Abstract
Mitogen-activated protein kinase (MAPK) cascades play pivotal roles in mediating biotic and abiotic stress responses. Cotton (Gossypium hirsutum) is the most important textile crop in the world, and often encounters abiotic stress during its growth seasons. In this study, a gene encoding a mitogen-activated protein kinase (MAPK) was isolated from cotton, and designated as GhMPK17. The open reading frame (ORF) of GhMPK17 gene is 1494 bp in length and encodes a protein with 497 amino acids. Quantitative RT-PCR analysis indicated that GhMPK17 expression was up-regulated in cotton under NaCl, mannitol and ABA treatments. The transgenic Arabidopsis plants expressing GhMPK17 gene showed higher seed germination, root elongation and cotyledon greening/expansion rates than those of the wild type on MS medium containing NaCl, mannitol and exogenous ABA, suggesting that overexpression of GhMPK17 in Arabidopsis increased plant ABA-insensitivity, and enhanced plant tolerance to salt and osmotic stresses. Furthermore, overexpression of GhMPK17 in Arabidopsis reduced H2O2 level and altered expression of ABA- and abiotic stress-related genes in the transgenic plants. Collectively, these data suggested that GhMPK17 gene may be involved in plant response to high salinity and osmotic stresses and ABA signaling.
Introduction
Plant growth is significantly affected by a range of environmental stresses, such as drought, high salinity, pathogen infection, cold and freezing. Higher plants are sessile organisms that can not move to escape diverse unfavorable environmental factors. In order to survive, plants have developed a great ability to adapt the changes in the environment. They are equipped with sophisticated mechanisms, such as the perception and transduction of stress stimuli. The mitogen-activated protein kinase (MAPK) signaling pathway is an elaborate and intricate complex that are highly conserved in eukaryotes and play important roles in response to various biotic and abiotic stresses [1], [2]. Each mitogen-activated protein kinase (MAPK) cascade is known to be one of the major phosphorylation pathways which are composed of three kinases, MAPK, MAPK kinase (MAPKK), MAPKK kinase (MAPKKK) and function downstream of sensors/receptors to regulate cellular responses. A highly conserved MAPK cascade consists of three steps: MAPKs are activated through phosphorylation on two threonine and tyrosine residues by upstream MAPKK, which are in turn phosphorylated on two serine/threonine residues by MAPKKK [3], [4]. Then activated MAPKs regulate the activities of diverse targets including transcription factors [5], cytoskeletal proteins [6], and other protein kinases [7].
Genome sequencing and annotation of Arabidopsis revealed the existence of 20 MAPKs, 10 MAPKKs and 80 MAPKKKs [8]. In addition, a similar repertoire of genes have been found in other plants, such as 17 MAPKs in rice [9], and 21 in poplar [10]. In plants, based on the phylogenetic analysis of amino acid sequences and phosphorylation motifs, MAPKs can be divided into two subtypes: one with a TEY amino acid motif and the other containing a TDY motif. The TEY subtype can be classified into three groups: A, B and C, while the TDY subtype forms group D. In the group D, MAPKs have a long C-terminal extension domain in relation to groups A, B and C, which serves as a docking site for MAPKKs, phosphatases and protein substrates [11], [12]. MAPKs in groups A and B are mostly involved in response to biotic, abiotic stresses and hormones [13]–[16]. MPK3, MPK6 and its apparent orthologs in other species, such as Arabidopsis MPK3 and MPK6 (belonging to group A), alfalfa SIMK and SAMK, and tomato LeMPK1/2 and LeMPK3 are all involved in defense-related signal transduction [17]. In Arabidopsis, group B MPK4 is involved in osmotic stress response pathways activated by hypoosmolarity [18]. The available information of group-C MAPKs is relatively limited, and it seems that part of them play a role in cell cycle regulation and environmental stress responses [19]. Microarray analysis detected circadian-rhythm-regulated expression of MPK7 [20]. Some members of group D MAPKs may be involved in pathogen infection, mechanical wounding processes and some may play roles in both biotic and abiotic stress responses. Two reported group D genes, OsBWMK1 and MsTDY1, are induced by blast fungus and wounding [21]–[23].
Cotton (Gossypium hirsutum) is an important fiber crop in the world. However, its growth and yield are severely impacted upon by various biotic and abiotic stresses. Previously, GhMPK2, a group C MAPK gene was identified in cotton. The expression of GhMPK2 is strongly induced by salinity, dehydration and ABA [24]. Here, a group D mitogen-activated protein kinase gene, GhMPK17 was identified in cotton. The GhMPK17 gene is preferentially expressed in anthers and cotyledons, and is also induced by salt, osmosis and ABA. Overexpression of GhMPK17 in Arabidopsis enhanced plant tolerance to salt and osmotic stresses, suggesting that GhMPK17 gene may be involved in cotton multiple abiotic tolerance.
Materials and Methods
Cotton Materials
Cotton (Gossypium hirsutum, cv. Xuzhou 142) seeds were surface-sterilized with 70% (v/v) ethanol for 1 min and 10% (v/v) H2O2 for 2 h, and then were washed with sterile water. The sterilized seeds were germinated on half strength of MS medium under a 16 h light/8 h dark cycle at 25°C for 5 days. Roots, cotyledons, and hypocotyls were cut from these sterile seedlings. Other tissues were derived from cotton plants grown in field for RNA extraction.
For ABA (abscisic acid), salt and osmotic treatments, the sterilized cotton seeds were germinated on MS medium under a 16 h light/8 h dark cycle at 25°C for 6 days, and then seedlings were transferred on MS agar medium containing 100 µM ABA, 200 mM NaCl, or 200 mM manntiol. The treated cotyledons and controls were harvested for RNA isolation.
Isolation of GhMPK17 Full-length cDNA and Gene
A cotton seedling cDNA library was constructed as described previously [25]. More than 4000 cDNA clones were randomly selected from the cDNA library for sequencing. One clone containing the full-length GhMPK17 sequence was identified from these cDNAs. Subsequently, the full-length GhMPK17 gene was amplified from cotton genome by PCR, using the proofreading Pfu DNA polymerase and gene-specific primers that were designed according to GhMPK17 cDNA sequence.
DNA and Protein Sequence Analysis
Nucleotide and amino acid sequences were analyzed using DNAstar (DNAstar Inc., USA). The peptide sequences were aligned with the ClustalW program (http://www.ebi.ac.uk). The conserved domains of the GhMPK17 were confirmed at the National Center for Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Identification of protein domains and significant sites was performed with Motifscan (http://myhits.isb-sib.ch/cgi-bin/motif_scan). The phylogenetic tree was constructed by the NJ (Neighbor- Joining) method using MEGA5. A bootstrap analysis with 1000 replicates was performed to assess the statistical reliability of the tree topology.
Quantitative RT-PCR Analysis
Expression of GhMPK17 gene in cotton under normal conditions and under NaCl, mannitol and ABA treatments, and the expression of ABA- and stress-responsive genes in Arabidopsis were analyzed by real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) using the fluorescent intercalating dye SYBR Green (Bio-Rad, USA) by the method described earlier [26]. A cotton ubiquitin gene (GhUBI1, GenBank accession number: EU604080) was used as a quantitative control in RT-PCR reactions. The primer sequences were as follows: GhMPK17 forward 5′-GTTGCAAGCATCCGTGGAACCAGAAT-3′ and reverse 5′-TAAGACAGATTAAGAACCTCCAGAGG-3′; GhUBI1 forward 5′-CTGAATCTTCGCTTTCACGTTATC-3′ and reverse 5′-GGGATGCAAATCTTCGTGAAAAC-3′.
