Abstract
Various adverse effects related to landfill leachate have made leachates an important issue in past decades, and it has been demonstrated that landfill leachate is an important source of environmental estrogens. In this study, we employed chemical analysis of some already evaluated estrogenic substances, in combination with a bioassay using several specific biomarkers (e.g., plasma vitellogenin and sex steroids, enzyme activity of gonad gamma-glutamyl transpeptidase, and gonadosomatic index) to evaluate the estrogenic activities in outlets from different stages of the leachate treatment process. The results indicated that 5 environmental estrogens (4-t-octylphenol, bisphenol A, di-ethyl phthalate, di-n-butyl phthalate, and diethylhexyl phthalate) were detected by a gas chromatography-mass spectrometry, and the concentrations in leachate samples were 6153 ng/L, 3642 ng/L, 2139 ng/L, 5900 ng/L, and 9422 ng/L, respectively. Leachate (1∶200 diluted) induced the synthesis of plasma vitellogenin and led to decreased enzyme activity of gonad gamma-glutamyl transpeptidase and gonadosomatic index in male goldfish (Carassius auratus) after a 28-day exposure, while increased circulating 17β-estradiol level was also observed in males exposed to treated effluent. Although the target EEs were partially removed with removal rates varying from 87.2% to 99.77% by the “membrane bioreactor+reverse osmosis+aeration zeolite biofilter” treatment process, the treated effluent is still estrogenic to fish. The method combined chemical techniques with the responses of test organisms allowing us to identify the group of estrogen-like chemicals so that we were able to evaluate the overall estrogenic effects of a complex mixture, avoiding false negative assessments.
Introduction
Currently, sanitary landfill has become the most practiced method for managing waste disposal due to its economic advantages. In 2011, approximately 6.36 billion tons of municipal solid waste (MSW) was deposited in landfills in China [1]. Landfill leachate is generated by the infiltration and percolation of rainfall, groundwater, or runoff water into and through the waste layers [2] and is a complex mixture of inorganic and organic compounds. It has already been established as a hazard to the environment [3]. Taking the risk of leachate leakage into account, leachate-induced biotoxicity has become of concern to societies throughout the world [4]–[6]. The proposed endocrine disrupting effects, or more specifically estrogenic effects, caused by landfill leachate were reported in Sweden [7]–[9], and landfill leachate has been considered an important source of environmental estrogens (EEs) in the aquatic habitat [10].
Various examinations regarding EEs in leachate are being conducted, and to our knowledge, the measuring methods used can be mainly classified into two categories: chemical analysis and biological based assays. Chemical analysis is a rapid and sensitive screen to measure a large number of compounds. A variety of chemical analysis methods, such as gas chromatography-mass spectrometry (GC-MS) [11], [12], gas chromatography-flame ionization detector (GC-FID) [13], and high performance liquid chromatography (HPLC) [14], have been developed to identify estrogenic substances that potentially interfere with the reproductive system. Based on several combinations of instruments and detectors, the analytical results could provide excellent quantitative information of target compounds. However, it is limited in describing the overall estrogenic effects engendered by complex environmental samples containing multiple EEs because of the existence of additive, synergistic, or even antagonistic influences. Moreover, the pretreatment of chemical analysis may lead to the loss of some unknown EEs. These limitations can be overcome by utilizing biological responses of tested organisms, cellular, or even non-cellular materials in a biotest. Biological assays are typically classified as either in vitro or in vivo bioassays. Currently, several in vitro bioassays have been established and conducted for analyzing EEs, such as a simplified yeast two-hybrid assay [15], an E-screen test and enzyme-linked receptor assay [16], and a reporter-gene-based assay with a human breast-cancer-derived cell line [10]. There were also reports using chemical analysis and in vitro bioassays together to investigate the xenoestrogens in leachate [17]–[19], while in vivo bioassays have been rarely used in the examination of the biotoxicology of landfill leachate. Compared to the in vivo test, the in vitro bioassay introduced in previous studies has obvious limitations because it does not take into account biological accumulation, biotransformation, or disruptions at other target sites in the reproductive axis, which could potentially result in a false negative evaluation or disappreciation of the EE concentrations [20], [21]. For example, monocrotophos pesticide showed no estrogenic activity in the in vitro MCF-7 cell proliferation assay [22]; however, this pesticide’s in vivo estrogenic potency was confirmed by a goldfish vitellogenin (Vtg) induction experiment in our previous study [23].
For the in vivo bioassay, the use of multiple biomarkers at different biological levels can be an effective tool in providing comprehensive and available information on EEs. As a female-specific protein, Vtg is a recognized indicator of exposure to estrogen-like substances [24]–[26]. However, Kime et al. [27] gave a suggestion that the result of evaluation using Vtg as an exclusive biomarker should be treated with caution, and some other specific indicators should be included. The concentrations of plasma sex steroids could help to reveal Vtg induction mechanism(s) [25], [28], and enzyme activity of gonad gamma-glutamyl transpeptidase (γ-GTP) and gonadosomatic index (GSI) are always associated with the adverse effects on reproductive health of organisms, making them excellent complementary biomarkers for the indication of the presence of EEs [29], [30].
In the past few years, the estrogenic contamination in certain lake, river, effluents of municipal wastewater treatment plant, and sediment has been evaluated by using chemical analysis combined with in vivo bioassays [31]–[35]. For example, Ribecco et al. [35] suggested the presence of estrogen-like substances in the sediment by revealing an increase in the expression of genes encoding estrogen receptor in Solea solea, though the levels of the primary pollutants present in the sediment did not exceed permissible Italian levels. In the present study, a valid method combining GC-MS and an in vivo bioassay was established to assess estrogenic activity in a real case study: a MSW landfill site in Qingdao. Frequently reported potential estrogenic compounds (natural and synthetic steroid estrogens, alkylphenols, phthalic acid esters, and BPA) were measured in outlets from different stages of the leachate treatment process, and then the removal ratios were calculated. Plasma Vtg and sex steroids, testis γ-GTP enzyme activity, and GSI were selected as specific bio-indicators to investigate the estrogenic effects in a semi-static exposure experiment.
Materials and Methods
Selection of the MSW Landfill Site
A MSW landfill site with a leachate treatment process in Qingdao was selected as a study model. It has been in operation since 2002 and mainly treats municipal solid waste and some bottom ash from MSW incinerators. The leachate treatment process used is an advanced membrane technology comprised of a membrane bioreactor treatment (MBR) with 4 bioreactors and an external ultrafiltration unit for sludge retention, a disctube reverse osmosis (DTRO) treatment, and a final aeration zeolite biofilter (see Figure 1). The leachate treatment facility is designed to collect and treat 900 m3 of leachate per day and the treated effluent discharged into the nearby river meets Grade one A standard of the Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant (GB18918-2002, China). The technical data of the leachate and the treated effluent samples employed in the experiment are shown in Table 1.
Figure 1. Schematic drawing of the treatment process for the leachate with sampling points (dots).
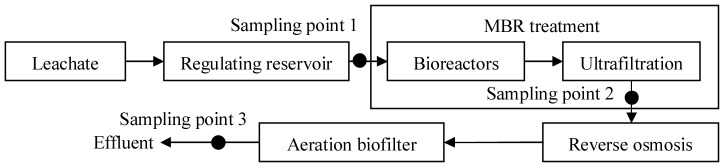
Table 1. Technical data of leachate and treated effluent.
| Technical data | Leachate | Treated effluent |
| CODCr (mg/L) | 15000 | ≤20 |
| BOD5 (mg/L) | 5000 | ≤10 |
| Ammonia nitrogen (mg/L) | 2900 | ≤1 |
| TN (mg/L) | 3100 | ≤8 |
| TP (mg/L) | 15 | ≤0.5 |
| TSS (mg/L) | 2000 | undetected |
CODCr: chemical oxygen demand; BOD5: five-day's biochemical oxygen demand; TN: total nitrogen; TP: total phosphorus; TSS: total suspended solids.
