Abstract
A large genomic deletion in human cardiac ryanodine receptor (RYR2) gene has been detected in a number of unrelated families with various clinical phenotypes, including catecholaminergic polymorphic ventricular tachycardia (CPVT). This genomic deletion results in an in-frame deletion of exon-3 (Ex3-del). To understand the underlying disease mechanism of the RyR2 Ex3-del mutation, we generated a mouse model in which the RyR2 exon-3 sequence plus 15-bp intron sequences flanking exon-3 were deleted. Heterozygous Ex3-del mice (Ex3-del+/−) survived, but no homozygous Ex3-del mice were born. Unexpectedly, the Ex3-del+/− mice are not susceptible to CPVT. Ex3-del+/− cardiomyocytes exhibited similar amplitude but altered dynamics of depolarization-induced Ca2+ transients compared to wild type (WT) cells. Immunoblotting analysis revealed markedly reduced expression of RyR2 protein in the Ex3-del+/− mutant heart, indicating that Ex3-del has a major impact on RyR2 protein expression in mice. Cardiac specific, conditional knockout of the WT RyR2 allele in Ex3-del+/− mice led to bradycardia and death. Thus, the absence of CPVT and other phenotypes in Ex3-del+/− mice may be attributable to the predominant expression of the WT RyR2 allele as a result of the markedly reduced expression of the Ex3-del mutant allele. The effect of Ex3-del on RyR2 protein expression is discussed in relation to the phenotypic variability in individuals with the RyR2 exon-3 deletion.
Introduction
The cardiac Ca2+ release channel (ryanodine receptor type 2, RyR2) plays an essential role in excitation-contraction (EC) coupling and sarcoplasmic reticulum Ca2+ handling in the heart [1]. RyR2 is also a key player in the pathogenesis of cardiac arrhythmias and cardiomyopathies [2]. To date, more than 150 mutations in RyR2 have been associated with different forms of cardiac arrhythmias, including catecholaminergic polymorphic ventricular tachycardia (CPVT), catecholaminergic idiopathic ventricular fibrillation, and atrial fibrillation [2]–[5]. Most patients with RyR2 mutations exhibit structurally normal hearts. However, some RyR2 mutations have been linked to cardiomyopathies as well as cardiac arrhythmias [6]–[11]. For instance, a large genomic deletion (1.1–3.6 kb) in the RYR2 gene that covers exon-3 was identified in a number of unrelated families [11]–[14]. Individuals with this exon-3 deletion display a wide spectrum of clinical phenotypes, including CPVT, sinoatrial node dysfunction, bradycardia, atrial fibrillation, AV block, dilated cardiomyopathy, and left ventricular non-compaction.
The deletion of exon-3 results in an in-frame deletion of 35 amino acid residues (Asn57-Gly91) in the NH2-terminal region of the RyR2 channel that is believed to be important for channel function [15]–[18]. Interestingly, the missing structural element due to exon-3 deletion can be rescued by a β-strand switching [16], [17]. As a result, the exon-3 deletion does not grossly affect the overall folding of the NH2-terminal domain of RyR2. Consistent with these observations, functional studies in HEK293 cells revealed that the exon-3 deleted RyR2 mutant remained functional, but displayed altered properties of Ca2+ release activation and termination [18].
Although the structural impact of the exon-3 deletion and its effect on spontaneous Ca2+ release in the heterologous HEK293 cells have been well characterized, the mechanisms by which the exon-3 deletion in RyR2 causes cardiac arrhythmias and cardiomyopathy are unknown. Genetically engineered mouse models harboring disease-associated mutations have been widely used for studying disease mechanisms. Indeed, a number of knock-in mice expressing RyR2 mutations linked to CPVT have been generated and characterized [19]–[26]. These RyR2 mutant mice have provided important insights into how RyR2 mutations cause CPVT. However, in contrast to CPVT-associated RyR2 mutations, there are few models that express cardiomyopathy-associated RyR2 mutations. In an attempt to understand the disease mechanism underlying RyR2-associated cardiomyopathies, in the present study, we generated a mouse model in which the entire exon-3 and part of the introns 2 and 4 were deleted using the knock-in approach. Unexpectedly, we found that this deletion has a dramatic impact on the expression of the mouse RyR2 protein. The observation that the exon-3 deletion alters the expression of RyR2 may provide some clues to the various clinical phenotypes and variable severities of patients with the RyR2 exon-3 deletion [11]–[14].
Materials and Methods
Generation of a mouse model harboring the RyR2 exon-3 deletion
A genomic DNA phage clone containing part of the mouse cardiac ryanodine receptor gene was isolated from the lambda mouse 129-SV/J genomic DNA library (Stratagene) and used to construct the RyR2 exon-3 deletion (Ex3-del) knock-in (KI) targeting vector. This genomic DNA fragment (about 15 kb) was released from the lambda vector by NotI, and subcloned into pBluescript to form the RyR2 genomic DNA plasmid. PCR-based site-directed mutagenesis was performed to generate a 660 bp DNA fragment containing the Ex3-del mutation using this RyR2 genomic DNA plasmid as a template. An XhoI site was created in the 5′ and a BamH I site in the 3′ in this DNA fragment. The modified XhoI-BamHI fragment was then subcloned into the targeting vector that contains a neomycin selection cassette flanked by FRT sites using BamH I and XhoI. The 2186 bp HindIII-HindIII and the 5994 bp AflII-AflII genomic DNA fragments were isolated from the RyR2 genomic DNA plasmid and inserted into the targeting vector to form the 5′ arm via the HindIII sites and the 3′ arm via the AflII sites, respectively. The DNA sequences of all PCR fragments used for constructing the targeting vector were confirmed by DNA sequencing. The targeting vector was linearized with NotI and subsequently electroporated into R1 embryonic stem (ES) cells.
