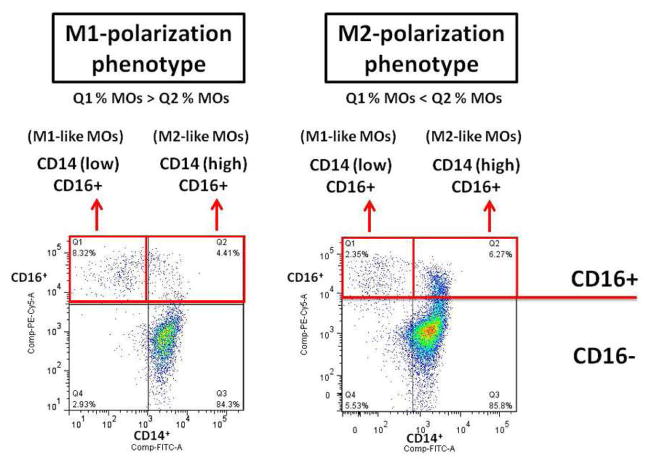
To better define the MO phenotype difference in the SCI patients, we analyzed the distributions of the two subsets of inflammatory MOs in each SCI patient. Consequently, a SCI patient with a higher frequency of CD14low/CD16+ MOs was referred to as having M1-dominance phenotype. Likewise, if the frequency of CD14high/CD16+ MOs was higher, then the patient was referred to as having the M2-dominance phenotype (see Figure 2)
Fig. 2. Defining MO phenotypes in SCI patients.
We used flow cytometry to further examine the CD14 & CD16 expression pattern and to analyze the distributions of the two subsets of inflammatory MOs in each SCI patient. Consequently, M1-like phenotype refers to samples where the frequency of CD14low/CD16+ MOs in Q1 was greater than that of CD14high/CD16+ MOs in Q2; whereas M2-like phenotype refers to that of CD14low/CD16+ MOs in Q1 less than that of CD14high/CD16+ MOs in Q2.
Comparison of SCI subjects’ inflammatory MOs to that of historical non-injury controls
The relative frequency of CD14+/CD16+ MOs in SCI patients was compared to that of the non-injury control reference. The non-injury control flow cytometry data was obtained from basal fasting blood samples from 18 healthy lean controls without recent injury/infection (age range 31–55 yrs old females) enrolled in an exercises-related metabolic study at the same academic medical center 20.
Analysis of circulating inflammatory mediators
25ul of plasma per sample in all serial blood samples collected were analyzed on a Luminex LX100 apparatus (MiraiBio, Austin, TX) using a Millipore, human 25-analyte cytokine/chemokine assay kit that included: EOTAXIN, GM-CSF, IFN-α2, IFN-γ, IL-1Ra, IL-1β, IL-2, SIL-2RA, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IP-10 (CXCL10), MCP-1, MIP-1α, MIP-1β, TNF-α, and MIG (CXCL9) (Millipore, Billerica, MA). Using the Luminex LX100, the median fluorescent value of each analyte was reported and compared to the fluorescent value of a standard curve with a range of 3.2 pg/ml to10,000 pg/ml for all analytes.
Statistical analysis
Student’s 2-tailed t-test was used when all 27 SCI subjects compared to the historical non-injury controls for analyzing the MO subsets and for the comparisons made at a single time point between the two phenotypes of patients. A one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test using SPSS software SYSTAT statistical program (Evanston, IL) was used for multiple comparisons: 1) for the simultaneous comparison of phenotypes and times on the concentrations of the inflammatory mediators in the first week post-SCI; 2) for the simultaneous comparisons of phenotypes and injury levels/ASIA on ISS. The results were expressed as mean ± SE. Differences were considered significant at p < .05.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
RESULTS
Phenotypical Difference of Circulating MOs in SCI Patients
The demographic information of the 27 SCI patients is shown in Table 1. Twenty of the patients had motor complete injury in accordance with the ASIA AIS A/B, and seven of the patients had motor incomplete motor injury ASIA AIS C/D (Table 1).
Table 1.