Chemical Analysis
In September 2011, composite aqueous samples were collected in solvent-clean brown glass bottles from storage tanks at different sampling points as shown in Figure 1 for the detection of target EEs, including natural and synthetic steroid estrogens [estrone (E1), 17β-estradiol (E2), estriol (E3), and 17α-ethynilestradiol (EE2)], bisphenol A (BPA), alkylphenols [nonylphenol (NP), 4-n-octylphenol (4-n-OP), and 4-t-octylphenol (4-t-OP)], and phthalic acid esters [di-ethyl phthalate (DEP), diethylhexyl phthalate (DEHP), di-n-butyl phthalate (DnBP), and di-n-octylphthalate (DNOP)]. All samples were stored at 4°C, protected from light, and analyzed within 1 week.
Natural and synthetic steroids, alkylphenols, and BPA were extracted as previously described by Diniz et al. [33], with a few modifications. Briefly, samples (500 mL) were filtered through 0.45 µm glass fiber filters, mixed with 40 mL methanol and acidified with HCl to pH 3.0. Samples were then extracted through a 3 mL C18 solid phase extraction (SPE) cartridge which was previously conditioned with methanol and deionized water. EEs were eluted with 2×10 mL dichloromethane for steroids and BPA and with 2×10 mL methanol for alkylphenols. Elution fractions were evaporated to dryness under a gentle stream of nitrogen and reconstituted with 10% methanol to a final volume of 0.5 mL. The extraction of phthalic acid esters was adopted as follows: Samples (500 mL) were filtered through 0.45 µm glass fiber filters and extracted with 2×15 mL n-hexane for 10 min. The elution fractions were mixed and evaporated to a final volume of 0.5 mL.
The target compounds were analyzed using an Agilent 6890N/5973i GC-MS system (Agilent Technologies Inc., Palo Alto, USA). GC separations were carried out using an Agilent DB-17MS column (30 m×0.25 mm×0.25 µm). A sample volume of 1 µL was injected in splitless mode at an inlet temperature of 230°C. The mobile phase was He at a constant flow of 1 mL/min. For the detection of natural and synthetic steroids, alkylphenols, and BPA, the GC column temperature was programmed from 80°C (initial equilibrium time 2 min) to 230°C via a ramp of 15°C/min, 230°C–250°C via a ramp of 15°C/min, 250°C–280°C via a ramp of 5°C/min, and finally maintained at 280°C for 5 min. For the detection of phthalic acid esters, the GC column temperature was programmed from 60°C (initial equilibrium time 1 min) to 220°C via a ramp of 20°C/min, 220°C–280°C via a ramp of 5°C/min, and maintained at 280°C for 7 min. Identification of compounds was achieved by comparing the mass spectra and retention times of the chromatographic peaks with those of standard samples. Calibration curves were constructed by analyzing aliquots of a stock solution of authentic standards and all the correlation coefficients (r2) were above 0.99. Table 2 shows the relevant parameters of the GC-MS. The removal ratios were calculated as following: the removal ratio = (1–concentration of a certain detected compound in treated effluent/the corresponding concentration in leachate)×100%.
Table 2. Relevant parameters of the GC-MS.
| EEs | linearequation | r 2 | Qualificationions (m/z) | Quantitationions (m/z) | Recovery | LODs (ng/L) |
| E1 | Y = 10.6X−18.7 | 0.9972 | 342 | 257, 218, 327 | ND | 10 |
| E2 | Y = 7.65X−16.0 | 0.9961 | 285 | 416, 232, 401 | 82.3 | 5 |
| E3 | Y = 2.05X−10.6 | 0.9946 | 285 | 368, 232, 218 | ND | 5 |
| EE2 | Y = 6.04X+40.9 | 0.9984 | 345 | 311, 504, 386 | ND | 3 |
| 4-t-OP | Y = 96.5X−1060 | 0.9984 | 207 | 208, 278, 209 | 112 | 3 |
| 4-n-OP | Y = 37.4X−292 | 0.9944 | 179 | 278, 180, 263 | 85.3 | 40 |
| NP | Y = 23.3X+308 | 0.9994 | 179 | 180, 372, 193 | 190 | 40 |
| BPA | Y = 86.3X−751 | 0.9954 | 357 | 372, 207, 179 | 235 | 40 |
| DEP | Y = 237X−241 | 1.0000 | 121 | 149, 205, 223 | ND | 40 |
| DnBP | Y = 443X−1620 | 1.0000 | 149 | 167, 205, 223 | ND | 10 |
| DEHP | Y = 202X−1820 | 1.0000 | 113 | 149, 167, 279 | 82.3 | 5 |
| DNOP | Y = 159X−2090 | 0.9996 | 149 | 167, 261, 279 | ND | 5 |
LODs: limits of detection; ND: not detected.
In vivo Biological Assays
The fish were handled according to the National Institute of Health guidelines for the handling and care of experimental animals. The animal utilization protocol was approved by the Institutional Animal Care and Use Committee of the Ocean University of China. All surgery was performed under MS-222 anesthesia, and all efforts were made to minimize suffering.
Goldfish (Carassius auratus) are widespread throughout the world, and are a member of one of the largest vertebrate families, the Cyprinidae. Goldfish are omnivorous, feeding on plants, small crustaceans, and other food, which can reflect the food chain accumulation effect. In addition, many studies have been carried out to develop goldfish which could be handled easily and affordably in the laboratory, as a model animal for the evaluation of the estrogenic activities of various environmental chemicals [23], [36]–[38]. Male goldfish (9.4±0.8 cm standard length; 27.1±4.2 g wet mass) were obtained from a local dealer in Qingdao, China, and were acclimated in the laboratory for 14 days. To estimate possible estrogenic activity on male fish, we simulated an accidental leachate release into groundwater resulting in a 1∶200 dilution of the leachate; it was previously determined that a leachate dilution greater than 1∶200 resulted in mortality (see Table S1 for data). Based on these data, male fish were exposed to 0.5% diluted leachate with dechlorinated tap water (sampling point 1), 0.5% diluted MBR outlet with dechlorinated tap water (sampling point 2), and 100% treated effluent (sampling point 3) for the in vivo bioassay. The semi-static toxicity test was conducted in 70 L aquaria containing 50 L solution water. Each group of fish (n = 24) were exposed in three aquaria (8 fish/tank). Additionally, 20 L exposure solutions were renewed daily and a male negative control group (dechlorinated tap water) was also set up. Male fish with intramuscular injection of E2 dissolved in ethanol and peanut oil (1∶1, v/v) at a concentration of 5 mg/mL was used as the positive control. The water temperature was 23±2°C and the dissolved oxygen was 7.0±0.1 mg/L. Fish were fed a non-estrogenic pelletized diet daily and no death was observed during exposure.
After a 28-day exposure in September 2011, male goldfish were anesthetized in 75 mg/L MS-222 (Sigma, St. Louis, MO, USA). Blood was taken from the caudal vein using chilled heparinized syringes and then goldfish were dissected. After centrifugation (3500×g, 10 min), plasma was frozen in liquid nitrogen for the detection of Vtg protein and sex steroids. Testis samples were removed, weighed, frozen in liquid nitrogen and stored at −80°C for enzyme activity analysis.