G418-resistant ES clones were screened for homologous recombination by Southern blotting using an external probe. Briefly, genomic DNA was extracted from G418-resistant ES cell clones. ES cell DNA was digested using BglII, separated on a 0.8% (wt/vol) agarose gel, and subsequently blotted onto a nitrocellulose membrane. A DNA probe (∼700 bp) was generated by PCR from mouse genomic DNA using the specific primers, forward: 5′-TGCCTTGTCGTCAATTAAGCTGT-3′; and reverse: 5′-TACATGTGTGCAGTTGCCCATA-3′. The PCR product was subsequently radiolabeled using [32P]dCTP by random priming (Invitrogen). DNA blots were hybridized with the radiolabeled probe and visualized by autoradiography. Eight positive homologous recombinants were detected out of 780 ES cell clones, two of which were microinjected into blastocysts from C57BL/6J mice to generate male chimeras. Male chimeras were bred with female 129sve mice to generate germline transmitted heterozygous Ex3-del neo knock-in mice. RyR2 Ex3-del neo male mice were bred with female mice that express Flp recombinase to remove the selectable marker (the neomycin resistant gene, neo). The genotypes from F1 generation without neo were determined by PCR using DNA from tail biopsy specimens using the DNeasy Tissue Kit from Qiagen and the DNA primers, forward: 5′-CACAGACACACAGTAAGGCATTAC-3′; reverse: 5′-GTATGTCTTTCAGACATCCTAAGC -3′.
All animal studies were approved by the Institutional Animal Care and Use Committees at the University of Calgary, and performed in accordance with NIH guidelines. RyR2 WT and mutant mice (1.5–9 months of age) were used for all experiments.
Confirmation of exon-3 deletion at the mRNA level using RT-PCR
Total RNA was isolated from about 100 mg of heart tissues from RyR2 exon-3 deletion mutant mice or RyR2 wild type mice using the RNA isolation kit (Invitrogen) according to the manufacturer's instructions. First-strand cDNA was synthesized from ∼5 µg of total RNA using Superscript II RNase H reverse transcriptase (Invitrogen) with gene-specific primer (5′-ATAGCCTTGGGCTGCTTCACTTCC) at 50°C. Amplification of cDNA fragments by polymerase chain reaction (PCR) was performed in a 50 µl mixture that contains 1 µl cDNA, 10 µl 5× PCR buffer, 0.5 µM of each forward or reverse gene-specific primer (Forward primer: 5′-TGATGCGGGCGAAGGCGAGGAT; Reverse primer:5′-GAAGGCAGCATCCACATGCCAGCT), 200 µM dNTP, and 0.01 U Q5 High-Fidelity DNApolymerase (New England Biolab). PCR was performed in a thermocycler with an initial denaturation at 98°C for 30 seconds, followed by 30 cycles (10 s at 98°C, 15 s at 68°C, and 30s at 72°C) of amplification, and a final cycle of 2 min at 72°C. PCR products were separated by 1.8% agarose gel electrophoresis, and desired DNA fragments were purified using QIAGEN DNA Extraction kit and cloned into pBluescript vector. Plasmid DNA was isolated using QIAGEN Plasmid Purification kit. Insert-positive clones were identified by restriction enzyme digestion (EcoRI and HindIII), and their sequences were determined by DNA sequencing.
Generation of inducible, cardiac specific, conditional RyR2 knockout mice containing RyR2 exon-3 deletion
The conditional RyR2 knockout (KO) mouse model (RyR2-floxed mice) was generated as described previously [27]–[29]. These RyR2-floxed mice were crossed with the myosin heavy chain (MHC)-mer-Cre-mer mice that express the tamoxifen-inducible, MHC promoter-controlled Cre-recombinase [30]. This breeding produced tamoxifen-inducible, cardiac-specific, heterozygous RyR2 conditional KO mice (iRyR2wt/flox), which were used to generate tamoxifen-inducible, cardiac-specific, homozygous RyR2 conditional KO mice (iRyR2flox/flox). These iRyR2flox/flox mice were used to conditionally and specifically diminish the expression of the WT RyR2 allele. To do this, we bred the iRyR2flox/flox mice with the RyR2 Ex3-del+/− mutant mice to generate the iRyR2flox/Ex3-del mice. For induction of knockout of the WT RyR2 allele, tamoxifen (sigma, 75 mg/kg/day) was injected (intraperitoneal, i.p.) into iRyR2flox/Ex3-del and iRyR2flox/flox mice (1.5–2 months) for 3 consecutive days. The mice were used for stress tests and immunoblotting analysis ∼12 days post tamoxifen treatment.