SCI patient (N=27) demographics.
| Case No. | Age (yrs) | Sex | Injury Level | ASIA grade | Cause of Injury | Days post-SCI |
|---|---|---|---|---|---|---|
| 1 | 77 | F | C-5 | A | fall | 7 |
| 2 | 26 | M | T-2 | A | MVC | 7 |
| 3 | 43 | M | T-12 | A | GSW | 6 |
| 4 | 27 | F | T-11 | A | sport accident | 5 |
| 5 | 28 | M | T-10 | A | sport accident | 3 |
| 6 | 19 | M | T-10 | A | MVC | 7 |
| 7 | 21 | M | T-12 | A | fall | 3 |
| 8 | 49 | M | T-3 | A | fall | 3 |
| 9 | 38 | F | C-4 | A | MVC | 3 |
| 10 | 63 | M | C-5 | A | fall | 4 |
| 11 | 25 | M | C-5 | A | fall | 4 |
| 12 | 21 | M | T-4 | A | MVC | 3 |
| 13 | 18 | M | C-7 | A | fall | 3 |
| 14 | 18 | M | C-5 | A | sport accident | 4 |
| 15 | 41 | M | T-9 | A | MCC | 2 |
| 16 | 57 | M | C-4 | A | fall | 2 |
| 17 | 54 | M | C-5 | A | fall | 2 |
| 18 | 21 | M | T-11 | A | GSW | 1 |
| 19 | 39 | M | T-11 | B | GSW | 3 |
| 20 | 54 | F | C-5 | B | MVC | 7 |
| 21 | 31 | M | L-1 | C | sport accident | 6 |
| 22 | 61 | M | C-4 | C | fall | 3 |
| 23 | 46 | M | C-5 | C | fall | 3 |
| 24 | 67 | M | C-4 | C | fall | 3 |
| 25 | 62 | M | T-9 | C | fall | 4 |
| 26 | 28 | M | C-6 | C | MVC | 2 |
| 27 | 67 | M | T-8 | D | MVC | 3 |
Abbreviations: MVC, motor vehicle crash; GSW, gunshot wound; MCC, motor cycle crash.
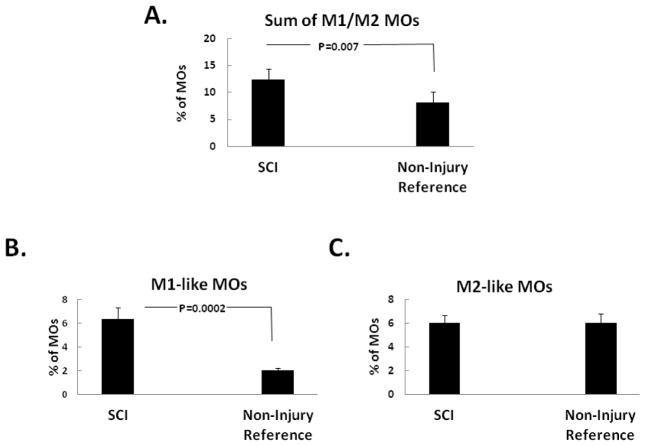
Flow cytometry analyses of these 27 SCI patients were compared to 18 historical non-injury controls. The inflammation-responsive CD14+ / CD16+ MOs were increased in frequency in response to traumatic SCI compared to the non-injury reference controls (Fig. 3A). And this seemed to be the result of the increased frequency of the CD14low/CD16+ MOs (Fig. 3B), which was higher in the injury, but not that of CD14high/CD16+ MOs, which was unchanged between the injury and the non-injury (Fig. 3C).
Fig. 3. Proportions of inflammatory monocyte in SCI patients.
The peripheral blood from SCI patients (n=27) was analyzed for the percentage of different MO subsets in total MOs. CD14high / CD16− MOs were not different between the SCI (n=27) and the non-injury (n=18) reference controls (data not shown). In contrast, the total inflammatory CD14+ / CD16+ MOs were significantly higher in the SCI patients than the non-injury reference controls (A.). This is mainly due to the difference in the M1-like subset of MOs, which was higher in the SCI group (B.), rather than that of the M2-like subset of MOs, which did not significantly differ between the two populations (C.).