Expression of ABA- and stress-responsive genes in Arabidopsis was assayed by quantitative RT-PCR described as the above, using gene-specific primers: AtABI1 (At4g26080) forward 5′-AGATGGCAAGGAAGCGGATT-3′, reverse 5′-CAACCACCACCACACTTATG-3′; AtABF4 (At3g19290) forward 5′-AACAACTTAGGAGGTGGTGGTCAT-3′, reverse 5′-TGTAGCAGCTGGCGCAGAAGTCAT-3′; AtSOS2 (At5g35410) forward 5′-GGCTTGAAGAAAGTGAGTCTCG-3′, reverse 5′-GCTACATAGTTCGGAGTTCCACA-3′; AtRAB18 (At5g66400) forward 5′-AGATGGCAAGGAAGCGGATT-3′, reverse 5′-CTTCTTCTCGTGGTGCTCAC-3′. Expression level of AtACTIN2 was monitored with forward 5′-GAAATCACAGCACTTGCACC-3′ and reverse 5′-AAGCCTTTGATCTTGAGAGC-3′ primers to serve as internal control. The RNA samples were extracted from 2-week-old seedlings treated with or without 50 µM ABA, 100 mM NaCl and 100 mM mannitol for 6 h. Then total RNAs were reversely transcribed into cDNAs which were used as templates in quantitative PCR reactions.
For all the above quantitative real-time PCR analysis, the assays were repeated three times along with three independent repetitions of the biological experiments, and means of three biological experiments were calculated for estimating gene expression levels.
Subcellular Location of GhMPK17 Protein
To construct GhMPK17::eGFP vector, the coding sequence (without stop codon) of GhMPK17 was cloned into pBI121 vector which had eGFP sequence at Xba I/BamH I sites, using primers P1 (5′-GGG TCTAGAATGTTTGGCAAAGACTTTTT-3′) and P2 (5′- CTTGGATCCAGCGACCAGTTTCTGCAACT-3′). Then the prepared vector was transferred into Agrobacterium tumefaciens strain LBA4404. The cultures were diluted to OD600 = 1 and infiltrated into the abaxial side of young tobacco (Nicotiana tabacum) leaves. For inducing transient expression of GhMPK17::eGFP fusion gene, tobacco plants were cultured for three days after infiltration. Then, the epithelial tissue of the tobacco leaves were observed under a SP5 Meta confocal laser microscope (Leica, Germany) with a filter set for GFP fluorescence (488 nm for excitation and 506–538 nm for emission). The digital images were taken and process by SP5 software (Leica, Germany).
Construction of Overexpression Vector and Arabidopsis Transformation
The coding sequence of GhMPK17 gene was amplified by PCR using proof-reading pfu DNA polymerase and cloned into pBI121 vector at BamH I and Sac I sites, replacing GUS gene to generate chimeric CaMV (cauliflower mosaic virus) 35Sp:GhMPK17 construct. The construct was introduced into Arabidopsis by the floral dip method. Transformed seeds were selected on MS medium containing 50 mg/L kanamycin. Homozygous lines of T3 and T4 generations were used for phenotypic analysis of transgenic plants.
Assay of Seed Germination and Cotyledon Greening/expansion
About 50 surface-sterilized seeds from each transgenic Arabidopsis line and wild type, respectively, were sowed on MS plates supplemented with or without different concentration of mannitol, ABA or NaCl, and placed at 4°C for 4 days, then moved to a growth room under a photoperiod of 16 h light/8 h dark at 22°C. The rate of seed germination (root emergence) was evaluated every day, and the number of seedlings whose cotyledons was green was counted after 10 days. Each experiment was repeated three times at least with identical results.
Assay of Primary Root Elongation and Lateral Root Length
For assaying primary root elongation, 7-day-old transgenic Arabidopsis seedlings and wild type were transferred and cultured on MS medium supplemented with different concentration of mannitol, ABA or NaCl for 7 days. Then, the elongation of roots (final root length minus original root length) was measured and calculated. All experiments were repeated at least three times.
For assaying lateral root length, 7-day-old transgenic Arabidopsis seedlings and wild type were transferred and cultured on MS medium supplemented with 100 mM mannitol for 7 days. Then, the quantity and length of lateral roots of wild type and GhMPK17 transgenic lines were measured and calculated. According to the data measured, lateral roots were artificially divided into eight groups. The percentage of each group occupied in all lateral roots of each transgenic line was calculated, using the wild type as a control. All experiments were repeated at least three times.
Histochemical Detection of H2O2
The substrate 3,3′-diaminobenzidine (DAB) was used to visually detect H2O2 in plants according to the method described previously [26]. Briefly, one-week-old Arabidopsis seedlings were treated with or without 50 µM ABA, 100 mM NaCl and 100 mM mannitol for 6 h. Then, the samples were infiltrated in 1 mg/mL DAB solution for 6 h, followed by the treatment with 70% ethanol to remove chlorophyll. The seedlings were examined and photographed.
Results
Characterization of GhMPK17
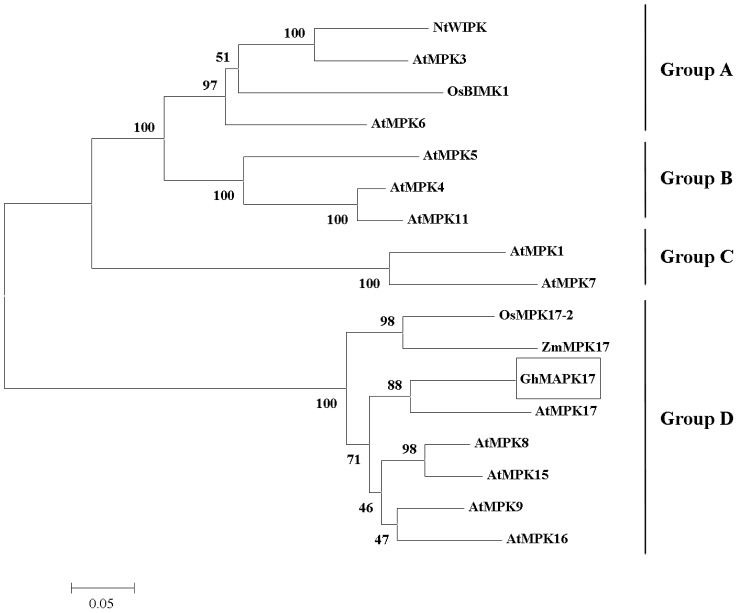
In order to identify genes involved in cotton stress response pathways, we randomly sequenced over 4000 cDNA clones from a cotton seedling cDNA library. Among these clones, one is a putative MAPK gene (cDNA) (designated as GhMPK17, GenBank accession number: KJ192192) that shares high similarity with other plant MAPKs (such as AtMPK17) in group D. The open reading frame (ORF) of GhMPK17 is 1494 bp in length, and encodes a protein of 497 amino acids. Multi-alignment analysis revealed that GhMPK17 is highly related to other group D MAPKs. As shown in Fig. 1, GhMPK17 and other group D MAPKs shared the same 11 conserved subdomains, an activation loop, a C-loop, a P-loop, a phosphorylation motif TDY between subdomains VII and VIII. Meanwhile, GhMPK17 and its homologs, OsMPK17-2 (DQ826423.2), AtMPK17 (NM001035863), ZmMPK17 (NM001035863) had an extension C-terminal region relative to groups A, B and C. This C-terminal region showed a high level of variation. Based on the amino acid sequences of GhMPK17 and other plant MAPKs, phylogenetic analysis indicated that GhMPK17 belongs to group D (Fig. 2).