Vtg is usually measured by immunoassay and detected in western blot using specific antibodies. In this study, the plasma vitellogenin analysis was carried out by western blot as previously described by Pan & Tian et al. [39]. Discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a resolving gel with 9% acrylamide and a stacking gel with 4% acrylamide. Rabbit anti-goldfish Vtg serum (prepared in previous studies) diluted 1∶600 in blocking buffers and goat anti-rabbit IgG horseradish peroxidase conjugate (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) diluted 1∶1600 in blocking buffers were used to detect the plasma Vtg protein.
Radioimmunoassay kits obtained from the Beijing North Institute of Biology Technology, China, were used to detect the plasma E2 and testosterone (T) levels. The detection of plasma steroids was performed according to the manufacturer’s instructions. The assay detection limits were 10 pg/mL for E2 and 0.1 ng/mL for T. The inter- and intra-assay coefficients of variation for E2 and T were controlled under 8% and 10%. The interaction ratio for E2 and T detection with other hormones is <0.03%.
γ-GTP testing kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used for the γ-GTP enzyme activity test. Gonads were homogenized in 9 volumes (w/v) of homogenate buffer on ice. The supernatant was prepared using centrifugation at 4000×g for 10 min at 4°C. Analyses of γ-GTP enzyme activity were performed on ice according to the testing kit instructions, and protein contents were measured according to Bradford’s method using bovine serum albumin (BSA) as the standard [40]. One unit (U) was defined as 0.01 OD produced by 1 mg protein in 1 h at 37°C. The assay detection limit was 0.08 U·mg/prot.
Statistics
The SPSS (version 16.0; USA) was employed to process all data. The data are expressed as the mean ± standard deviation (SD). Multiple comparisons were performed using one-way ANOVA analysis after checking for normality and equal variance. All differences were considered significant at p<0.05.
Results
Occurrence of EEs
The occurrence of EEs in outlets from different stages of the treatment process during this study is summarized in Table 3. 5 environmental estrogens (4-t-OP, BPA, DEP, DnBP, and DEHP) were detected whereas natural and synthetic steroid estrogens, NP, and DNOP were under detection limits. The highest concentrations were found in leachate and reached 6153 ng/L, 3642 ng/L, 2139 ng/L, 5900 ng/L, and 9422 ng/L, respectively. The removal ratios of 4-t-OP, BPA, DEP, DnBP, and DEHP were 99.7%, 99.04%, 99.69%, 87.2%, and 92.9%, respectively.
Table 3. Mean concentrations of target EEs measured by GC-MS and EEQs of different samples.
| Samples | Selected EEs (ng/L) | EEQ (ng/L) | ||||
| 4-t-OP | BPA | DEP | DnBP | DEHP | ||
| Effluent | 14.3 | 34.8 | 6.66 | 757 | 666 | 0.01 |
| MBR outlet | 13.41 | 5.88 | 2.87 | 198.5 | 530 | 0.008 |
| Leachate | 6153 | 3642 | 2139 | 5900 | 9422 | 3.78 |
Induction of Plasma Vtg
Plasma samples were analyzed for Vtg by western blot (Figure 2). The results revealed the presence of one Vtg protein subunit in the plasma of 0.5% leachate exposed fish and 100% treated effluent exposed fish, which was also observed in the E2 induced male but absent from the control male fish, indicating the induction of Vtg protein synthesis by 0.5% leachate and treated effluent exposure. In contrast, Vtg protein was absent from plasma of fish exposed to 0.5% MBR outlet.
Figure 2. Western blot of plasma from treated and control male fish.
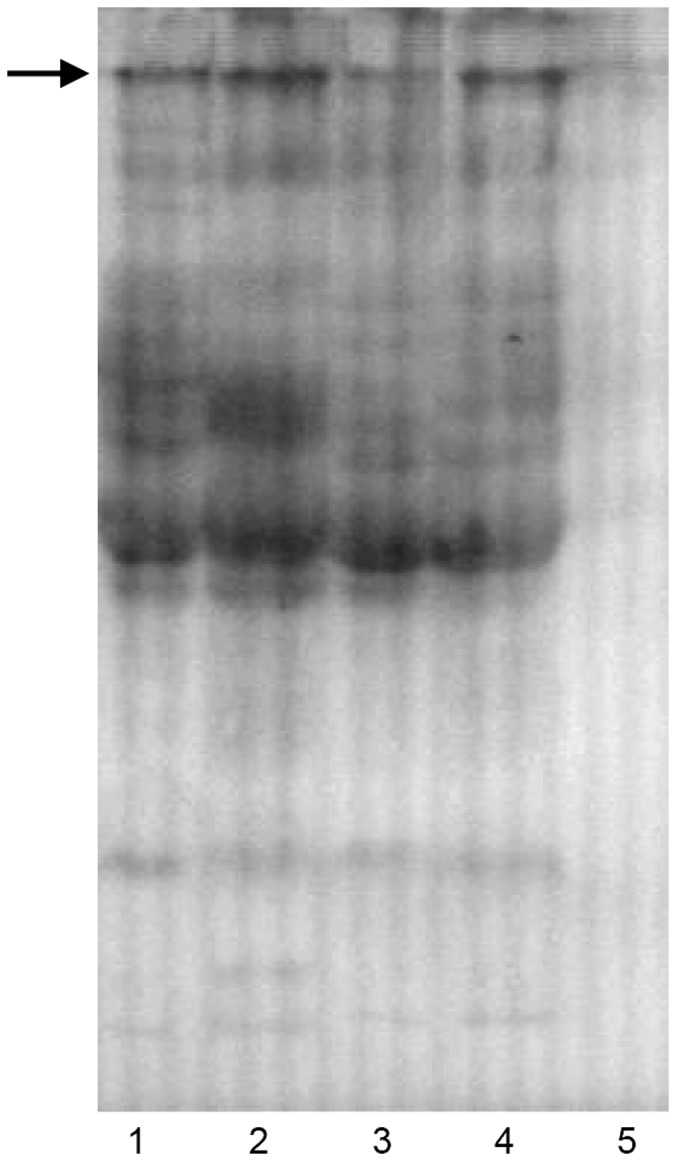
Lane 1: plasma from E2 injected fish (20×); Lane 2: plasma from effluent exposed fish; Lane 3: plasma from 0.5% diluted MBR outlet exposed fish; Lane 4: plasma from 0.5% diluted leachate exposed fish; Lane 5: plasma from negative control male. Primary antibody: rabbit anti-goldfish Vtg serum (diluted 1∶600). Secondary antibody: goat anti-rabbit IgG horseradish peroxidase conjugate (diluted 1∶1600).