ECG recordings
RyR2 Ex3-del+/− mutant mice and their WT littermates, iRyR2flox/Ex3-del, and iRyR2flox/flox mice were assessed for their susceptibility to stress-induced ventricular tachyarrhythmias using ECG recording [24]. Briefly, mice were lightly anesthetized with isoflurane vapor (0.5–1%) and 95% O2. Anesthetized mice were placed on a heating pad (27°C) and needle electrodes were inserted subcutaneously into the right upper limb and left lower abdomen for ECG recording (BIOPAC MP System, Goleta, CA). The animals′ ECG was continuously monitored under anesthesia until the heart rate became stabilized. Baseline ECG was recorded for 5–10 minutes. For induction of ventricular arrhythmias, the mice were subjected to intraperitoneal injection of a mixture of epinephrine (1.6 mg/kg) and caffeine (120 mg/kg). ECG was continuously recorded for 30 minutes after the injection of epinephrine and caffeine.
Western blotting
Mouse hearts were crushed by a Wollenberger clamp pre-cooled in liquid nitrogen. The crushed heart tissues were stored at −80°C until use. Frozen heart tissues were pulverized in liquid nitrogen and homogenized immediately in 6 volumes of homogenizing buffer containing 30 mM KH2PO4 (pH 7.0), 40 mM NaF, 5 mM EDTA, 300 mM sucrose, 4 µM leupeptin, 1 mM benzamidine, 100 µM PMSF, and 0.5 mM DTT, aliquoted, frozen with liquid nitrogen and stored at −80°C until use. Aliquots of homogenates were solubilized in a final 500 µl of solubilizing buffer containing 50 mM Tris-HCl (pH 7.4) plus 3% SDS for 1 h at room temperature and then incubated at 55°C for 10 min. The insoluble materials were removed by centrifugation at 12,000×g for 10 min [31]. The protein concentration of the supernatant was determined using a BioRad detergent-compatible protein assay kit. Solubilized proteins (15–20 µg) were used for SDS-PAGE [32]. RyR2 proteins resolved in 6% SDS-PAGE were transferred to nitrocellulose membranes at 45 V for 18–20 h at 4°C in the presence of 0.01% SDS according to the method of Towbin et al [33]. For the detection of β-actin, proteins were separated in 15% SDS-PAGE and transferred at 110 V for 1 h at 4°C. The nitrocellulose membranes containing the transferred proteins were blocked for 30 min with phosphate buffered saline (PBS) containing 0.5% Tween-20 and 5% skim milk powder. The blocked membrane was incubated with anti-RyR2 or anti-β-actin antibodies, and washed with PBS containing 0.5% Tween-20 for three times with shaking, each time for 5 min. The membrane was then incubated with the secondary anti-mouse or anti-rabbit IgG (H&L) antibodies conjugated to horseradish peroxidase (1∶20,000) for 30 min. After washing with PBS containing 0.5% Tween-20 for three times, the bound antibodies were detected using an enhanced chemiluminescence kit from Pierce. The intensity of each band was determined from its intensity profile obtained by ImageQuant LAS 4000 (GE Healthcare Life Sciences), analyzed by using the Image-J software, and normalized to that of β-actin.
Single cell Ca2+ imaging of isolated ventricular myocytes
Ventricular myocytes were isolated using retrograde aortic perfusion as described previously [34]. Isolated cells were kept at room temperature in Krebs-Ringers-HEPES (KRH) buffer (in mM: 125 NaCl, 12.5 KCl, 25 HEPES, 6 glucose, and 1.2 MgCl2, pH 7.4) containing 20 mM taurine, 20 mM 2,3-butanedione monoxime (BDM), 5 mg/ml albumin, and 1 mM free Ca2+ until use. Freshly isolated mouse ventricular myocytes were added to glass coverslips pre-coated with 50 µg/ml laminin, and loaded with 5 µM Rhod-2, AM (Molecular Probes, USA) in KRH buffer containing 1 mM Ca2+ for 20 min at room temperature as described previously [35]. The glass coverslip pre-mounted to a recording chamber was then placed onto an inverted microscope (Nikon ECLIPSE Ti) equipped with a Nikon CFI Plan Apo VC 60xWI objective. Then Rhod-2 loaded cells were perfused with KRH buffer containing 2 mM extracellular Ca2+ and 5 µM blebbistatin. The cells were paced by field stimulation using the S88X electric stimulator (Grass, USA) at 3 Hz. Confocal line-scanning (512 pixels and 1.9 ms per line) were performed along the longitudinal axis of cells for 10 seconds using the Nikon A1R confocal system. The Rhod-2 loaded myocytes were excited using the 561 nm diode laser and the fluorescence emission at 570–620 nm was recorded. The line-scan images were processed and analyzed with the NIS-Elements AR 4.0.
Statistical analysis
All values shown are mean ± SEM unless indicated otherwise. To test for differences between groups, we used unpaired Student's t tests (2-tailed). A P value <0.05 was considered to be statistically significant.