When we further analyzed the 27 SCI subjects and compared them at similar injury severities, we found that the relative frequency of the CD14low/CD16+ or CD14high/CD16+ MOs subset in the total inflammatory MO population differed in SCI individuals despite comparable injury severity or injury levels (data not shown), and thus displayed M1-dominance or M2-dominance phenotype. For the further assessments made later of the circulating profile of the inflammatory mediators in relation to the M1/M2, we divided the SCI patients into two groups: one with motor-complete ASIA A/B SCI, and the other with motor-incomplete ASIA C/D SCI. The total inflammatory MO population was not significantly different between groups of M1 polarized and M2 polarized SCI patients (data not shown). However, in the ASIA A/B group, the M1-dominance phenotype displayed ~65% of CD14low/CD16+ MOs versus ~35% CD14high/CD16+ MOs; on the other hand, the M2-dominance phenotype consisted of ~30% CD14low/CD16+ MOs and ~70% CD14high/CD16+ MOs within the inflammatory MOs. A similar distribution of MOs was noted also in ASIA C/D M1 or M2 dominance phenotype.
The ASIA A/B complete motor function deficit group had 12 subjects (7 cervical; 5 thoracic) with M1-dominance phenotype and 8 subjects (3 cervical; 5 thoracic) with the M2-dominance phenotype, whereas the incomplete motor deficit group ASIA C/D had 2 subjects (2 cervical; 0 thoracic) with the M1-dominance phenotype and 5 (2 cervical; 3 thoracic) with the M2-dominance phenotype. In contrast, only one patient in the non-injury control group demonstrated a M1-dominance phenotype, whereas the other 17 subjects demonstrated the M2-dominance phenotype.
Although the calculated scale of ISS includes SCI, the ISS scale also includes the injuries of the other systems of the body at the time of SCI. As expected, the ISS showed significant differences between the motor complete ASIA A/B and the motor incomplete ASIA C/D (p=.016) whereas the ISS showed no statistically significant difference between the patients with M1 or M2 phenotype or cervical vs. thoracic/lumbar levels (data not shown). Therefore, the M1/M2 phenotypic differences in motor complete or incomplete SCI patients is not explained by different ISSs, injury levels or sampling timing (data not shown).
Correlation between Circulating Cytokine Profiles and Monocyte Phenotypes in the Acute Response to SCI
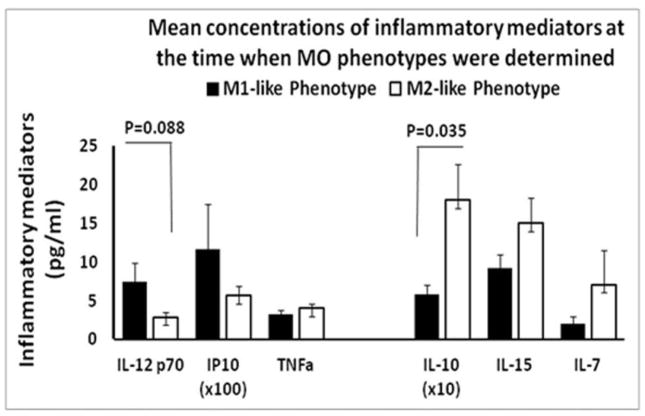
Due to limitation of sample size in the ASIA C/D group (n=2 M1-dominace and n=5 M2-dominace), only the ASIA A/B were included in the analysis of the circulating profile of the inflammatory mediators in relationship to the M1/M2 phenotypes. The motor complete ASIA A/B SCI patients who had M1-dominace of circulating MOs also exhibited a trend towards higher circulating levels of the pro-inflammatory cytokine IL-12p70 and the chemokine Interferon gamma-induced protein 10 kDa (IP-10/CXCL10), especially on the same day when the MO phenotype was analyzed (Fig. 4). In contrast, the SCI patients who had M2-dominace of circulating MOs had higher levels of the anti-inflammatory cytokine IL-10, the reported anti-apoptotic cytokine IL-15, and T-cell differentiation-stimulating cytokine IL-7. Those different profiles were present not only on the day when MOs were obtained (Fig. 4), but also in the serial blood samples in the first week post-SCI (Fig. 5). During this time period, the SCI patients with M1-dominace MOs also exhibited a pattern moving toward higher TNFα levels.