Effects of Sex Steroids Levels in Plasma
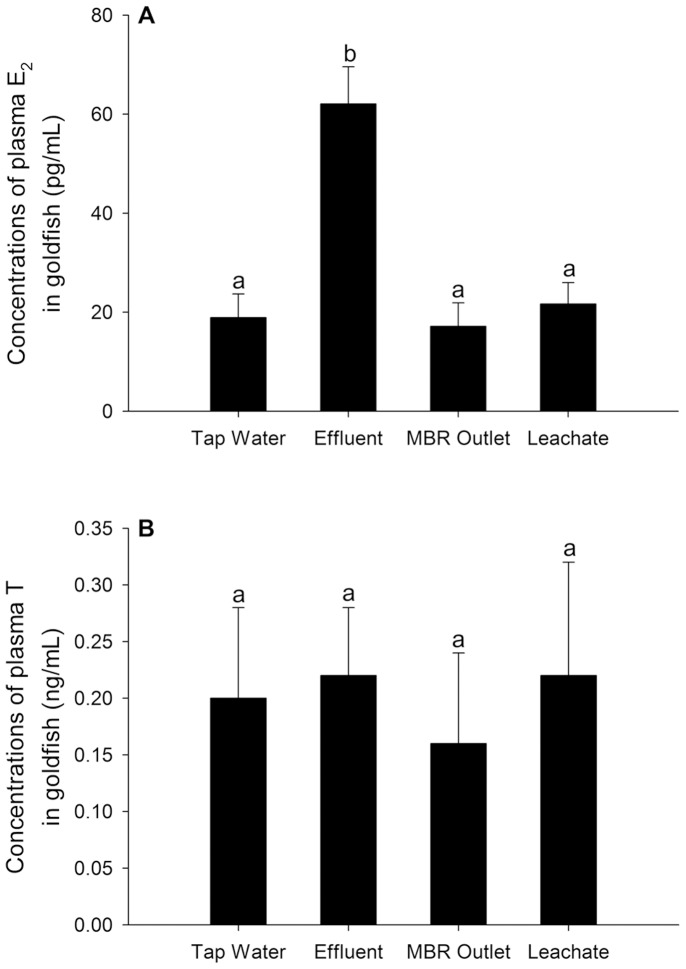
As shown in Figure 3A, the level of E2 in the plasma from treated effluent exposed fish (62.06±7.49 pg/mL) was significantly increased (p<0.05), while that of the other two groups was unchanged compared to the control male fish. Furthermore, the plasma T concentrations were not affected by any of the outlets from different stages of the treatment process (Figure 3B).
Figure 3. Quantification of plasma sex steroid levels in exposed male fish.
Male goldfish were exposed to dechlorinated tap water, 100% treated effluent, 0.5% diluted MBR outlet, and 0.5% diluted landfill leachate (designated Tap Water, Effluent, MBR Outlet, and Leachate, respectively) for 28 days in a semi-static exposure system. Data are presented as the mean ± standard deviation (n = 24). Values with different letters denote statistically significant differences according to a one-way ANOVA analysis (p<0.05).
Effects on Gonad γ-GTP Activity and GSI
Enzymatic activities of γ-GTP in the gonad were down-regulated after a 28-day exposure of outlets from different stages of the treatment process (Figure 4A), among which the γ-GTP activity in the male fish exposed to 0.5% leachate was close to the detection limit (0.08 U·mg/prot).
Figure 4. γ-GTP activity in gonad (A) and GSI (B) of exposed male goldfish.
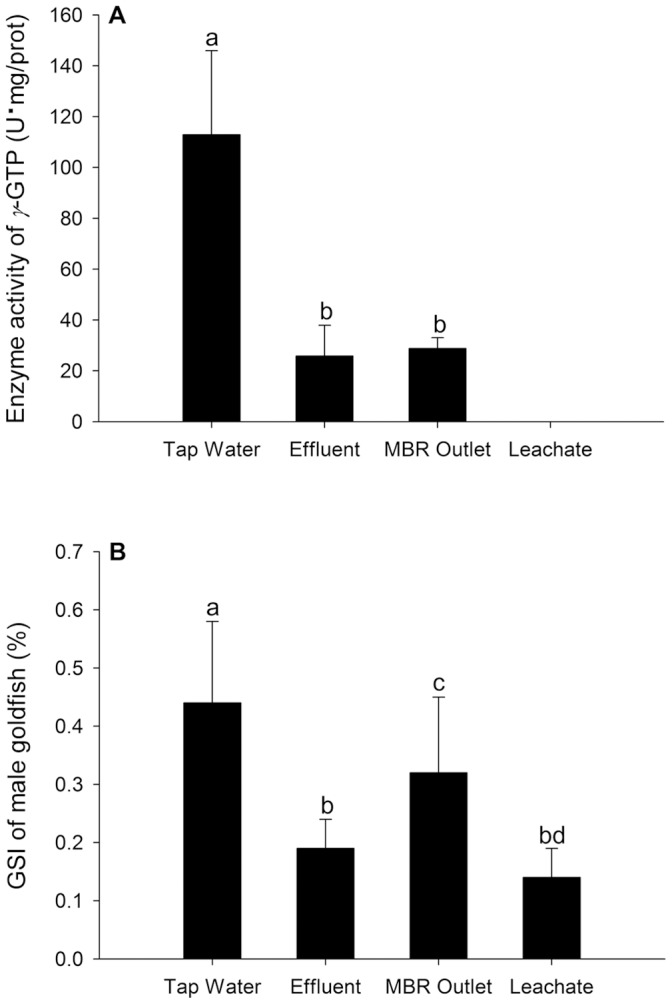
Male goldfish were exposed to dechlorinated tap water, 100% treated effluent, 0.5% diluted MBR outlet, and 0.5% diluted landfill leachate (designated Tap Water, Effluent, MBR Outlet, and Leachate, respectively) for 28 days in a semi-static exposure system. Data are presented as the mean ± standard deviation (n = 24). Values with different letters denote statistically significant differences according to a one-way ANOVA analysis (p<0.05).
The gonadosomatic index (GSI) was calculated by: GSI = (total gonad weight/wet mass)×100%. As shown in Figure 4B, GSIs of male fish exposed to 0.5% leachate, outlet of MBR treatment, and treated effluent were significantly lower than control after a 28-day exposure.
Discussion
Chemical analysis was utilized to identify the chemical components of leachate samples. Yasuhara et al. [41], [42] investigated approximately 190 types of chemical substances and more than 100 types of organic compounds including 1, 4-dioxane, phthalates, and bisphenol A in leachate samples in Japan. 41 sorts of phenols including low concentrations of 4-t-OP to high concentrations of BPA were detected in 38 municipal solid waste landfill sites [43]. Chemical analysis can serve as a useful tool to identify a group of EEs in leachate, by which the possible source of these compounds can be investigated. In this study, 5 identified EEs (4-t-OP, BPA, DnBP, DEP, and DEHP) were detected in leachate samples collected from selected landfill sites, and high detection rates have been frequently reported for them in previous studies [11], [12], [17]. 4-t-OP is the most representative final breakdown product of alkylphenol polyethoxylates (APEOs), which are widely used as wetting and emulsion agents in industrial and household applications [33]. BPA is employed during the production of polycarbonate and epoxy resins, which are important raw materials of commodity products [44]. PAEs are mainly applied as plasticizers in the production of dope, binder, and a variety of plastics [45]. It has been suggested that the occurrence of these detected estrogen-like chemicals may be a result of the degradation of household appliances, more importantly household plastics. In addition, the bottom ash from MSW incinerators could be another source [43].
Of more importance, chemical analysis is recommended as a prevailing methodological approach for the quantification of known or suspected EEs. In this study, measured concentrations of 5 types of estrogenic compounds were found to be present in concentration ranges reported in the literature. Teuten et al. [46] reported that the highest concentration range of BPA was 0.18–4300 µg/L in leachates from tropical Asian countries. Jonsson et al. [47] found the occurrences of DEHP (5–460 µg/L), DnBP (1–23 µg/L), and DEP (1–33 µg/L) in 17 European landfill leachates. However, the concentration of 4-t-OP found in this study was a little higher (0.03–3.4 µg/L by Teuten et al. [46]; 0.005–1.2 µg/L by Kurata et al. [43]; 0.04–0.31 µg/L by Asakura et al. [48]). The difference in 4-t-OP levels may be due to the composition of the landfill waste or the landfilling method.