Results
Generation of a mouse model expressing the RyR2 exon-3 deletion mutant
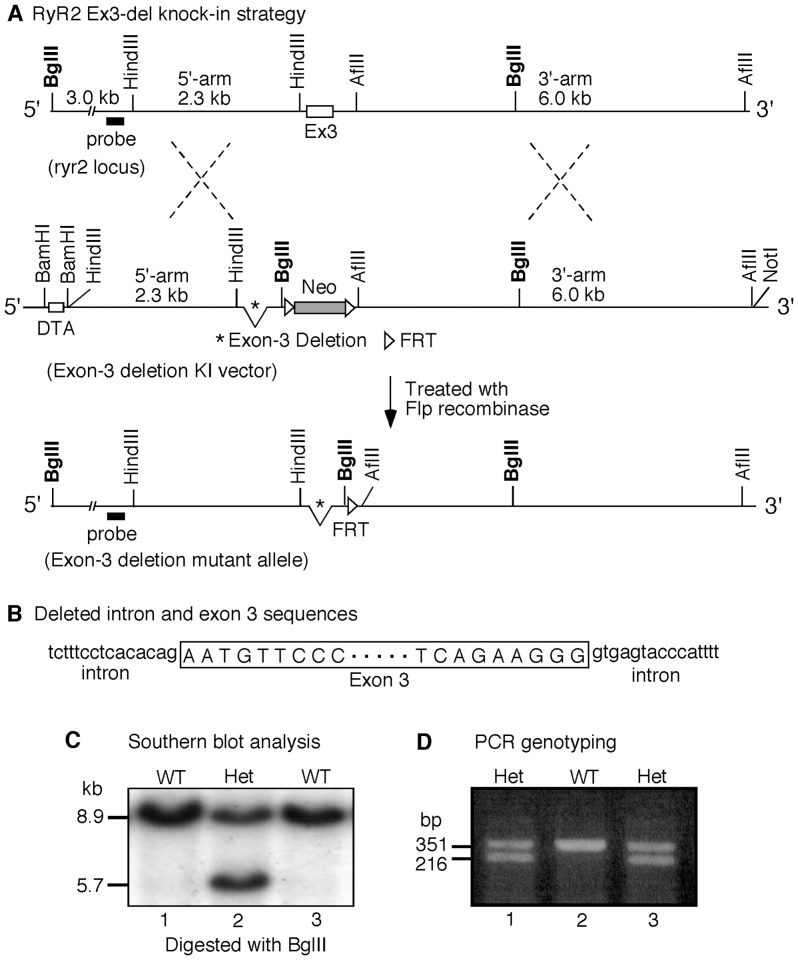
Naturally occurring deletion of exon-3 (Ex3-del) encoding residues Asn57-Gly91 in the NH2-terminal region of the RyR2 channel has been linked to various cardiac abnormalities in humans, but their causal mechanisms are unknown [11]–[14]. To determine the physiological consequence of this deletion, we generated a mouse model harboring the RyR2 Ex3-del mutant. As shown in Fig. 1, the 105-bp exon-3 sequence and the intron sequences (15-bp on both sides) that flank exon-3 were removed from an RyR2 genomic DNA fragment. This modified RyR2 DNA fragment was then used to construct the Ex3-del knock-in plasmid (Fig. 1A,B). Mouse embryonic stem (ES) cells were transfected with the Ex3-del knock-in plasmid. Southern blot screening of transfected ES cells identified 8 positive recombinant ES cell clones (Fig. 1C). One of these positive ES cell clones was then used to produce heterozygous RyR2 Ex3-del mutant mice (Ex3-del+/−) (Fig. 1D). Interestingly, while heterozygous Ex3-del mice survived and showed no obvious gross defects, no homozygous RyR2 Ex3-del mice were ever detected, suggesting that homozygous RyR2 exon-3 deletion is embryonically lethal in mice.
Figure 1. Generation of the RyR2 Ex3-del mutant mouse model.
(A) The mouse ryr2 locus (top line), the construction of the knock-in (KI) vector containing the deletion of the exon-3 and flanking intron sequences in the mouse ryr2 gene, the selectable markers neo (neomycin resistant gene) and DTA (diphtheria toxin A), the FRT sites (middle line), the generation of the exon-3 deletion mutant allele, and the removal of the neo gene via homologous recombination (bottom line) are depicted. (B) Intron and exon sequences that were deleted in the RyR2 Ex3-del mutant mice. (C) Southern blot analysis of recombinant embryonic stem cells (WT, wild type; Het, heterozygous). (D) PCR genotyping using tail samples from WT and Ex3-del heterozygous (Het) mice.