Figure 1. Characterization of GhMPK17 protein.
Alignment of the deduced amino acid sequence of GhMPK17 with MAPKs from other plant species: AtMPK17 (NM001035863), ZmMPK17 (NM001154688.1), OsMPK17-2 (DQ826423.2). Protein kinase subdomains are shown by lines with numerals (I – XI), activation-loop motifs are boxed, and the conserved motif TDY is indicated by arrows.
Figure 2. Phylogenetic relationship of GhMPK17 and other plant MAPK proteins.
All 17 MAPK proteins can be divided into four groups (A, B, C, and D) based on their sequence similarities. Numbers above or below branches indicate bootstrap values from 1000 replicates. The plant MAPK proteins used for the phylogenetic tree are AtMPK1(NP172492), AtMPK3((NP190150), AtMPK4((NP192046), AtMPK5((NP567378), AtMPK6(NP181907), AtMPK7(NP179409), AtMPK8(NP173253), AtMPK9(NP566595), AtMPK11(NP001117210), AtMPK12(NP182131), AtMPK13(NP172266), AtMPK15(NP565070), AtMPK16(NP197402), AtMPK17(NP001030941), OsBIMK1(AAK01710.1), OsBIMK2(AAS18417), NtWIPK(BAA09600).
Expression of GhMPK17 in Cotton is Induced by Salt, Mannitol and ABA
To determine the expression profile of GhMPK17 gene in cotton, total RNA was isolated from different tissues of cotton. Expression levels of GhMPK17 in cotton different organs or tissues were quantified by real-time quantitative RT-PCR (Fig. 3A). The results showed that GhMPK17 was expressed at relatively high levels in both anthers and cotyledons, and at moderate levels in petals and leaves, whereas at relatively low levels in roots, hypocotyls, ovules and fibers.
Figure 3. Quantitative RT-PCR analysis of GhMPK17 expression in cotton under normal conditions and different stresses.
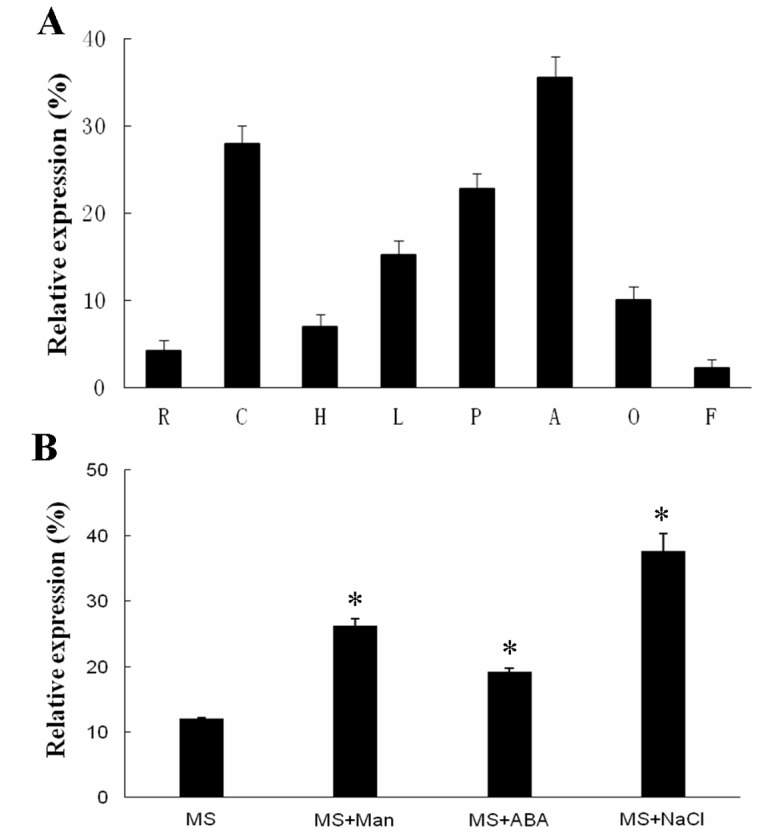
(A) Quantitative RT-PCR analysis of GhMPK17 expression in cotton tissues. Total RNA was isolated from roots (R), hypocotyls (H), cotyledons (C), leaf (L), anthers (A), petals (P), ovules (O), and fibers (F). (B) Quantitative RT-PCR analysis of GhMPK17 expression in cotton under different stresses. Total RNA was isolated from 6-day-old cotyledons treated with 100 µM abscisic acid (ABA), 200 mM NaCl and 200 mM manntiol (Man), respectively, using the untreated 6-day-old cotyledons as control (MS). Relative value of the expression of GhMPK17 in cotton was shown as percentage of GhUBI1 expression activity. Mean values and standard errors (bar) were shown from three independent experiments. *, P<0.05.
It has been supposed that some members of MAPK group D are involved in response to abiotic stress and abscisic acid (ABA) treatment [27], [28]. To study whether expression of the isolated GhMPK17 gene is regulated by abiotic stress and ABA, cotton seedlings were subjected to NaCl, mannitol and ABA treatments. As shown in Fig. 3B, GhMPK17 expression in cotyledons was up-regulated by NaCl, mannitol and ABA treatments, suggesting that GhMPK17 gene may be involved in plant response to abiotic stress and ABA signaling.
Subcellular Localization of GhMPK17
It has been reported that MAPKs can phosphorylate target proteins in both the cytoplasm and nucleus, and the subcellular localization of MAPKs is extraordinary correlated with the resulting cellular response [29]. To reveal the intracellular localization of GhMPK17 protein, an eGFP reporter gene was fused to GhMPK17 under the control of the cauliflower mosaic virus (CaMV) 35 S promoter. This 35 S:GhMPK17:eGFP construct was introduced into tobacco (Nicotiana tabacum) epidermal cells by agroinfiltration (see Methods). After a subculture of 48 hours, the GFP fluorescence was observed by confocal microscopy. As shown in Fig. 4, the GFP fluorescence signals were detected in cytoplasm of the transformed tobacco cells, suggesting that GhMPK17 protein may localize in the cytoplasm of cells for performing its function in cotton.
Figure 4. Subcellular localization of GhMPK17 protein.
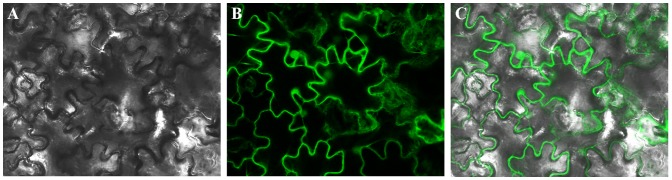
eGFP gene fused to C-terminal of GhMPK17 gene was expressed in epidermal cells of transformed tobacco leaves. GFP fluorescence signals were mainly detected in cytoplasm of cells harboring the GhMPK17-eGFP reporter construct. Transmission image (A), GFP-derived fluorescence (B) and fluorescence superimposed over the transmission image (C) are shown.