Fig. 4. Plasma concentrations of pro-inflammatory mediators IL-12p70, IP-10 and TNF-α on the left and anti-inflammatory or pleiotropic cytokines IL-10, IL-15 and IL-7 on the right in M1 or M2 dominance SCI patients measured on the day of MO sampling (see Materials and Methods for details).
All values are mean ± SE. Statistically significant differences were indicated in the figure.
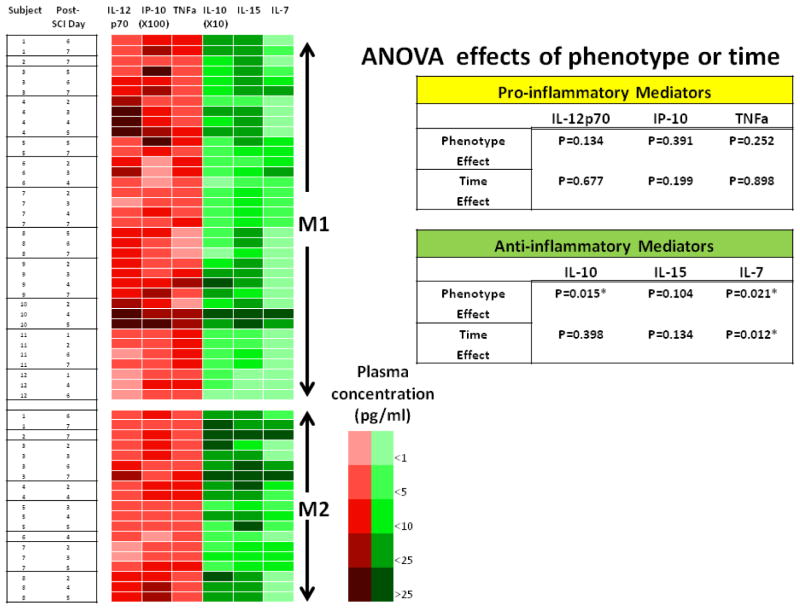
Fig. 5. Patterns of pro-inflammatory mediators IL-12p70, IP-10 and TNFa and anti-inflammatory or pleiotropic cytokines IL-10, IL-15 and IL-7 distributed in M1 or M2 dominance SCI patients in the first week post-SCI.
This figure illustrates the phenotype-dependent pattern of inflammatory mediators in individual patient in serial samples within the first week post-SCI. The concentrations of the each mediator are illustrated by heat-map according to the color grades indicated. Statistically significances related to phenotype or time by ANOVA are indicated in the table. See Materials and Methods for details.
In particular, statistically significant differences (P=.035) in circulating levels of IL-10 between SCI patients with M1-dominace or M2-dominace were not only observed on the day when MOs were analyzed (Fig. 4), but also in the following week: ANOVA showed significant differences in IL-10 between patients with M1 dominance and M2 dominance (P=.015), whereas the timing of sampling after injury had little association with the levels of IL-10 (Fig. 5). The association of IL-10 with monocyte phenotype persisted into the second week post-SCI (data not shown). The difference in IL-7, however, may due to both phenotype and time effects. Although the pro-inflammatory mediators IL-12p70, IP-10, and TNFα did not reach statistically significant differences between SCI patients with M1- or M2-dominance, the patterns between M1 and M2 tended to be opposing (Fig. 4 & 5). Other known MO-related mediators, e.g. IL-1β, IL-6, IL-8, Macrophage Inflammatory Proteins (MIP)-1α, and MIP-1β did not exhibit any clear patterns between M1/M2 phenotypes.
DISCUSSION
Our pilot study suggests that the altered balance between the M1/M2 MO subsets may give rise to different immunologic phenotypes reflecting individual differences in acute responses to traumatic SCI and those different phenotypes correlate with different profiles in circulating inflammatory mediators, particularly the anti-inflammatory cytokine IL-10.