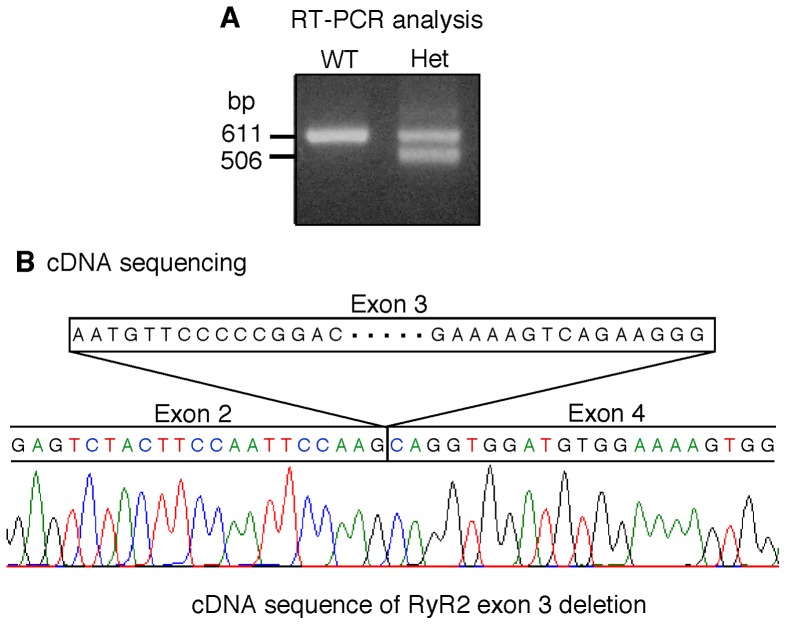
To confirm the absence of exon-3 in the Ex3-del+/− mice at the mRNA level, we isolated mRNAs from the wild type (WT) and Ex3-del+/− mouse hearts and generated cDNA fragments covering exon-3 via RT-PCR. Sequencing analysis of these cDNA fragments shows that the RyR2 exon-3 sequence in the Ex3-del+/− mouse heart has indeed been deleted (Fig. 2).
Figure 2. Deletion of exon-3 in the RyR2 mRNA from heterozygous RyR2 Ex3-del mice.
A fragment of the mouse RyR2 mRNA covering exon-3 was converted to cDNA and amplified using RT-PCR from total RNAs isolated from wild type (WT) and heterozygous RyR2 Ex3-del (Het) mutant mice (A). The RT-PCR products were isolated and sequenced. The sequence of the RyR2 Ex3-del cDNA was shown (B). Note that the exon-2 sequence is directly followed by the exon-4 sequence, i.e. the exon-3 sequence has been deleted.
Heterozygous RyR2 Ex3-del mutant mice show no stress-induced ventricular tachyarrhythmias
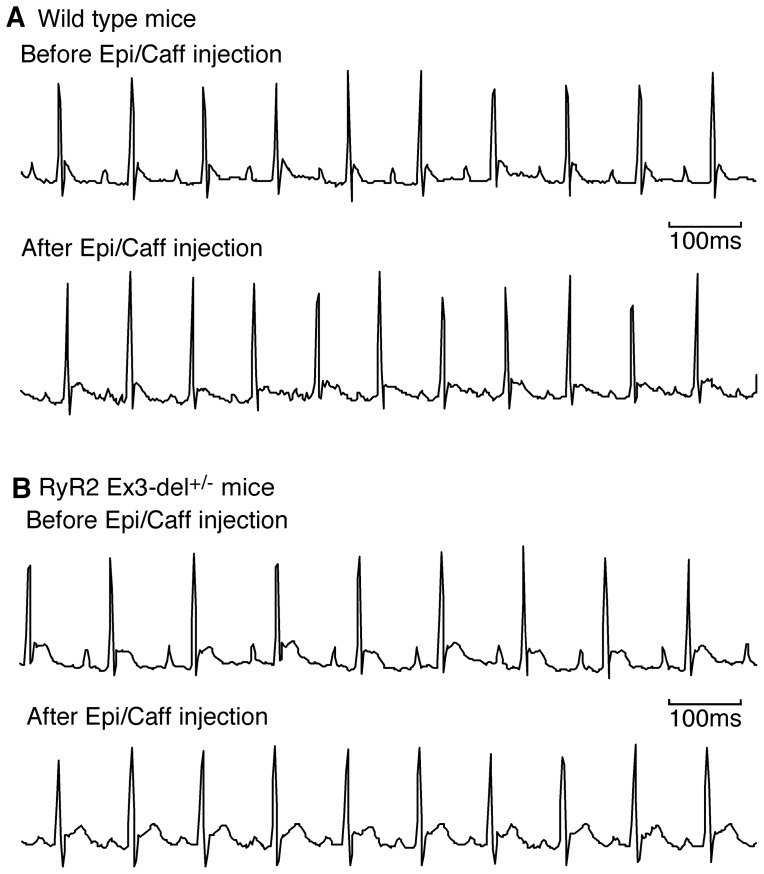
Deletion of exon-3 in RyR2 has been associated with CPVT in humans. To assess whether Ex3-del+/− mutant mice are susceptible to stress-induced ventricular tachyarrhythmias (VTs), we monitored the ECG of these mice before and after the injection of pharmacological triggers (caffeine/epinephrine). We have previously shown that these triggers readily induced VTs in the CPVT-linked RyR2 R4496C+/− mutant mice [24]. Unexpectedly, we found that caffeine and epinephrine did not induce VTs in either RyR2 Ex3-del+/− mutant mice (n = 14) or their WT littermates (n = 16) at 1.5–3 months of age (Fig. 3). To assess whether their susceptibility to CPVT is age-dependent, we performed the same caffeine/epinephrine stress tests on mice at 3–5 months of age (5 WT and 10 Ex3-del+/− mice) and 8–9 months of age (8 Ex3-del+/− mice). Similarly, we found that none of these older WT or Ex3-del+/− mutant mice showed stress-induced VTs. Thus, CPVT susceptibility in Ex3-del+/− mutant mice is unlikely to be age-dependent.
