Abstract
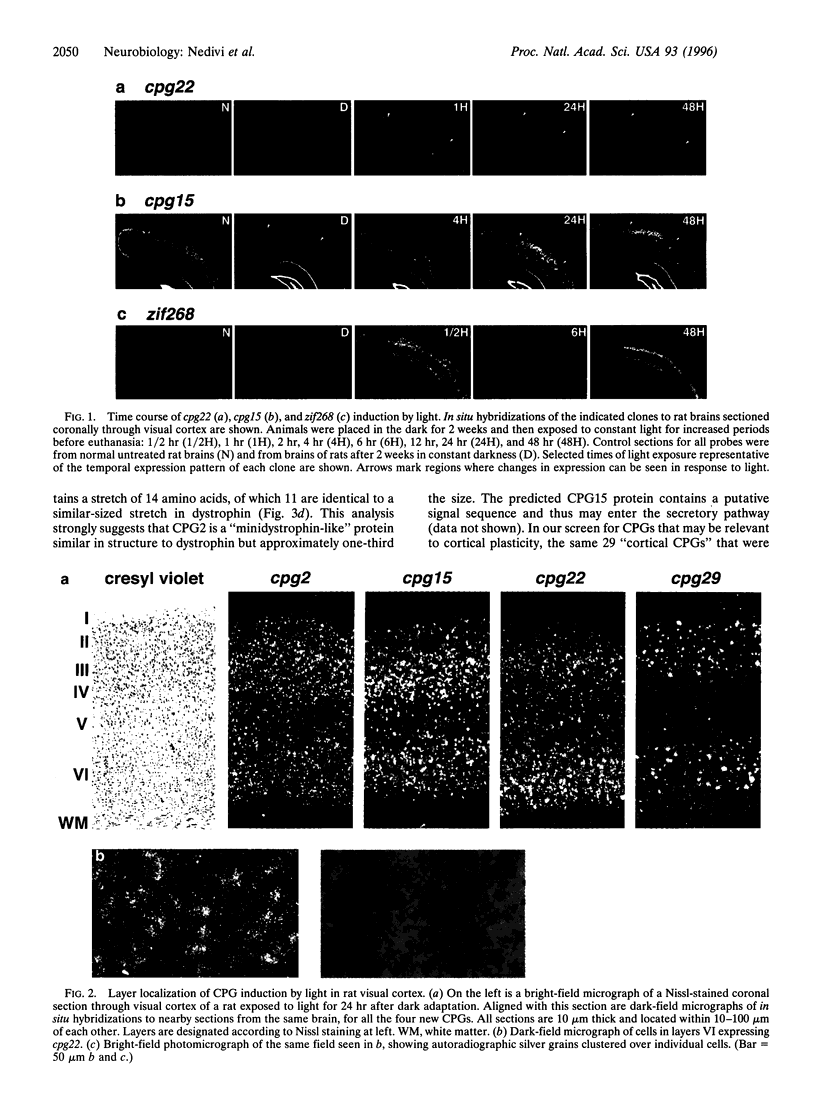
Activity-dependent plasticity is thought to underlie both formation of appropriate synaptic connections during development and reorganization of adult cortical topography. We have recently cloned many candidate plasticity-related genes (CPGs) induced by glutamate-receptor activation in the hippocampus. Screening the CPG pool for genes that may contribute to neocortical plasticity resulted in the identification of six genes that are induced in adult visual cortical areas in response to light. These genes are also naturally induced during postnatal cortical development. CPG induction by visual stimulation occurs primarily in neurons located in cortical layers II-III and VI and persists for at least 48 hr. Four of the visually responsive CPGs (cpg2, cpg15, cpg22, cpg29) are previously unreported genes, one of which (cpg2) predicts a "mini-dystrophin-like" structural protein. These results lend molecular genetic support to physiological and anatomical studies showing activity-dependent structural reorganization in adult cortex. In addition, these results provide candidate genes the function of which may underlie mechanisms of adult cortical reorganization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard T., Clark S. A., Jenkins W. M., Merzenich M. M. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol. 1991 Sep;66(3):1048–1058. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Diamond M. E., Ebner F. F. An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons. J Neurosci. 1994 Nov;14(11 Pt 2):6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Barinaga M. The brain remaps its own contours. Science. 1992 Oct 9;258(5080):216–218. doi: 10.1126/science.1411520. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Cabelli R. J., Hohn A., Shatz C. J. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995 Mar 17;267(5204):1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Campanelli J. T., Roberds S. L., Campbell K. P., Scheller R. H. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994 Jun 3;77(5):663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Castrén E., Zafra F., Thoenen H., Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos J. E., Golarai G., Sutula T. P. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991 Sep;11(9):2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A., Cynader M. S. Activity-dependent expression of the transcription factor Zif268 reveals ocular dominance columns in monkey visual cortex. Brain Res. 1993 Mar 12;605(2):349–353. doi: 10.1016/0006-8993(93)91765-k. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C., Gilbert C. D. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994 Apr 21;368(6473):737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Diamond M. E., Huang W., Ebner F. F. Laminar comparison of somatosensory cortical plasticity. Science. 1994 Sep 23;265(5180):1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Funakoshi H., Belluardo N., Arenas E., Yamamoto Y., Casabona A., Persson H., Ibáez C. F. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995 Jun 9;268(5216):1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Gall C. M., Isackson P. J. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989 Aug 18;245(4919):758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Gee S. H., Montanaro F., Lindenbaum M. H., Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994 Jun 3;77(5):675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Shatz C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993 Jan;72 (Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Hwang H. M., Gorman C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4549–4552. doi: 10.1073/pnas.82.13.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J. H., Krubitzer L. A., Chino Y. M., Langston A. L., Polley E. H., Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990 Apr 13;248(4952):229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- Kaas J. H. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- Kirkwood A., Dudek S. M., Gold J. T., Aizenman C. D., Bear M. F. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993 Jun 4;260(5113):1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lidov H. G., Byers T. J., Watkins S. C., Kunkel L. M. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 1990 Dec 20;348(6303):725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Nedivi E., Hevroni D., Naot D., Israeli D., Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993 Jun 24;363(6431):718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Nguyen P. V., Abel T., Kandel E. R. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994 Aug 19;265(5175):1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Patterson S. L., Grover L. M., Schwartzkroin P. A., Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992 Dec;9(6):1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Recanzone G. H., Schreiner C. E., Merzenich M. M. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993 Jan;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B. L., Fox K., O'Leary D. D. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993 Aug 12;364(6438):623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Tauck D. L., Nadler J. V. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985 Apr;5(4):1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley P. F., Cole A. J., Murphy T. H., Christy B. A., Nakabeppu Y., Baraban J. M. Synaptic regulation of immediate-early genes in brain. Cold Spring Harb Symp Quant Biol. 1990;55:213–223. doi: 10.1101/sqb.1990.055.01.023. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Sanders L. K., Kaufmann W. E., Yee W., Barnes C. A., Nathans D., Worley P. F. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem. 1994 Jun 10;269(23):16333–16339. [PubMed] [Google Scholar]
- Zarzecki P., Witte S., Smits E., Gordon D. C., Kirchberger P., Rasmusson D. D. Synaptic mechanisms of cortical representational plasticity: somatosensory and corticocortical EPSPs in reorganized raccoon SI cortex. J Neurophysiol. 1993 May;69(5):1422–1432. doi: 10.1152/jn.1993.69.5.1422. [DOI] [PubMed] [Google Scholar]