It is generally accepted that infiltrating macrophages consist of destructive M1 and repairing M2 macrophages 8, 13. Similarly, the precursors of macrophages in blood also consist of M1-like, pro- inflammatory CD14low / CD16+ and M2-like, anti-inflammatory CD14high / CD16+ subsets of MOs. This categorization of cell subsets is supported by intracellular cytokine staining data from in vitro studies 16–18. In the present study, although the frequency of total CD14+/CD16+ MOs was similar in all subjects, we found that SCI patients had either M1 (CD14low/CD16+) or M2 (CD14high/CD16+) dominance, and that such phenotypic differences were not dependent on ISS, injury levels or sampling timing, and correlated with different patterns of inflammatory mediators, suggesting distinct inflammatory responses to acute SCI in these two different subpopulations.
The heterogeneous phenotype of activated MOs/macrophages is thought to be required for the divergent function of MOs/macrophages in a given inflammatory process 8, 13. Thus both M1 and M2 MOs/macrophages are necessary for inflammatory reactions post-SCI. The M1 MOs/macrophages are likely ultimately essential in removing cell debris from lesion areas, whereas the M2 MOs/macrophages may help with repair. Furthermore, the balance between the two macrophage subsets may affect functional recovery after SCI 9–11. It has been shown that the M1 response overwhelms the transient M2 response after 7 days post-SCI in a rodent model of SCI 8. Therefore, we chose to analyze M1/M2 dominance during the first week following the injury. It is possible that MO dominance (M1 vs. M2) in SCI patients may change late in the course of healing, and while this could not be addressed with the current study design, it should be addressed in future studies.
The mechanisms that control the dynamic balance between M1/M2 MOs/macrophages remain to be elucidated. What is known, however, is that favorable MO dominance could be induced by human mesenchymal stem cells 21, and that immune tolerance driven in part by alternatively activated M2 macrophages could be modulated through recognition of self-antigen 22. This may suggest the M2-dominant phenotype, if it is related to better outcomes, could be induced. In a rodent model of SCI, improved motor function was noted along with axonal regeneration when autologous activated macrophages were injected into the injured spinal cord 10, 23. The analogous human translational clinical trials along this line showed promise in a phase 1 study 11. However, a phase 2 trial did not show any significant improvement compared to the controls 24. It is possible that the human study, unlike the rodent study, did not show any favorable outcomes due to greater variability in human immune responses in the acute phase of SCI. Therefore, identifying M1/M2 dominant phenotypes may facilitate future personalized medicine approaches by targeting a subset of SCI patients who are more likely to respond to immune-modulating therapy.
The MOs/macrophages that infiltrate the injured spinal cord generate secondary damage by releasing pro-inflammatory chemokines and cytokines. Reducing such pro-inflammatory mediators is critical in limiting the progression of secondary damage to tissue spared from the initial injury 7, 8. In the current study, we hypothesized that circulating levels of inflammatory mediators may be related to the predominance of M1 or M2 infiltrating blood MOs that arises shortly post-SCI in individual patients. Accordingly, we investigated the correlation between the circulating inflammatory mediators and the M1/M2 MO phenotype following SCI. We find that with motor-complete injury (ASIA A/B), the MO phenotype segregates individual SCI patients into two subgroups with distinguishable patterns of inflammatory mediators. The subjects with a higher frequency of CD14 (low) CD16+ MOs (M1 phenotype) were associated with tendency of higher circulating levels of the pro-inflammatory mediators IL-12p70 and IP-10, and lower levels of the anti-inflammatory cytokine IL-10, IL-15, as well as of the pleiotropic cytokine IL-7. In contrast, subjects with a higher frequency of CD14(high) CD16+ MOs (M2 phenotype) exhibited the significantly higher levels of IL-10 and IL-7 along with a pattern moving towards lower levels of IL-12p70 and IP-10 and higher levels of IL-15..