GhMPK17 is Involved in ABA Signaling in the Transgenic Arabidopsis Seedlings
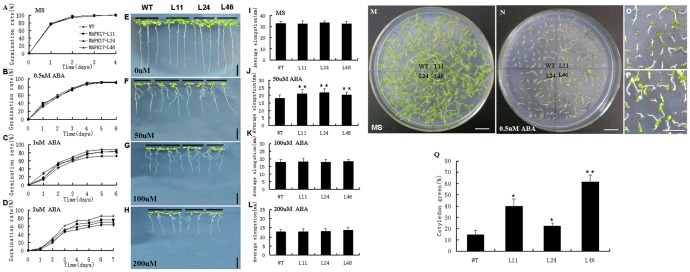
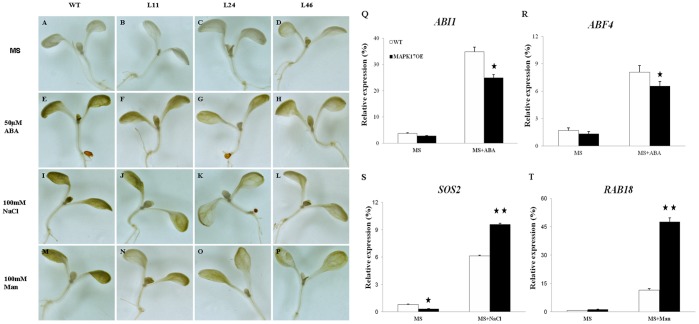
To investigate the biological function of GhMPK17 in plants, GhMPK17 gene under the control of CaMV 35 S promoter was introduced into Arabidopsis via Agrobacterium-mediated DNA transfer (see Methods). Sixty-four transgenic Arabidopsis plants were obtained on a selection MS medium with 50 mg/L kanamycin, and the homozygous transgenic progeny lines (T2– T4 generations) were selected through kanamycin-resistance assay and PCR analysis (data not shown). GhMPK17 expression levels in the transgenic lines were examined by quantitative RT-PCR analysis using gene-specific primers, and three homozygous transgenic lines (L11, L24 and L46) with different expression levels of GhMPK17 were selected for further analysis. Under normal conditions, GhMPK17 overexpression transgenic lines were phenotypically indistinguishable from wild type. To study the ABA-tolerance of GhMPK17 overexpression transgenic Arabidopsis, we tested the seed germination rate of wild type and GhMPK17 overexpression lines on MS medium containing 0, 0.5, 1 or 2 µM ABA. As shown in Fig. 5A, in the absence of exogenous ABA, GhMPK17 overexpression seeds germinated normally, as did wild type seeds. The seed germination rate of both transgenic lines and wild type reached almost 100% after four days. In the presence of 0.5 µM ABA, seed germination rate of GhMPK17 overexpression lines was slightly better, compared with that of wild type (Fig. 5B). When sowing on MS medium containing 1 µM ABA, however, seed germination rate of GhMPK17 overexpression lines was significantly higher than that of wild type. Statistically, wild type had 71.5% seed germination rate, while the transgenic lines L11, L24 and L46 kept 83.5%, 81.5% and 87.5% seed germination rate, respectively (Fig. 5C). Similarly, three transgenic lines still showed significantly higher germination rate (76%, 70% and 86%, respectively) on MS with 2 µM ABA, compared with that (63.5%) of wild type in the same conditions (Fig. 5D).
Figure 5. Assays of seed germination and root growth of transgenic Arabidopsis expressing GhMPK17 under abscisic acid (ABA) treatment.
(A to D) Seed germination rate of GhMPK17 overexpression transgenic lines and wild type on MS medium containing different concentrations of ABA. Each curve represents an average of three replicates. (E to H) Phenotype of wild type and GhMPK17 overexpression seedlings under ABA treatment. Seeds were germinated on MS medium under normal condition for 7 days and then the seedlings were transferred and cultured on MS medium supplemented with 0, 50, 100 and 200 µM ABA for 7 days. Each experiment was repeated at least three times with identical results. Bars = 1 cm. (I to L) Statistical analysis of root elongation of wild type and GhMPK17 overexpression plants. Seeds were germinated on MS medium under normal condition for 7 days, and then the seedlings were transferred and cultured on MS medium with 0, 50, 100 and 200 µM ABA for 7 days. Data were shown from three independent experiments. (M and N): Post-germination seedling establishment analysis of GhMPK17 overexpressing plants. (M) Seedlings of wild type and GhMPK17 transgenic lines grew on MS medium without ABA for 10 days. (N) Seedlings of wild type and GhMPK17 transgenic lines grew on MS medium with 0.5 µM ABA for 10 days. (O and P) Partial magnified drawing of wild type (O) and transgenic line 46 (P) in N. Bars = 1 cm. (Q) statistical analysis of the cotyledon greening/expansion ratio of wild type and transgenic seedlings grown on MS medium containing 0.5 µM ABA. *, P<0.05 and **, P<0.01. WT, wild type; L11, L24 and L46, GhMPK17 transgenic line 11, 24 and 46.
In addition, Arabidopsis seeds were germinated on MS medium under normal conditions for 7 days, and then the seedlings were transferred to grow on MS medium supplemented with 0, 50, 100 and 200 µM ABA for 7 days. Root phenotypes of wild type and transgenic lines were observed and statistically analyzed. As shown in Fig. 5E and 5I, when seedlings grow on MS medium, there was no difference in root elongation between GhMPK17-overexpressing lines and wild type. On the contrary, roots of the transgenic plants were much longer than those of wild type in the presence of 50 µM ABA (Fig. 5F). The average root elongations of transgenic seedlings were 20.9 mm (L11), 21.6 mm (L24) and 20.2 mm (L46), respectively, while wild type was 18.1 mm (Fig. 5J). With the increased ABA concentration (100–200 µM), root elongation of both transgenic lines and wild type were severely hindered (Fig. 5G, 5H, 5K and 5L), suggesting that too high ABA concentration may greatly inhibit plant growth.
Furthermore, GhMPK17 transgenic seedlings on MS medium grew normally, like wild type (Fig. 5M). While sowing on MS medium containing 0.5 µM ABA, cotyledon greening/expansion of both GhMPK17 transgenic lines and wild type was remarkably inhibited. However, GhMPK17 transgenic plants showed a higher cotyledon greening/expansion ratio than the wild type (Fig. 5N –5P). Measurement and statistical analysis revealed that there was very significant difference in cotyledon greening/expansion between GhMPK17 transgenic lines and wild type. 22.5% (L24), 39.8% (L11) and 61.2% (L46) cotyledons of GhMPK17 transgenic plants turned green/expansion, respectively, whereas only 14.5% of wild type turned green/expansion (Fig. 5Q). These data indicated that overexpression of GhMPK17 in Arabidopsis increased ABA-insensitivity of the transgenic plants, implying that GhMPK17 may be involved in ABA signaling in the transgenic Arabidopsis.