Figure 3. Heterozygous RyR2 Ex3-del mutant mice are not susceptible to CPVT.
Representative ECG recordings of wild type (WT) (A) and RyR2 Ex3-del+/ − mutant (B) mice (1.5–3 months of age) before (top) and after (bottom) the injection of epinephrine (1.6 mg/kg) and caffeine (120 mg/kg). Note that no VTs were detected in either WT (n = 16) or mutant (n = 14) mice during the 30-min period of ECG recording after the injection of the triggers.
Depolarization-induced Ca2+ transients in WT and heterozygous Ex3-del mutant cardiomyocytes
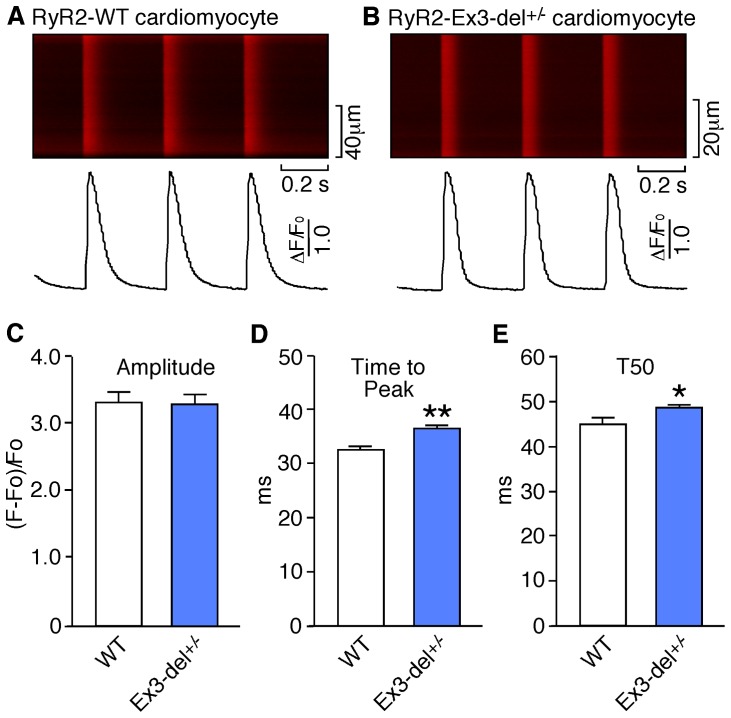
To determine whether Ex3-del+/− mutant cardiomyocytes exhibit altered excitation-contraction coupling, we analyzed the amplitude and dynamics of Ca2+ release evoked by depolarization in cardiac myocytes isolated from RyR2 WT and Ex3-del+/− mutant mice using confocal line-scan Ca2+ imaging. As shown in Fig. 4, there was no significant difference (P = 0.472) in the Ca2+ transient amplitude (ΔF/F0; WT: 3.32±0.17 vs. Ex3-del+/−: 3.29±0.14) between RyR2 WT (n = 35) and Ex3-del+/− mutant (n = 58) ventricular myocytes. On the other hand, the time-to-peak (WT: 32.5±0.7 ms vs. Ex3-del+/−: 36.4±0.5 ms, P<0.001) and time-to-50% decay (T50; WT: 44.8±1.5 ms vs. Ex3-del+/−: 48.3±0.9 ms, P<0.05) in Ex3-del+/− mutant cells were slightly but significantly increased compared to those in the RyR2 WT cells.
Figure 4. Depolarization-induced Ca2+ transients in WT and heterozygous RyR2 Ex3-del mutant cardiomyocytes.
Ventricular myocytes isolated from RyR2 WT and Ex3-del+/ − mutant hearts were loaded with Rhod-2-AM and perfused with 2 mM extracellular Ca2+ in KRH solution and paced at 3Hz. Ca2+ transients were monitored by line-scan confocal Ca2+ imaging. Representative images/traces of WT (A) and Ex3-del+/ − mutant (B) cardiomyocytes, and average data of the amplitude (C), time to peak (D), and time to 50% decay (E) of Ca2+ transients in WT and Ex3-del+/ − mutant cells are shown. Data shown are mean ± SEM from 35 WT and 58 mutant cells (**P<0.001; *P<0.05).