Because post-injury inflammation is a dynamic process that might be associated with time-dependent changes of the inflammatory mediators, we used days post-SCI as a covariant in the ANOVA analyses when evaluating the contributions of each key component to the differences between M1 and M2 phenotypes. In this analysis, we found that the time factor only significantly influenced IL-7, but did not explain the dynamic variability in the concentrations of the other inflammatory mediators examined. In addition, the levels of IL-10 depended solely on the MO phenotype, with little of effect of time post-SCI. Although the source of these cytokines cannot be surmised from the current study, prior ex vivo studies using secretion and depletion assay identified MO as the source of inflammatory cytokines in a human in vivo glomerulopathy model 25. However, we did not provide direct evidence for the circulating MOs acting as the source of the secreted cytokines, and therefore we cannot exclude the contributions of other immune cells to the circulating levels of the inflammatory mediators, which is a limitation of the current study.
The most striking finding in the correlation between MOs phenotype and inflammatory mediators is the significant difference in IL-10 between the M1/M2 dominance SCI patients, which not only appeared on the same day that the MO phenotypes were determined, but also persisted up to 14 days post-SCI. IL-10 is generally considered a potent anti-inflammatory cytokine that is produced primarily by MOs. The potent anti-inflammatory action of IL-10, particularly its inhibition of inflammatory cytokine production has been well-documented 26. This finding is relevant to SCI, since it has been previously shown that a subset of IL-10-positive MO-derived macrophages to be anti-inflammatory and essential for tissue repair in a rodent model of SCI 7. More importantly, it has been demonstrated that over expression of IL-10 in spinal cord results in increased neuronal survival, which is associated with improved motor function up to 6 weeks after injury in a rodent model of SCI 27. Interestingly, in the current study subjects with M2-like phenotypes showed elevation of IL-10, while TNF-α demonstrated a pattern moving toward lower expression, which would represent an overall favorable inflammatory profile. This relationship has been further demonstrated after systemic administration of IL-10, which resulting in improved hind limb motor function 2 months after SCI injury in rodent 28. Together, those studies provide in vivo data suggesting neuroprotective effects of IL-10.
In the current study, we were particularly focused on those cytokines/proteins known to be secreted by MOs. Although some known MO-associated cytokines/proteins, e.g. IL-1β, IL-6, IL-8, MIP-1α and MIP-1β were not different between M1/M2, it may have been due to the limitation in sample size studied, and represents an important consideration to be investigated in future larger scale studies.
As expected, ISS significantly differed between the motor complete and the incomplete groups (but not between the phenotypes or injury levels by ANOVA), therefore we segregated the SCI patients accordingly for the analyses. Due to the smaller sample size for ASIA C/D subjects, the same formal association of MO phenotypes with circulating cytokine levels could not be determined. However, consistent with a previous report 29, ASIA scales influences levels of some circulating inflammatory mediators in SCI patients, suggesting possible different inflammatory mediators might be correlated to the MO phenotypes in the motor-incomplete SCI patients.
Our observations suggest in acute response to SCI, individual differences exist in the MO predominance and inflammatory profiles. Those individual differences may be important considerations for the acute care of the SCI and the related clinical trials 30. However, the clinical relevance of this observation as it relates to neurologic recovery or outcomes needs to be established. Our future goal is to compare these circulating cellular and biochemical markers with clinical outcomes on a much larger scale so as to determine if the makers will have prognostic utility.
The current study is limited by the small size of the SCI patient group, as well as the predominance of ASIA A/B SCI subjects. The results presented in this study must therefore be evaluated in larger and more varied SCI populations to elucidate other co-variants, such as age, mechanism of injury, surgical intervention and/or complications. In addition, the control subjects to which the SCI subjects were compared differed in gender, which may affect the interpretation of the results. In the current study, we focused our analysis on cytokines/chemokines known to be associated with MO. However, although outside of the scope of the current study, it is possible that cytokines/chemokines associated with other immune cells, e.g., T cells, could also demonstrate relevant associations. It is also possible that additional cytokines and chemokines outside of the 25 selected for analysis in this study could demonstrate relationships, and this represents an area for future study.