Overexpression of GhMPK17 in Arabidopsis Enhances Plant Tolerance to Salt Stress
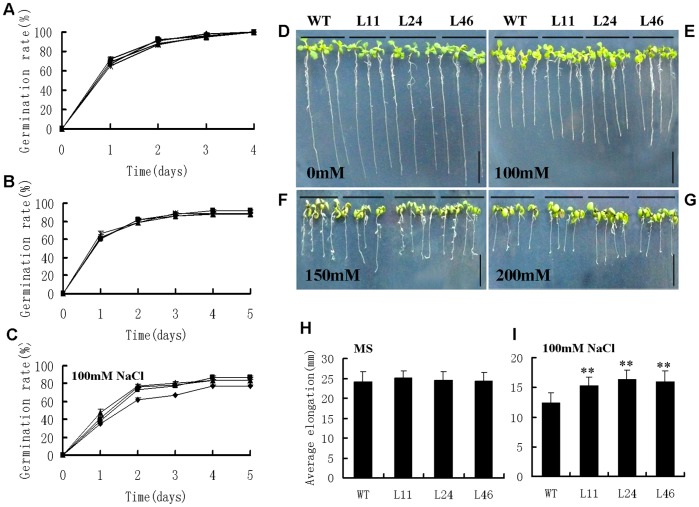
To determine whether the GhMPK17 was involved in high salinity stress in plants, we tested the seed germination capacity on MS medium with 0, 50 and 100 mM NaCl. On MS medium supplemented 0 (control) and 50 mM NaCl, there was no difference in seed germination rate between wild type and transgenic seedlings (Fig. 6A and 6B). On MS with 100 mM NaCl, however, the GhMPK17-overexpressing lines displayed the enhanced tolerance to salt stress, compared with the wild type. The germination rates of transgenic lines were 86.5% (L11), 83% (L24) and 84% (L46), while wild type was 77% (Fig. 6C).
Figure 6. Analysis of seed germination and root elongation of GhMPK17 overexpression transgenic Arabidopsis under NaCl stress.
(A to C) Seed germination rate of GhMPK17 overexpression lines and wild type on MS medium containing different concentrations of NaCl. (A) Seeds germinated on MS medium (control). (B) Seeds germinated on MS medium with 50 mM NaCl. (C) Seeds germinated on MS medium with 100 mM NaCl. Each curve represents an average of three replicates. (D to G): Phenotype of wild type and GhMPK17 overexpression seedlings under NaCl treatment. Seeds were germinated on MS medium under normal condition for 7 days and then the seedlings were transferred onto MS medium supplemented with 0 (D), 100 (E), 150 (F) and 200 (G) mM NaCl for 7 days. Each experiment was repeated at least three times with identical results. Bars = 1 cm. (H to I) Statistical analysis of root elongation of wild type and GhMPK17 overexpression seedlings grown on MS medium as control (H) or MS medium supplemented 100 mM NaCl (I). Data were shown from three independent experiments, and bars indicate SDs. **, P<0.01. WT, wild type; L11, L24 and L46, GhMPK17 transgenic line 11, 24 and 46.
In addition, we also measured and analyzed the root elongation of transgenic plants and wild type when they were cultured on MS medium with different NaCl concentration. On MS medium, root elongation of GhMPK17-overexpressing lines was as same as that of wild type (Fig. 6D and 6H). On 100 mM NaCl medium, the root elongation of transgenic plants was significantly longer than that of wild type (Fig. 6B and 6I). With the increased NaCl concentration (150 and 200 mM), root growth of both wild type and transgenic lines was inhibited dramatically (Fig. 6F and G), compared with those under normal conditions. These results indicated that GhMPK17 enhance plant resistance to salt stress during early plant development.
Overexpression of GhMPK17 in Arabidopsis Improves Plant Tolerance to Osmotic Stress
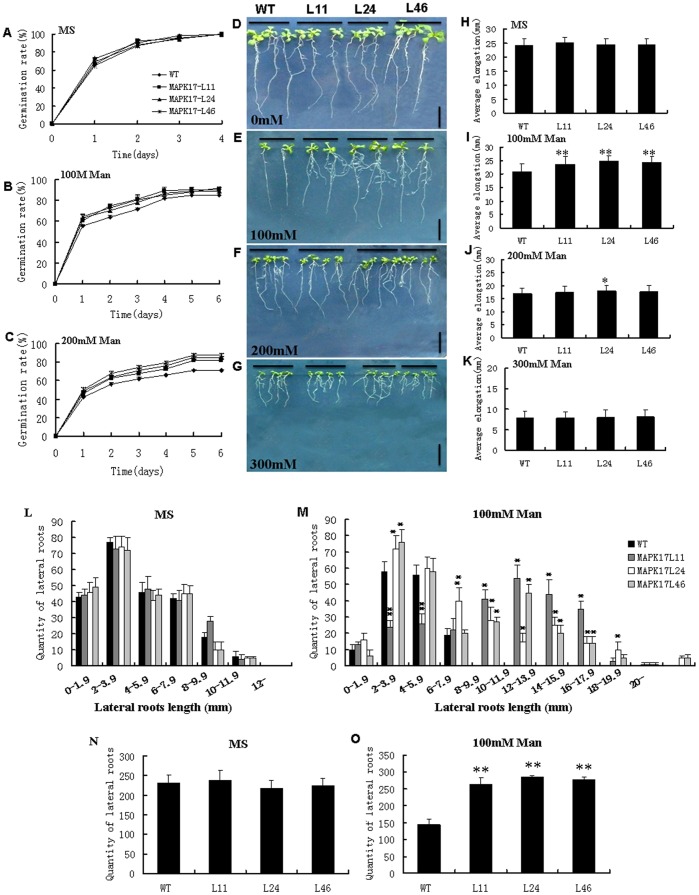
To examine if GhMPK17 responds to osmotic stress in plants, we tested the seed germination capacity on MS medium with 0, 100 and 200 mM mannitol to mimic hyperosmotic condition. In the absence of mannitol, there was little difference in seed germination rate between GhMPK17 overexpression transgenic lines and wild type (Fig. 7A). In the presence of 100 mM mannitol, the difference in seed germination rate between the transgenic lines and wild type was still not significant (Fig. 7B). Under 200 mM mannitol stress, however, the germination rate of the transgenic seeds was higher than that of wild type. Seed germination percentage of the transgenic lines reached to 82% (L11), 85% (L24) and 87.5% (L46), respectively, while the wild type was 71% on MS medium containing 200 mM mannitol (Fig. 7C).
Figure 7. Analysis of seed germination and root elongation of GhMPK17 overexpression transgenic Arabidopsis under mannitol treatment.
(A to C) Seed germination rate of GhMPK17 overexpression lines and wild type on MS medium containing 0 (A), 100 (B) and 200 (C) mM mannitol. Germination rate (root emergence) was evaluated at indicated time after sowing. (D to G) Phenotype of wild type and GhMPK17 overexpression seedlings under mannitol treatment. Arabidopsis seeds were germinated on MS medium under normal condition for 7 days and then the seedlings were transferred onto MS medium supplemented with 0 (D), 100 (E), 200 (F) and 300 (G) mM mannitol for 7 days. Bars = 1 cm. (H to K) Average root elongation histogram of wild type and GhMPK17 overexpression transgenic lines grown on MS medium (H), or MS medium supplemented 100 (I), 200 (J) and 300 (K) mM mannitol, respectively. (L and M) Statistical analysis on the quantity and length of the lateral roots of GhMPK17 transgenic lines and wild type on MS medium (L) or supplemented 100 mM mannitol (M). The lateral roots of GhMPK17 transgenic lines and wild type were divided into several groups according to their length (mm) and the quantity that each group occupied is shown. (N and O) Histogram of lateral roots quantity of wild type and GhMPK17 overexpression lines on MS medium (N) or supplemented 100 mM mannitol (O). Data were shown from three independent experiments, and bars indicate SDs. *, P<0.05 and **, P<0.01. WT, wild type; L11, L24 and L46, GhMPK17 transgenic line 11, 24 and 46.