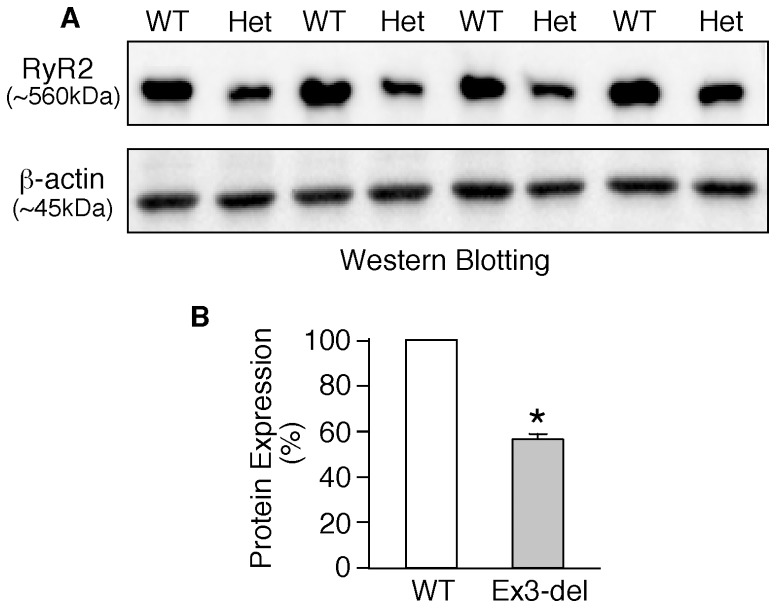
Heterozygous RyR2 Ex3-del mutant hearts display markedly reduced RyR2 protein expression
The lack of CPVT phenotype in RyR2 Ex3-del+/− mutant mice is surprising given the impact of Ex3-del on RyR2 function in heterologous systems and its link to CPVT and other cardiac abnormalities in patients [11]–[14], [18]. This lack of phenotypes could result from poor expression of the RyR2 Ex3-del mutant protein. To test this possibility, we performed immunoblotting analysis to assess the level of the RyR2 protein expressed in Ex3-del+/− mutant and WT hearts. It should be noted that it is difficult to distinguish the RyR2 Ex3-del mutant protein from the RyR2 WT, as there are currently no specific probes available for the detection of the RyR2 Ex3-del mutant protein. Thus, we examined the total RyR2 protein expressed in the WT and mutant hearts. As shown in Fig. 5, heterozygous Ex3-del mutant hearts (n = 4) exhibited a markedly reduced level of RyR2 protein (58±3%) as compared to WT hearts (n = 4) (P<0.001). Thus, these data indicate that deletion of exon-3 significantly reduces the expression of RyR2 protein in mice. Since the expression of the normal WT RyR2 allele in the heterozygous Ex3-del mutant heart is unlikely to be markedly altered, the reduction in RyR2 expression observed in the heterozygous Ex3-del mutant heart would mostly result from the impaired expression of the Ex3-del mutant allele. Hence, the absence of CPVT phenotype in RyR2 Ex3-del+/− mutant mice may be attributable to the lack of expression of the Ex3-del mutant RyR2 allele.
Figure 5. Reduced RyR2 protein expression in heterozygous RyR2 Ex3-del mutant hearts.
(A) Whole heart homogenates were prepared from wild type (WT) (n = 4) and RyR2 Ex3-del−/− mutant (n = 4) mice (2–3 months) and used for immunoblotting analysis using antibodies against RyR2 or β-actin. (B) The expression of RyR2 in the Ex3-del hearts was significantly reduced (58±3%) as compared to that in WT hearts (*P<0.001).
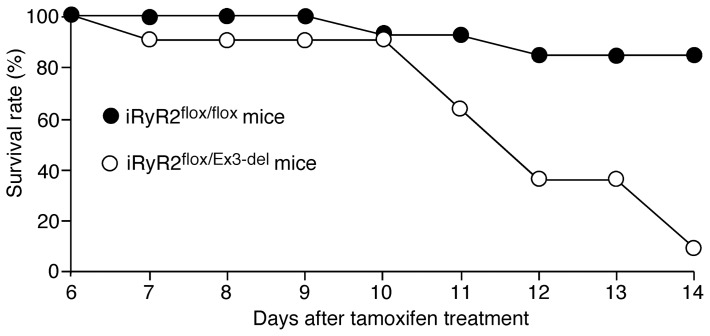
Cardiac-specific, conditional knockout of the WT RyR2 allele in heterozygous RyR2 Ex3-del mutant mice
To test the possibility that the predominant expression of the WT allele over the mutant allele in Ex3-del+/− mutant mice may mask the impact of the RyR2 Ex3-del mutation, we employed the Cre-Lox recombination technology to conditionally and specifically reduce the expression level of the WT RyR2 allele in the Ex3-del+/− mutant heart. We reasoned that by specifically reducing the expression of the WT allele, we might be able to reveal the impact of the Ex3-del mutation. To this end, we bred the RyR2-floxed mice with the myosin heavy chain (MHC)-mer-Cre-mer mice that express the tamoxifen-inducible, MHC promoter-controlled Cre-recombinase [28]–[30]. Through this breeding, we obtained tamoxifen-inducible, cardiac-specific RyR2 conditional KO mice (iRyR2flox/flox) [28], [29]. We then bred these iRyR2flox/flox mice with our RyR2 Ex3-del+/− mutant mice to generate the iRyR2flox/Ex3-del mice. To induce knockout of the WT RyR2 allele, we injected the iRyR2flox/Ex3-del (n = 11) and iRyR2flox/flox (n = 13) mice with tamoxifen. We found that the iRyR2flox/Ex3-del mice died starting ∼11 days post tamoxifen treatment. By 14 days post tamoxifen treatment, most of the iRyR2flox/Ex3-del mice (10 out 11) had died. On the other hand, only 2 of the 13 iRyR2flox/flox mice died 14 days post tamoxifen treatment (Fig. 6). Thus, upon reducing the expression of the WT RyR2 allele, heterozygous Ex3-del mutant mice displayed a higher rate of death than the RyR2-floxed mice.