Because of complex matrices of landfill leachate, the chemical analysis is limited in providing comprehensive knowledge of the overall estrogenic effects that might be engendered by leachate. This limitation can be overcome by the in vivo bioassay which utilized a series of specific biomarkers. In this study, a battery of bio-indicators including plasma Vtg and sex steroids, testis γ-GTP enzyme activity, and GSI were employed to evaluate the estrogenic activity at the molecular level, biochemical level, and tissue level.
Vtg is a female-specific phospholipoglycoprotein synthesized in the liver as nutrients for the developing embryo and is induced by endogenous estrogens [49], [50]. Male and juvenile fish normally produce minute amounts of Vtg due to the lack of endogenous estrogens. Therefore, an exposure to estrogenic compounds would lead to an abnormal induction of plasma Vtg, which makes it an ideal biomarker to test for exogenous estrogen exposure [24], [26], [51]. Also as mentioned in the preliminary draft guideline “The Fish Screening Assay for Endocrine Active Substances” within Organisation for Economic Co-operation and Development (OECD), Vtg is employed as a core biomarker endpoint for detecting endocrine active chemicals (unpublished). Currently, the Vtg induction assay has been widely used in assessing the estrogenic activity of effluent from wastewater treatment plants [52]–[54]. Our current study demonstrated that vitellogenin secretion was significantly induced in male goldfish after exposure to 0.5% diluted leachate and 100% treated effluent, suggesting significant estrogenic properties of these outlets in vivo. It is likely that estrogenic substances present in leachate and treated effluent were taken up by the fish and thereby stimulated the biosynthesis of Vtg. Considering the weak Vtg induction found in plasma of male fish exposed to 0.5% MBR outlet, the estrogenic activity was thought to be relatively low. Because the normal concentrations of sex steroids were not affected in 0.5% leachate for exposed male fish, it appears that various components of landfill leachate may bind to the estrogen receptor directly to induce Vtg synthesis. Evidence from in vitro assays has indicated that growth hormone and prolactin can also induce Vtg [55], [56].
Exposure to EEs is always associated with altered concentrations of plasma sex hormones, which play a decisive role in the regulation of sex differentiation, sexual characteristics, reproductive behavior, and reproductive capacity [8]. According to Lu et al. [32], male goldfish exposed to water from the Nanjing section of the Yangzi River had an elevated plasma E2 concentration. Similarly, plasma and testicular T levels in prepubertal male rats were decreased after subcutaneous administration of BPA, a recognized environmental estrogen, as described by Nakamura et al. [57]. In the present study, exposure of goldfish to treated effluent resulted in significantly increased circulating E2 levels compared to the negative control group, indicating a disturbance of sexual hormone homeostasis in male goldfish. The increased endogenous estradiol is one of the possible explanations of Vtg synthesis. In addition, this lower estrogenic potency of MBR outlet is further supported by the fact that the concentration of plasma E2 in 0.5% MBR exposed males was not affected. Endogenous E2 is mainly generated by the conversion of T [58] and the raised E2 level is always accompanied by a decreased T level. In the current study, exposure to 100% treated effluent for 28-days caused significant increases in plasma E2 levels but not in the T level, thus indicating the possible existence of androgenic activity. Studies in recent years have demonstrated that landfill leachate, municipal waste water, and industrial waste water may contain androgenic compounds [59]–[62]. For example, nine androgens and nine progestogens were detected in the influent and effluent of wastewater treatment plants in Beijing, China [63]. In addition, the biotransformation among androgens can be another reason for the unaffected T levels in plasma, as there are many types of androgens in male fish, such as androstenedione, 17α, 20β-algestone, and 11-ketotestosterone. Further detailed analysis is required to confirm this hypothesis.
The γ-GTP enzyme is mainly localized in the Sertoli cells of testis and the activity of the γ-GTP enzyme is a frequently used biomarker to indicate a lesion in the Sertoli cells and damage to the reproduction organs of male fish by xenobiotics at the biochemical level [30], [64]. Previous studies have suggested that exposure to 4-t-OP or NP may lead to structural damage of Sertoli cells and decreased enzyme activity of γ-GTP in eelpout (Zoarces viviparus), the effect of which is similar to E2 [65], [66]. Because the γ-GTP activity in testis of male fish exposed to treated effluent was decreased, it is reasonable to conclude that the Sertoli cells might be damaged due to the remarkable estrogenic potency. The enzyme activity of γ-GTP of male fish exposed to 0.5% leachate was close to the detection limit (0.08 U·mg/prot), which indicates the high biotoxicity of leachate due to the existence of hazardous substances.
GSI is a highly comprehensive index to assess the damage of reproductive function at the tissue level. Generally speaking, the exposure to environmental estrogens always results in a pronounced decline in GSI of adult male fish [67]–[69]. The reproductive toxicity of leachate and treated effluent was certified by the testicular atrophy of males in this study. These significantly lower GSIs are likely to be a result of Vtg synthesis. Jobling et al. [70] reported the inverse correlation between plasma Vtg and GSI in male roaches (Rutilus rutllus). Considering that Vtg synthesis is an energy-wasting process, the reproduction and growth of exposed fish tends to be delayed.
The method using estrogen equivalent quantity (EEQ) is recommended for hazard assessment of different classes of estrogenic chemical mixtures. By the use of this approach, Muller et al. [71] assessed the removal efficiency of estrogens by a municipal wastewater treatment plant and found that the biological treatment process possessed the highest removal ratio. The overall estrogenic equivalents of a mixture are defined by the concentration of individual compounds in a mixture times their relative potencies to E2, as reported by Safe [72]. Thus, the overall estrogenic potency of a complex mixture, for example, leachate, can be calculated and compared. According to previous studies, the estrogen equivalent factors of the detected EEs were 5.6×10−4 for BPA, 6×10−5 for OP, 2.4×10−5 for DnBP, 2.0×10−7 for DEHP, and 0.5×10−7 for DEP [17], [73]–[76]. The EEQs of leachate, MBR outlet, and treated effluent when calculated by this method were 3.78 ng/L, 0.008 ng/L, and 0.01 ng/L, which seemed to be relatively low. However, remarkable estrogenic activities that led to the induction of Vtg, and depression of γ-GTP activity and GSI were observed in leachate and treated effluent exposed male fish. The biological responses in male goldfish did not agree either with the chemical data obtained by GC-MS. Similar results were also reported by Blankvoort et al. [60], and Gunnarsson et al. [77]. Leachate is supposed to compromise the validity of the EEQ approach based on chemical analysis because of the cross talk between multiple endocrine pathways [72], and hence it should be noted that an underestimation may be made if only using the results of chemical analysis. However, in vivo bioassays mostly provide an integrated result of the total potency of complex mixtures to induce some biological responses, just as mentioned above that due to the presence of some substances with androgenic activities, the plasma T levels were unexpectedly not changed. Thus batteries of biomarkers at the molecular level, biochemical level, and tissue level, may prove more useful than single biomarkers in characterizing certain endocrine disrupting effect. Accordingly such in vivo experimentation might be complicated to operate and costly in financial terms, thereby making it valuable in study rather than in routine controls. Taking these findings together, a combination of chemical analysis and an in vivo bioassay is necessary to provide comprehensive knowledge and valid assessments of estrogenicity of a complicated mixture, such as landfill leachate.