Additionally, we measured root elongation as an indicator of osmotic stress tolerance. Seeds of both wild type and transgenic lines were germinated on MS medium for 7 days, and then the seedlings were transferred and cultured on MS medium containing 0, 100, 200 and 300 mM mannitol. On MS medium without mannitol (control), there was no difference in root elongation between the transgenic lines and wild type (Fig. 7D and 7H). When treated with 100 mM mannitol, root growth of wild type was inhibited to a greater extent than that of the transgenic seedlings (Fig. 7E). The root elongation of wild type was 20.9 mm, while the transgenic plants reached to 23.6 mm (L11), 25.0 mm (L24) and 24.4 mm (L46), respectively (Fig. 7I). With high concentrations (such as 200 and 300 mM) of mannitol treatment, plant growth was severely inhibited, and no statistical significant differences were detected between transgenic plants (L11 and L46) and wild type (Fig. 7F, 7G, 7J and 7K).
Intriguingly, we observed that the number of lateral roots in the transgenic lines was much more, compared with wild type. Also, the length of lateral roots of the transgenic lines was much longer than that of wild type. After measuring lateral root length, we divided the lateral roots into several groups according to their length (see Methods). As shown in Fig. 7L and 7M, we found that on MS medium, the distribution of the lateral root length of both wild type and GhMPK17 overexpression lines were almost the same. In each length group, their lateral root quantity did not have obvious distinction. However, when they were treated with 100 mM mannitol, there was significant difference in the number and length of lateral roots between the transgenic lines and wild type. The length of wild type lateral roots was mainly between 2–5.9 mm and not longer than 8 mm, while the length of L11 lateral roots mainly distributed among 8–5.9 mm, and the distribution trend of L24 and L46 was similar to L11. Lateral root length and quantity of the transgenic lines was greater than those of wild type. We separately counted lateral roots of 50 seedlings of each GhMPK17 transgenic line and wild type. Statistic analysis revealed that there was no difference in root quantity between wild type and transgenic lines on MS medium (Fig. 7N). In contrast, on MS medium supplemented with 100 mM mannitol, the quantity of lateral roots/50 seedlings in the transgenic lines was 263 (L11), 286 (L24) and 277 (L46), respectively, while 143 in wild type. More and longer lateral roots might help the transgenic plants to absorb more water for improving the ability of plant resistance to osmotic stress.
Overexpression of GhMPK17 in Arabidopsis Reduces H2O2 Level and Alters Expression of ABA- and Abiotic Stress-related Genes
The accumulation of reactive oxygen species (ROS) is one of the responses of plants under abiotic stress. H2O2, as one of the ROS, was examined in the transgenic plants and wild type under ABA, NaCl and mannitol treatments. As shown in Fig. 8A–8D, both transgenic seedlings and wild type displayed low levels of H2O2, and there was little difference in H2O2 level between the transgenic plants and wild type. On the contrary, the H2O2 levels of both wild type and transgenic lines were significantly increased on MS medium supplemented with ABA, NaCl and mannitol, but the H2O2 content in the transgenic seedlings were lower than that in wild type, especially under mannitol treatment (Fig. 8E–8P).
Figure 8. Assays of H2O2 accumulation and the expression levels of ABA- and abiotic stress-related genes in GhMPK17 overexpression transgenic Arabidopsis.
(A to P) Histochemical assay of H2O2 accumulation in wild type (A, E, I, M) and GhMPK17 transgenic seedlings (B to D, F to H, J to L, N to P) on MS medium (A to D), MS medium supplemented with 50 µM ABA (E to H), 100 mM NaCl (I to L) and 100 mM mannitol (M to P). (Q to T) Quantitative RT-PCR analysis of expression of ABA-related genes (Q and R) and abiotic stress-related genes (S and T) in wild type and GhMPK17 transgenic seedlings. Total RNA was isolated from two-week-old Arabidopsis seedlings grown under normal conditions (MS) and under ABA (MS+ABA), NaCl (MS+NaCl), mannitol (MS+Man) treatments for 6 h. Relative value of the expression of ABI1, ABF4, SOS2 and RAB18 in Arabidopsis was shown as percentage of AtACTIN2 expression activity. Mean values and standard errors (bar) were shown from three independent experiments. *, P<0.05 and **, P<0.01. WT, wild type; L11, L24 and L46, GhMPK17 overexpression transgenic line 11, 24 and 46; MAPK17OE, GhMPK17 overexpression transgenic lines.
Seed germination, root elongation and H2O2 level detection indicated an increased ABA-, salt- and osmotic stress-tolerance of the GhMPK17 overexpressing Arabidopsis plants. To investigate the role of H2O2 in response to abiotic stress, the expression patterns of the ABA and abiotic stress related genes (ABI1, ABF4, SOS2 and RAB18) were examined in the transgenic seedlings treated with ABA, NaCl and mannitol, using wild type as control. As shown in Fig. 8Q and 8R, the expression levels of ABI1 and ABF4 were up-regulated in the transgenic lines and wild type after ABA treatment. However, the expression levels of ABI1 and ABF4 in the transgenic seedlings were lower than those in wild type. Similarly, the expression levels of SOS2 and RAB18 were dramatically increased in the transgenic lines and wild type under NaCl and mannitol treatments, respectively, and gene expression levels in the transgenic plants were remarkably higher than those in wild type (Fig. 8S and 8T).
Discussion
Crops in many parts of the world, especially the land of high soil concentration of NaCl, are severely affected by salt stress [30]. Hyperosmotic stress can exacerbate the effects of sodium toxicity in cases where the salt concentration in the soil is particularly high. Several signals are triggered by high salinity and hyperosmotic stresses including ROS formation, PLD-mediated phosphatidic acid production, increment of cytosolic Ca2+ concentrations and accumulation of nitric oxide [31]–[34]. Salt and hyperosmotic stresses-induced secondary messengers activate diverse signaling proteins including various members of the MAPK family, which have been implicated in the transduction of the abiotic signals to elicit responses in plants. In Arabidopsis, MPK4 and MPK6 are activated by various stresses including salt, drought, cold and wounding [35]. MPK3 is also activated by osmotic stress [36]. MEKK1 mRNA accumulated in response to high salinity [37]. Functional and interaction analysis in yeast revealed protein-protein interactions between MEKK1 and MKK2/MEK1, between MKK2/MEK1 and MPK4, and between MPK4 and MEKK1 [38]. Further studies proved that environmental stress signals are transmitted to at least two MAPK cascades. One is the MPK4 cascade (MEKK1-MEK1/MKK2-MPK4) and the other involves MPK6 and p44MAPK [35]. After identifying MKK2 in a yeast-based screen for activators of MPK4 and MPK6, it was found that MKK2 is activated by salt and cold stress in transient protoplast assays [39]. mkk2-null mutant is hypersensitive to cold and salt stresses, and its overexpression transgenic plants are more tolerant to these stresses. MEKK1 can activate MPK4 and MPK6 in an MKK2-dependent manner [40]. Hyperosmotic stress as well as high salinity causes differential activation of the stress-related Arabidopsis MPKs [41]. In particular, the activation of MPK6 has been shown to play the central role in the early stage of sodium detoxification, since it rapidly phosphorylates the SOS1 Na+/H+ antiporter and thereby induces sodium efflux at the expense of cytosolic acidification [42]. A 46-kDa SIMK (salt stress-induced MAPK) in alfalfa was reported to be activated by salt [43]. Yeast-two hybrid assay identified an upstream activator kinase SIMKK that interacts specifically with SIMK and enhanced salt tolerance [44]. Tobacco protoplasts that were exposed to salt and osmotic stresses resulted in enhancement of a 48-kDa kinase, SIPK (salicylic acid-induced protein kinase) [45]. Recently, some salt and hyperosmotic stress-induced MAPKs have been identified in various plants [1]. Likewise, our data revealed that cotton MPK17 is induced in cotton by salt and mannitol treatments, suggesting it may be involved in plant response to salt and osmotic stresses.