Conclusion
In summary, the current pilot study suggests 1) that there are individual differences in the immune responses to acute traumatic SCI, represented by different phenotypes of MO dominance in patients with comparable injury severities and levels; and 2) that there is a correlation between the M1 and M2 MO phenotypes and the pro- and anti-inflammatory cytokine/protein profiles, respectively. The circulating monocyte phenotype in light of its correlation with circulating inflammatory mediators may prove to be a feasible biomarker for evaluating the outcomes and the treatments of SCI, and may lead to a target for immune modulation for treatment after traumatic spinal cord injury.
Acknowledgments
Funding source: NIDRR (RERC on Spinal Cord Injury grant number: H133E070024 and NIH grant P50-GM-53789).
We would like to express our gratitude to all the SCI patients participated in this study and the nurses in the UPMC intensive care units for their support. Tina Harrison and Ian J. Smith collected all participants’ ISS and their efforts are greatly appreciated.
This project is funded by NIDRR (Rehabilitation Engineering Research Center on Spinal Cord Injury grant number: H133E070024 and NIH grant P50-GM-53789) and Albert B. Ferguson, M.D. Orthopaedic Fund of the Pittsburgh Foundation (M2009-0122).
Footnotes
Preliminary versions of this report were presented at the 50th International Spinal Cord Society Annual Meeting, Washington, DC, USA, May 2011 and the Annual Meeting of the Association of Academic Physiatrists, Las Vegas, NV, USA, March 2012.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tator CH. Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med. 1996;19:206–14. doi: 10.1080/10790268.1996.11719436. [DOI] [PubMed] [Google Scholar]
- 2.Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712–24. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang HT. Subacute human spinal cord contusion: few lymphocytes and many macrophages. Spinal Cord. 2007;45:174–82. doi: 10.1038/sj.sc.3101910. [DOI] [PubMed] [Google Scholar]
- 4.Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–69. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 5.Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–65. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 6.Blight AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. 1994;60:263–73. doi: 10.1016/0306-4522(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 7.Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin Y, Cui Q, Li Y, et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–93. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapalino O, Lazarov-Spiegler O, Agranov E, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–21. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 11.Knoller N, Auerbach G, Fulga V, et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3:173–81. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- 12.Shechter R, Raposo C, London A, Sagi I, Schwartz M. The glial scar-monocyte interplay: a pivotal resolution phase in spinal cord repair. PLoS One. 2011;6:e27969. doi: 10.1371/journal.pone.0027969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 14.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 16.Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7. [PubMed] [Google Scholar]
- 17.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 18.Skrzeczynska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol. 2008;67:152–9. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 19.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 20.Corn PMGB. Circulating inflammatory Monocytes (CD14+CD16+) are elevated in severe obesity. Diabetes. 2010;59 [Google Scholar]
- 21.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–53. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bomstein Y, Marder JB, Vitner K, et al. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J Neuroimmunol. 2003;142:10–6. doi: 10.1016/s0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 23.Lazarov-Spiegler O, Solomon AS, Zeev-Brann AB, Hirschberg DL, Lavie V, Schwartz M. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J. 1996;10:1296–302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- 24.Lammertse DP, Jones LA, Charlifue SB, et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord. 2012;50:661–71. doi: 10.1038/sc.2012.39. [DOI] [PubMed] [Google Scholar]
- 25.De Serres SA, Vadivel N, Mfarrej BG, et al. Monocyte-secreted inflammatory cytokines are associated with transplant glomerulopathy in renal allograft recipients. Transplantation. 2011;91:552–9. doi: 10.1097/TP.0b013e318205b3c1. [DOI] [PubMed] [Google Scholar]
- 26.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol. 2009;220:183–90. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bethea JR, Nagashima H, Acosta MC, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–63. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 29.Kwon BK, Stammers AM, Belanger LM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010;27:669–82. doi: 10.1089/neu.2009.1080. [DOI] [PubMed] [Google Scholar]
- 30.Lammertse DP. Clinical trials in spinal cord injury: lessons learned on the path to translation. The 2011 International Spinal Cord Society Sir Ludwig Guttmann Lecture. Spinal Cord. 2013;51:2–9. doi: 10.1038/sc.2012.137. [DOI] [PubMed] [Google Scholar]