Based on the comprehensive assessment results, the efficiency of unit operations in removing the previously selected compounds can be well evaluated. Due to the heavily contaminated property and poor biodegradability of landfill leachate, conventional wastewater treatment cannot fulfill treatment requests. Advanced wastewater treatments, especially the membrane process, for example, reverse osmosis treatment, are widely applied as a landfill leachate treatment. Coors et al. [10] used a reporter-gene-based bioassay with a human breast-cancer-derived cell line to study the removal rate of two different membrane employing processes and found that the concentrated effluent of the reverse osmosis treatment still showed an estrogenic response. The selected leachate treatment facility employed a “membrane bioreactor+reverse osmosis+aeration zeolite biofilter” treatment process, with qualified removal ratios of conventional parameters (i.e., CODCr, BOD5, TN, TP, and TSS), and the quality of the treated effluent meets Grade one A standard of the Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant (GB18918-2002, China). A relatively high rejection ratio of detected EEs, which can reach 87.2% or above, was achieved by the landfill leachate treatment process. However, the treated effluent still showed estrogenic activity in bioassays, indicating the existence of environmental estrogens and the potential risk to the aquatic habitat.
Conclusions
To achieve both qualitative and quantitative analysis results for estrogenic potency of leachate and treated effluent, a combined approach incorporating two types of analyses was proposed and applied to a real case study in Qingdao. The following are the main findings: the method combined chemical techniques with the responses of test organisms can be effectively applied for analyzing estrogen compounds, and resulted in a more comprehensive characterization of the estrogenic activity of environmentally complex samples. In a concentration of 200 times diluted, the leachate still showed strong estrogenic activity and led to bio-toxicity effects on male goldfish. The removal rate of “membrane bioreactor+reverse osmosis+aeration zeolite biofilter” treatment process for the target EEs can reach 87.2% or above; however, 5 types of EEs were still present in the effluent and contribute to an estrogen-like effect.
Supporting Information
Biological responses of goldfish exposed to different dilutions of leachate. Male goldfish (12 fish each group) were exposed to different dilutions of the leachate (1∶10, 1∶20, 1∶50, 1∶100, and 1∶200) in a semi-static exposure system after acclimated in the lab for 14 days. The leachate was diluted with dechlorinated tap water and a negative control group (dechlorinated tap water) was also set up. The mortality rate, survival time, and abnormal behavior were recorded.
(DOCX)
The ARRIVE guidelines checklist for animal research: reporting in vivo experiments.
(PDF)
Acknowledgments
The authors are grateful to all members in the lab for their help.
Funding Statement
This study was supported by Natural Science Foundation of China (31170322), www.nsfc.gov.cn and Natural Science Foundation of Jiangsu Province (BK2011165), www.jsnsf.gov.cn. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ministry of Environmental Protection of The People’s Republic of China (2011) Chinese environmental statistics annual book. Available: http://www.zhb.gov.cn.
- 2. Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, et al. (2002) Present and long-term composition of MSW landfill leachate: a review. Critical reviews in environmental science and technology 32: 297–336. [Google Scholar]
- 3. Lei L, Piao M, Nishizaki H, Nakamura H, Aoyama I (2006) Toxicity identification evaluation on industrial and municipal solid waste landfill leachates. Japanese Journal of Environmental Toxicology 9: 11–21. [Google Scholar]
- 4. Sisinno CL, Oliveira-Filho EC, Dufrayer MC, Moreira JC, Paumgartten FJ (2000) Toxicity evaluation of a municipal dump leachate using zebrafish acute tests. Bull Environ Contam Toxicol 64: 107–113. [DOI] [PubMed] [Google Scholar]
- 5. Pivato A, Gaspari L (2006) Acute toxicity test of leachates from traditional and sustainable landfills using luminescent bacteria. Waste Manag 26: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 6. Klauck CR, Rodrigues MA, da Silva LB (2013) Toxicological evaluation of landfill leachate using plant (Allium cepa) and fish (Leporinus obtusidens) bioassays. Waste Manag Res 31: 1148–1153. [DOI] [PubMed] [Google Scholar]
- 7. Noaksson E, Tjarnlund U, Bosveld AT, Balk L (2001) Evidence for endocrine disruption in perch (Perca fluviatilis) and roach (Rutilus rutilus) in a remote Swedish lake in the vicinity of a public refuse dump. Toxicol Appl Pharmacol 174: 160–176. [DOI] [PubMed] [Google Scholar]
- 8. Noaksson E, Linderoth M, Bosveld AT, Norrgren L, Zebuhr Y, et al. (2003) Endocrine disruption in brook trout (Salvelinus fontinalis) exposed to leachate from a public refuse dump. Sci Total Environ 305: 87–103. [DOI] [PubMed] [Google Scholar]
- 9. Noaksson E, Linderoth M, Tjarnlund U, Balk L (2005) Toxicological effects and reproductive impairments in female perch (Perca fluviatilis) exposed to leachate from Swedish refuse dumps. Aquat Toxicol 75: 162–177. [DOI] [PubMed] [Google Scholar]
- 10. Coors A, Jones PD, Giesy JP, Ratte HT (2003) Removal of estrogenic activity from municipal waste landfill leachate assessed with a bioassay based on reporter gene expression. Environ Sci Technol 37: 3430–3434. [DOI] [PubMed] [Google Scholar]
- 11. Asakura H, Matsuto T, Tanaka N (2007) Analytical study of endocrine-disrupting chemicals in leachate treatment process of municipal solid waste (MSW) landfill sites. Environ Sci 14: 79–87. [PubMed] [Google Scholar]
- 12. Asakura H, Matsuto T (2009) Experimental study of behavior of endocrine-disrupting chemicals in leachate treatment process and evaluation of removal efficiency. Waste Manag 29: 1852–1859. [DOI] [PubMed] [Google Scholar]
- 13. He PJ, Zheng Z, Zhang H, Shao LM, Tang QY (2009) PAEs and BPA removal in landfill leachate with Fenton process and its relationship with leachate DOM composition. Sci Total Environ 407: 4928–4933. [DOI] [PubMed] [Google Scholar]
- 14. Joseph L, Zaib Q, Khan IA, Berge ND, Park YG, et al. (2011) Removal of bisphenol A and 17alpha-ethinyl estradiol from landfill leachate using single-walled carbon nanotubes. Water Res 45: 4056–4068. [DOI] [PubMed] [Google Scholar]
- 15. Kawagoshi Y, Tsukagoshi Y, Fukunaga I (2002) Determination of estrogenic activity in landfill leachate by simplified yeast two-hybrid assay. J Environ Monit 4: 1040–1046. [DOI] [PubMed] [Google Scholar]