Emerging evidence suggests that hormone signaling pathways (such as abscisic acid (ABA), salicylic acid (GA), jasmonic acid (JA), auxin and ethylene), as well as ROS signaling pathway, play crucial roles in the crosstalk between biotic and abiotic stress signaling [46]. These hormones may interact with one another in regulating stress signaling and plant stress tolerance. Especially, ABA is an important hormone that mediates the adaptation of plants to unfavorable environmental conditions including salinity, cold, drought and pathogen attacks. Treating plants with ABA induces the transcriptional regulation, protein accumulation and stability, kinase activity of several components of distinct MAPK signaling cascades in many plant species, indicating the involvement of MAP kinase cascades in ABA transduction pathway [47], [48]. In Arabidopsis, treatment with ABA induced the transcriptional regulation of MPK3, MPK5, MPK7, MPK18, MPK20, MKK9, MAPKKK1, MAPKKK10, MAPKKK14, MAPKKK15, MAPKKK16, MAPKKK17, MAPKKK18, MAPKKK19, Raf6, Raf12, and Raf35 [49]. In other plant species, several MAPKs have also been reported to be transcriptionally activated by ABA. In rice, the ABA-inducible OsMAPK5 was shown to act as a positive regulator in abiotic stress tolerance and as a negative regulator of PR gene expression and broad-spectrum disease resistance [13]. In maize, ZmMKK3 was involved in osmotic stress and ABA responses [50]. ABA has also been shown to induce transcription of MAPK genes in other plant species [51]–[53]. Three isoforms of catalase, CAT1, CAT2 and CAT3, have been discovered in Arabidopsis [54]. It was assumed that ABA-induced expression of CAT1 was regulated by Arabidopsis MKK1 in response to H2O2 signaling [55]. Another component that was found to be involved in this signaling pathway is MPK6. It is therefore likely that the MKK1/MPK6 module is an important component of the ABA-dependent signaling pathway that is responsible for H2O2 production and stress responses [47]. Similarly, we found that GhMPK17 expression was up-regulated in cotton by ABA, and overexpression of GhMPK17 in Arabidopsis increased ABA-tolerance of the transgenic plants, implying that GhMPK17 may be involved in ABA signaling during cotton development.
In the present study, we identified a MAPK protein (GhMPK17) of group D in cotton. GhMPK17 contains a TDY activation motif in its T-loop, the extended C-terminal region and the lack of the CD domain, compared with the TEY subtypes of MAPKs. Intriguingly, GhMPK17 was up-regulated in cotton seedlings under NaCl, mannitol and ABA treatments. Overexpression of GhMPK17 in Arabidopsis enhanced plant tolerance to salt and osmotic stresses, and altered H2O2 level and expression of abiotic stress-related genes in the transgenic plants. These data suggested that GhMPK17 may be involved in cotton response and tolerance to salt and osmotic stresses in ABA-dependant manner.
Funding Statement
This work was supported by the projects from the Ministry of Agriculture of China for transgenic research (Grant No. 2014ZX08009-027B, 2014ZX08009-003-004) and National Natural Sciences Foundation of China (Grant No. 31171174). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sinha AK, Jaggi M, Raghuram B, Tuteja N (2011) Mitogen-activated protein kinase signaling plants under abiotic stress. Plant Signal Behav 6: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rasmussen MW, Roux M, Petersen M, Mundy J (2012) MAP kinase cascades in Arabidopsis innate immunity. Front Plant Sci 3: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonak C, Okresz L, Boger H, Hirt H (2002) Complexity cross talk and integration of plant MAP kinase signaling. Curr Opin Plant Biol 5: 415–424. [DOI] [PubMed] [Google Scholar]
- 4. Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci 10: 339–346. [DOI] [PubMed] [Google Scholar]
- 5. Ishihama N, Yoshioka H (2012) Post-translational regulation of WRKY transcription factors in plant immunity. Curr Opin Plant Biol 15: 431–437. [DOI] [PubMed] [Google Scholar]
- 6. Šamajová O, Komis G, Šamaj J (2013) Emerging topics in the cell biology of mitogen-activated protein kinases. Trends Plant Sci 18: 140–148. [DOI] [PubMed] [Google Scholar]
- 7. Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, et al. (2009) MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev 23: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signaling network involved in multiple biological processes. Biochem J 413: 217–226. [DOI] [PubMed] [Google Scholar]
- 9. Reyna NS, Ynag Y (2006) Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol Plant Microbe Interact 19: 530–540. [DOI] [PubMed] [Google Scholar]
- 10. Nicole MC, Hamel LP, Morency MJ, Beaudoin N, Ellis BE, et al. (2006) MAP-ping genomic organization and organ-specific expression profiles of poplar MAP kinases and MAP kinase kinases. BMC Genomics 7: 223–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, et al. (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7: 301–308. [DOI] [PubMed] [Google Scholar]
- 12. Opdenakker K, Remans T, Vangronsveld J, Cuypers A (2012) Mitogen-activated protein (MAP) kinases in plant metal stress: regulation and responses in comparison to other biotic and abiotic stresses. Int J Mol Sci 13: 7828–7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acidinducible mitogen-activated protein kinase. Plant Cell 15: 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonak C, Nakagami H, Hirt H (2004) Heavy metal stress. Activation of destinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol 136: 3276–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seo S, Sano H, Ohashi Y (1999) Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ouaked F, Rozhon W, Lecourieux D, Hirt H (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO J 22: 1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ren D, Yang KY, Li GJ, Liu Y, Zhang S (2006) Activation of Ntf4, a tobacco mitogen-activated protein kinase, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol 141: 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Droillard MJ, Boudsocq M, Barbier-Brygoo H, Lauriere C (2004) Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Lett 574: 42–48. [DOI] [PubMed] [Google Scholar]
- 19. Baogre L, Calderini O, Binarova P, Matlauch M, Till S, et al. (1999) A MAP kinase is activated late in mitosis and becomes localised to the plane of cell division. Plant Cell 11: 5–14. [PMC free article] [PubMed] [Google Scholar]
- 20. Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, et al. (2001) Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 13: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He C, Fong SHT, Yang D, Wang GL (1999) BWMK1, a novel MAP kinase induced by fungal infection and mechanical wounding in rice. Mol Plant Microbe Interact 12: 1064–1073. [DOI] [PubMed] [Google Scholar]
- 22. Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, et al. (2003) BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol 132: 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Champin A, Picaud A, Henry Y (2005) Reassessing the MAP3K and MAP4K relationships. Trends in plant Sci 9: 123–128. [DOI] [PubMed] [Google Scholar]
- 24. Zhang L, Xi DM, Li SW, Gao Z, Zhao SL, et al. (2011) A cotton group C MAP kinase gene, GhMPK2, positively regulates salt and drought tolerance in tobacco. Plant Mol Biol 77: 17–31. [DOI] [PubMed] [Google Scholar]
- 25. Li DD, Tai FJ, Zhang ZT, Zheng Y, Li Y, et al. (2009) A cotton gene encodes a tonoplast aquaporin that is involved in cell tolerance to cold stress. Gene 438: 26–32. [DOI] [PubMed] [Google Scholar]
- 26. Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96: 6553–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li XB, Fan XP, Wang XL, Cai L, Yang WC (2005) The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. Plant Cell 17: 859–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi J, Zhang L, An HL, Wu CA, Guo XQ (2011) GhMPK16, a novel stress-responsive group D MAPK gene from cotton, is involved in disease resistance and drought sensitivity. BMC Mol Biol 12: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan JW, Zhang MY, Kong XP, Xing X, Liu YK, et al. (2012) ZmMPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta 235: 661–676. [DOI] [PubMed] [Google Scholar]
- 30. Mutalik VK, Venkatesh KV (2006) Effect of the MAPK cascade structure, nuclear translocation and regulation of transcription factors on gene expression. Biosystems 85: 144–157. [DOI] [PubMed] [Google Scholar]
- 31. Yadav S, Irfan M, Ahmad A, Hayat S (2011) Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol 32: 667–685. [PubMed] [Google Scholar]
- 32. Miller GAD, Suzuki N, Ciftci-Yilmaz S, Mittler RON (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33: 453–467. [DOI] [PubMed] [Google Scholar]
- 33. Hong Y, Zhang W, Wang X (2010) Phospholipase D and phosphatidic acid signalling in plant response to drought and salinity. Plant Cell Environ 33: 627–635. [DOI] [PubMed] [Google Scholar]
- 34. Laohavisit A, Colaco R, Davies J (2012) Cytosolic Ca(2+) determinations in studying plant responses to salinity and oxidative stress. Methods Mol Biol 913: 163–171. [DOI] [PubMed] [Google Scholar]
- 35. Khan MN, Siddiqui MH, Mohammad F, Naeem M (2012) Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 27: 210–218. [DOI] [PubMed] [Google Scholar]
- 36. Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24: 655–665. [DOI] [PubMed] [Google Scholar]
- 37. Droillard MJ, Boudsocq M, Barbier-Brygoo H, Laurière C (2002) Different protein kinase families are activated by osmotic stresses in Arabidopsis thaliana cell suspensions. Involvement of the MAP kinases AtMPK3 and AtMPK6. FEBS Lett 527: 43–50. [DOI] [PubMed] [Google Scholar]
- 38. Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, et al. (1996) A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ichimura K, Mizoguchi T, Irie K, Morris P, Giraudat J, et al. (1998) Isolation of ATMEKK1 (a MAP Kinase kinase kinase)-interacting proteins and analysis of a MAP kinase cascade in Arabidopsis. Biochem Biophys Res Commun 253: 532–543. [DOI] [PubMed] [Google Scholar]
- 40. Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, et al. (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15: 141–152. [DOI] [PubMed] [Google Scholar]
- 41. Matsuoka D, Nanmori T, Sato K, Fukami Y, Kikkawa U, et al. (2002) Activation of AtMEK1, an Arabidopsis mitogen-activated protein kinase kinase, in vitro and in vivo: analysis of active mutants expressed in E. coli and generation of the active form in stress response in seedlings. Plant J 29: 637–647. [DOI] [PubMed] [Google Scholar]
- 42. Kim SH, Woo DH, Kim JM, Lee SY, Chung WS, et al. (2011) Arabidopsis MKK4 mediates osmotic-stress response via its regulation of MPK3 activity. Biochem Biophys Res Commun 412: 150–154. [DOI] [PubMed] [Google Scholar]
- 43. Yu L, Nie J, Cao C, Jin Y, Yan M, et al. (2010) Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol 188: 762–773. [DOI] [PubMed] [Google Scholar]
- 44. Munnik T, Ligterink W, Meskiene II, Calderini O, Beyerly J, et al. (1999) Distinct osmo-sensing protein kinase pathways are involved in signaling moderate and severe hyper-osmotic stress. Plant J 20: 381–388. [DOI] [PubMed] [Google Scholar]
- 45. Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, et al. (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12: 2247–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mikołajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G (2000) Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant cell 12: 165–178. [PMC free article] [PubMed] [Google Scholar]
- 47. Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, et al. (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin in Plant Biol 9: 436–442. [DOI] [PubMed] [Google Scholar]
- 48. Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54: 440–451. [DOI] [PubMed] [Google Scholar]
- 49. Zong XJ, Li DP, Gu LK, Li DQ, Liu LX, et al. (2009) Abscisic acid and hydrogen peroxide induce a novelmaize group CMAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 229: 485–495. [DOI] [PubMed] [Google Scholar]
- 50. Wang XJ, Zhu SY, Lu YF, Zhao R, Xin Q, et al. (2010) Two coupled components of the mitogen-activated protein kinase cascade MdMPK1 and MdMKK1 from apple function in ABA signal transduction. Plant Cell Physiol 51: 754–766. [DOI] [PubMed] [Google Scholar]
- 51. Zhang M, Pan J, Kong X, Zhou Y, Liu Y, et al. (2012) ZmMKK3, a novel maize group B mitogen-activated protein kinase kinase gene, mediates osmotic stress and ABA signal responses. J Plant Physiol 169: 1501–1510. [DOI] [PubMed] [Google Scholar]
- 52. Ghawana S, Kumar S, Ahuja PS (2010) Early low-temperature responsivemitogen activated protein kinases RaMPK1 and RaMPK2 from Rheum australe D. Don respond differentially to diverse stresses. Mol Biol Rep 37: 933–938. [DOI] [PubMed] [Google Scholar]
- 53. Kumar KR, Srinivasan T, Kirti PB (2009) A mitogen-activated protein kinase gene, AhMPK3 of peanut: molecular cloning, genomic organization, and heterologous expression conferring resistance against Spodoptera litura in tobacco. Mol Genet Genomics 282: 65–81. [DOI] [PubMed] [Google Scholar]
- 54. Liu Y, Li X, Tan H, Liu M, Zhao X, et al. (2010) Molecular characterization of RsMPK2, a C1 subgroup mitogen-activated protein kinase in the desert plant Reaumuria soongorica. Plant Physiol Biochem 48: 836–844. [DOI] [PubMed] [Google Scholar]
- 55. Frugoli JA, Zhong HH, Nuccio ML, McCourt P, McPeek MA, et al. (1996) Catalase is encoded by a multigene family in Arabidopsis thaliana (L.) Heynh. Plant Physiol 112: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]