- 16. Dizer H, Fischer B, Sepulveda I, Loffredo E, Senesi N, et al. (2002) Estrogenic effect of leachates and soil extracts from lysimeters spiked with sewage sludge and reference endocrine disrupters. Environ Toxicol 17: 105–112. [DOI] [PubMed] [Google Scholar]
- 17. Behnisch PA, Fujii K, Shiozaki K, Kawakami I, Sakai S (2001) Estrogenic and dioxin-like potency in each step of a controlled landfill leachate treatment plant in Japan. Chemosphere 43: 977–984. [DOI] [PubMed] [Google Scholar]
- 18. Kawagoshi Y, Fujita Y, Kishi I, Fukunaga I (2003) Estrogenic chemicals and estrogenic activity in leachate from municipal waste landfill determined by yeast two-hybrid assay. J Environ Monit 5: 269–274. [DOI] [PubMed] [Google Scholar]
- 19. Baderna D, Maggioni S, Boriani E, Gemma S, Molteni M, et al. (2011) A combined approach to investigate the toxicity of an industrial landfill's leachate: chemical analyses, risk assessment and in vitro assays. Environ Res 111: 603–613. [DOI] [PubMed] [Google Scholar]
- 20. Folmar LC, Hemmer M, Denslow ND, Kroll K, Chen J, et al. (2002) A comparison of the estrogenic potencies of estradiol, ethynylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro . Aquat Toxicol 60: 101–110. [DOI] [PubMed] [Google Scholar]
- 21. Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty G, et al. (2003) Relative potencies and combination effects of steroidal estrogens in fish. Environ Sci Technol 37: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 22. Zheng G, Zhou ZJ, Dai XF (2004) Detection of estrogenic effects of organphosphorus pesticides on MCF-7 human breast cancer cells. Chinese Journal of Industrial Medicine 17: 273–276. [Google Scholar]
- 23. Tian H, Ru S, Wang Z, Cai W, Wang W (2009) Estrogenic effects of monocrotophos evaluated by vitellogenin mRNA and protein induction in male goldfish (Carassius auratus). Comp Biochem Physiol C Toxicol Pharmacol 150: 231–236. [DOI] [PubMed] [Google Scholar]
- 24. Tyler CR, van Aerle R, Hutchinson TH, Maddix S, Trip H (1999) An in vivo testing system for endocrine disruptors in fish early life stages using induction of vitellogenin. Environmental Toxicology and Chemistry 18: 337–347. [Google Scholar]
- 25. Sole M, Raldua D, Piferrer F, Barcelo D, Porte C (2003) Feminization of wild carp, Cyprinus carpio, in a polluted environment: plasma steroid hormones, gonadal morphology and xenobiotic metabolizing system. Comp Biochem Physiol C Toxicol Pharmacol 136: 145–156. [DOI] [PubMed] [Google Scholar]
- 26. Marin MG, Matozzo V (2004) Vitellogenin induction as a biomarker of exposure to estrogenic compounds in aquatic environments. Marine pollution bulletin 48: 835–839. [DOI] [PubMed] [Google Scholar]
- 27. Kime D, Nash J, Scott A (1999) Vitellogenesis as a biomarker of reproductive disruption by xenobiotics. Aquaculture 177: 345–352. [Google Scholar]
- 28. Diniz MS, Peres I, Castro L, Freitas AC, Rocha-Santos TA, et al. (2009) Effects of ECF-Kraft pulp mill effluent treated with fungi (Rhizopus oryzae) on reproductive steroids and liver CYP1A of exposed goldfish (Carassius auratus). Ecotoxicology 18: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 29. Komen J, Lodder P, Huskens F, Richter C, Huisman E (1989) Effects of oral administration of 17α-methyltestosterone and 17β-estradiol on gonadal development in common carp, (Cyprinus carpio). Aquaculture 78: 349–363. [Google Scholar]
- 30. Rasmussen TH, Teh SJ, Bjerregaard P, Korsgaard B (2005) Anti-estrogen prevents xenoestrogen-induced testicular pathology of eelpout (Zoarces viviparus). Aquat Toxicol 72: 177–194. [DOI] [PubMed] [Google Scholar]
- 31. Lu G, Yan Z, Wang Y, Chen W (2011) Assessment of estrogenic contamination and biological effects in Lake Taihu. Ecotoxicology 20: 974–981. [DOI] [PubMed] [Google Scholar]
- 32. Lu GH, Song WT, Wang C, Yan ZH (2010) Assessment of in vivo estrogenic response and the identification of environmental estrogens in the Yangtze River (Nanjing section). Chemosphere 80: 982–990. [DOI] [PubMed] [Google Scholar]
- 33. Diniz MS, Mauricio R, Petrovic M, Lopez de Alda MJ, Amaral L, et al. (2010) Assessing the estrogenic potency in a Portuguese wastewater treatment plant using an integrated approach. J Environ Sci (China) 22: 1613–1622. [DOI] [PubMed] [Google Scholar]
- 34. Ribecco C, Baker ME, Šášik R, Zuo Y, Hardiman G, et al. (2011) Biological effects of marine contaminated sediments on Sparus aurata juveniles. Aquat Toxicol 104: 308–316. [DOI] [PubMed] [Google Scholar]
- 35. Ribecco C, Hardiman G, Šášik R, Vittori S, Carnevali O (2012) Teleost fish (Solea solea): A novel model for ecotoxicological assay of contaminated sediments. Aquat Toxicol 109: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tian H, Ru S, Bing X, Wang W (2010) Effects of monocrotophos on the reproductive axis in the male goldfish (Carassius auratus): Potential mechanisms underlying vitellogenin induction. Aquat Toxicol 98: 67–73. [DOI] [PubMed] [Google Scholar]
- 37. Tian H, Ru S, Wang W, Bing X (2010) Effects of monocrotophos on the reproductive axis in the female goldfish (Carassius auratus). Comp Biochem Physiol C Toxicol Pharmacol 152: 107–113. [DOI] [PubMed] [Google Scholar]
- 38. Zhang X, Tian H, Wang W, Ru S (2013) Exposure to monocrotophos pesticide causes disruption of the hypothalamic–pituitary–thyroid axis in adult male goldfish (Carassius auratus). Gen Comp Endocrinol 193: 158–166. [DOI] [PubMed] [Google Scholar]
- 39. Pan Z, Tian H, Wang W, Wang J, Ru S (2012) Identification, purification, and immunoassay of stone flounder (Kareius bicolouratus) vitellogenin. Journal of the Korean Society for Applied Biological Chemistry 55: 219–227. [Google Scholar]
- 40. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 41. Yasuhara A, Shiraishi H, Nishikawa M, Yamamoto T, Uehiro T, et al. (1997) Determination of organic components in leachates from hazardous waste disposal sites in Japan by gas chromatography-mass spectrometry. Journal of Chromatography A 774: 321–332. [Google Scholar]
- 42. Yasuhara A, Shiraishi H, Nishikawa M, Yamamoto T, Nakasugi O, et al. (1999) Organic components in leachates from hazardous waste disposal sites. Waste Management and Research 17: 186–197. [Google Scholar]
- 43. Kurata Y, Ono Y, Ono Y (2008) Occurrence of phenols in leachates from municipal solid waste landfill sites in Japan. Journal of material cycles and waste management 10: 144–152. [Google Scholar]
- 44. Furhacker M, Scharf S, Weber H (2000) Bisphenol A: emissions from point sources. Chemosphere 41: 751–756. [DOI] [PubMed] [Google Scholar]
- 45.Giam C, Atlas E, Powers M Jr, Leonard J (1984) Phthalic acid esters. Anthropogenic compounds: Springer. 67–142.
- 46. Teuten EL, Saquing JM, Knappe DR, Barlaz MA, Jonsson S, et al. (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci 364: 2027–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jonsson S, Ejlertsson J, Ledin A, Mersiowsky I, Svensson BH (2003) Mono- and diesters from o-phthalic acid in leachates from different European landfills. Water Res 37: 609–617. [DOI] [PubMed] [Google Scholar]
- 48. Asakura H, Matsuto T, Tanaka N (2004) Behavior of endocrine-disrupting chemicals in leachate from MSW landfill sites in Japan. Waste Manag 24: 613–622. [DOI] [PubMed] [Google Scholar]
- 49. Wallace RA, Begovac PC (1985) Phosvitins in Fundulus oocytes and eggs. Preliminary chromatographic and electrophoretic analyses together with biological considerations. J Biol Chem 260: 11268–11274. [PubMed] [Google Scholar]
- 50. Gerber-Huber S, Nardelli D, Haefliger JA, Cooper DN, Givel F, et al. (1987) Precursor-product relationship between vitellogenin and the yolk proteins as derived from the complete sequence of a Xenopus vitellogenin gene. Nucleic Acids Res 15: 4737–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sumpter JP, Jobling S (1995) Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect 103 Suppl 7 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Purdom C, Hardiman P, Bye V, Eno N, Tyler C, et al. (1994) Estrogenic effects of effluents from sewage treatment works. Chemistry and Ecology 8: 275–285. [Google Scholar]
- 53. Li CR, Lee SH, Kim SS, Kim A, Lee KW, et al. (2009) Environmental estrogenic effects and gonadal development in wild goldfish (Carassius auratus). Environ Monit Assess 150: 397–404. [DOI] [PubMed] [Google Scholar]
- 54. Wang R, Liu J, Yang X, Lin C, Huang B, et al. (2013) Biological response of high-back crucian carp (Carassius auratus) during different life stages to wastewater treatment plant effluent. Environ Sci Pollut Res Int 20: 8612–8620. [DOI] [PubMed] [Google Scholar]
- 55. Carnevali O, Mosconi G, Yamamoto K, Kobayashi T, Kikuyama S, et al. (1992) Hormonal control of in vitro vitellogenin synthesis in Rana esculenta liver: effects of mammalian and amphibian growth hormone. Gen Comp Endocrinol 88: 406–414. [DOI] [PubMed] [Google Scholar]
- 56. Carnevali O, Mosconi G, Yamamoto K, Kobayashi T, Kikuyama S, et al. (1993) In-vitro effects of mammalian and amphibian prolactins on hepatic vitellogenin synthesis in Rana esculenta . J Endocrinol 137: 383–389. [DOI] [PubMed] [Google Scholar]
- 57. Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A, et al. (2010) Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol Lett 194: 16–25. [DOI] [PubMed] [Google Scholar]
- 58. Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, et al. (1994) Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 15: 342–355. [DOI] [PubMed] [Google Scholar]
- 59. Svenson A, Allard AS, Junestedt C, Cerne O, Ek M (2004) Assessment of androgenicity in leachates from Swedish landfills and treatments for its elimination. J Environ Sci Health A Tox Hazard Subst Environ Eng 39: 2817–2825. [PubMed] [Google Scholar]
- 60. Blankvoort BM, Rodenburg RJ, Murk AJ, Koeman JH, Schilt R, et al. (2005) Androgenic activity in surface water samples detected using the AR-LUX assay: indications for mixture effects. Environ Toxicol Pharmacol 19: 263–272. [DOI] [PubMed] [Google Scholar]
- 61. Leusch FD, Chapman HF, van den Heuvel MR, Tan BL, Gooneratne SR, et al. (2006) Bioassay-derived androgenic and estrogenic activity in municipal sewage in Australia and New Zealand. Ecotoxicol Environ Saf 65: 403–411. [DOI] [PubMed] [Google Scholar]
- 62. Wen R, Xie Y, Wan C, Fang Z (2013) Estrogenic and androgenic effects in mosquitofish (Gambusia affinis) from streams contaminated by municipal effluent in Guangzhou, China. Aquat Toxicol 132–133: 165–172. [DOI] [PubMed] [Google Scholar]
- 63. Chang H, Wan Y, Wu S, Fan Z, Hu J (2011) Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: comparison to estrogens. Water Res 45: 732–740. [DOI] [PubMed] [Google Scholar]
- 64. Miura T, Yamauhi K, Nagahama Y, Takahashi H (1991) Induction of Spermatogenesis in Male Japanese Eel, Anguilla japonica, by a Single Injection of Human Chorionic Gonadotropin (Endocrinology). Zoological science 8: 63–73. [Google Scholar]
- 65. Christiansen L, Pedersen K, Korsgaard B, Bjerregaard P (1998) Estrogenicity of xenobiotics in rainbow trout (Oncorhynchus mykiss) using in vivo synthesis of vitellogenin as a biomarker. Marine Environmental Research 46: 137–140. [Google Scholar]
- 66. Rasmussen TH, Korsgaard B (2004) Estrogenic octylphenol affects seminal fluid production and its biochemical composition of eelpout (Zoarces viviparus). Comp Biochem Physiol C Toxicol Pharmacol 139: 1–10. [DOI] [PubMed] [Google Scholar]
- 67. Jobling S, Sumpter JP, Sheahan D, Osborne JA, Matthiessen P (1996) Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environmental Toxicology and Chemistry 15: 194–202. [Google Scholar]
- 68. Toft G, Baatrup E (2001) Sexual characteristics are altered by 4-tert-octylphenol and 17beta-estradiol in the adult male guppy (Poecilia reticulata). Ecotoxicol Environ Saf 48: 76–84. [DOI] [PubMed] [Google Scholar]
- 69. Cardinali M, Maradonna F, Olivotto I, Bortoluzzi G, Mosconi G, et al. (2004) Temporary impairment of reproduction in freshwater teleost exposed to nonylphenol. Reprod Toxicol 18: 597–604. [DOI] [PubMed] [Google Scholar]
- 70.Jobling S, Tyler C, Nolan M, Sumpter J (1998) The identification of oestrogenic effects in wild fish. Research and development Technical Report W 119.
- 71. Muller M, Rabenoelina F, Balaguer P, Patureau D, Lemenach K, et al. (2008) Chemical and biological analysis of endocrine-disrupting hormones and estrogenic activity in an advanced sewage treatment plant. Environ Toxicol Chem 27: 1649–1658. [DOI] [PubMed] [Google Scholar]
- 72. Safe SH (1998) Hazard and risk assessment of chemical mixtures using the toxic equivalency factor approach. Environ Health Perspect 106 Suppl 4 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Api A (2001) Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food and Chemical Toxicology 39: 97–108. [DOI] [PubMed] [Google Scholar]
- 74. Körner W, Bolz U, Süβmuth W, Hiller G, Schuller W, et al. (2000) Input/output balance of estrogenic active compounds in a major municipal sewage plant in Germany. Chemosphere 40: 1131–1142. [DOI] [PubMed] [Google Scholar]
- 75. ter Veld MG, Schouten B, Louisse J, van Es DS, van der Saag PT, et al. (2006) Estrogenic potency of food-packaging-associated plasticizers and antioxidants as detected in ERalpha and ERbeta reporter gene cell lines. J Agric Food Chem 54: 4407–4416. [DOI] [PubMed] [Google Scholar]
- 76. Liu ZH, Kanjo Y, Mizutani S (2009) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment-physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407: 731–748. [DOI] [PubMed] [Google Scholar]
- 77. Gunnarsson L, Adolfsson-Erici M, Bjorlenius B, Rutgersson C, Forlin L, et al. (2009) Comparison of six different sewage treatment processes-reduction of estrogenic substances and effects on gene expression in exposed male fish. Sci Total Environ 407: 5235–5242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biological responses of goldfish exposed to different dilutions of leachate. Male goldfish (12 fish each group) were exposed to different dilutions of the leachate (1∶10, 1∶20, 1∶50, 1∶100, and 1∶200) in a semi-static exposure system after acclimated in the lab for 14 days. The leachate was diluted with dechlorinated tap water and a negative control group (dechlorinated tap water) was also set up. The mortality rate, survival time, and abnormal behavior were recorded.
(DOCX)
The ARRIVE guidelines checklist for animal research: reporting in vivo experiments.
(PDF)