Figure 6. Cardiac-specific, conditional knockout of the WT RyR2 allele in heterozygous RyR2 Ex3-del mutant mice results in early death.
iRyR2flox/flox (n = 13, black circles) and iRyR2flox/Ex3-del (n = 11, white circles) mice were injected with tamoxifen (75 mg/kg/day) for 3 consecutive days. The percentage of live mice (survival rate) on day 6–14 post tamoxifen treatment is shown.
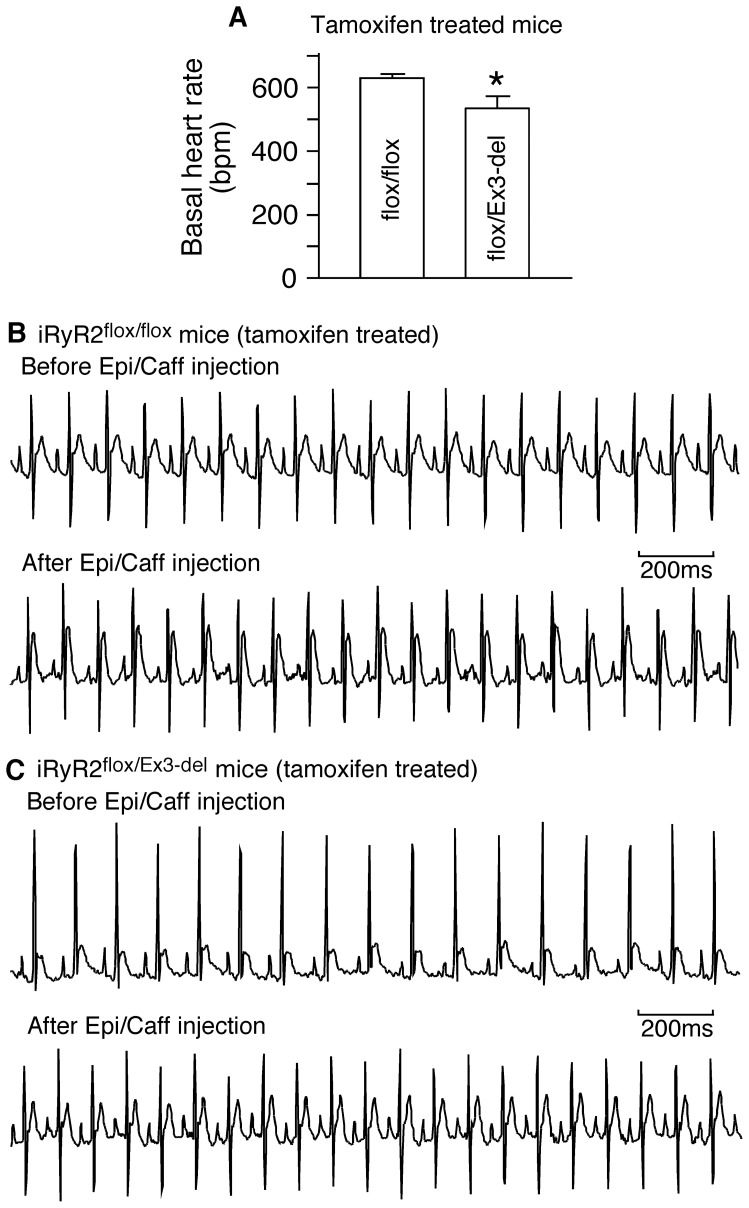
To gain some insight into the cause of death, we monitored the ECG of iRyR2flox/Ex3-del and iRyR2flox/flox mice ∼12 days post tamoxifen treatment. We found that the basal heart rate of the iRyR2flox/Ex3-del mice (535±38 bpm) (n = 8) was significantly reduced as compared to that of iRyR2flox/flox mice (629±13 bpm) (n = 11) (P = 0.012). We also performed caffeine/epinephrine stress tests on these iRyR2flox/Ex3-del and iRyR2flox/flox mice. We found that caffeine/epinephrine challenge did not induce VTs in either the iRyR2flox/Ex3-del (n = 8) or iRyR2flox/flox (n = 11) mice ∼12 days post-tamoxifen treatment (Fig. 7). Thus, upon reducing the expression of the WT RyR2 allele, heterozygous Ex3-del mutant mice displayed bradycardia but no stress-induced VTs.
Figure 7. Heterozygous RyR2 Ex3-del mutant mice with cardiac specific, conditional KO of the WT RyR2 allele exhibit bradycardia, but no CPVT.
iRyR2flox/flox (n = 11) and iRyR2flox/Ex3-del (n = 8) mice were treated with tamoxifen. ECG recording was performed 12 days post tamoxifen treatment to determine their basal heart rates (before epinephrine/caffeine challenge) (A) and their susceptibility to CPVT (B, C). Representative ECG recordings of the tamoxifen-treated iRyR2flox/flox mice (B) and the tamoxifen-treated iRyR2flox/Ex3-del mice (C) before (top panel) and after (bottom panel) the injection of epinephrine (1.6 mg/kg) and caffeine (120 mg/kg). Note that no VTs were detected in either the tamoxifen-treated iRyR2flox/flox mice (B) or the tamoxifen-treated iRyR2flox/Ex3-del mice during the 30-min period of ECG recording after the injection of the triggers (*P<0.05).
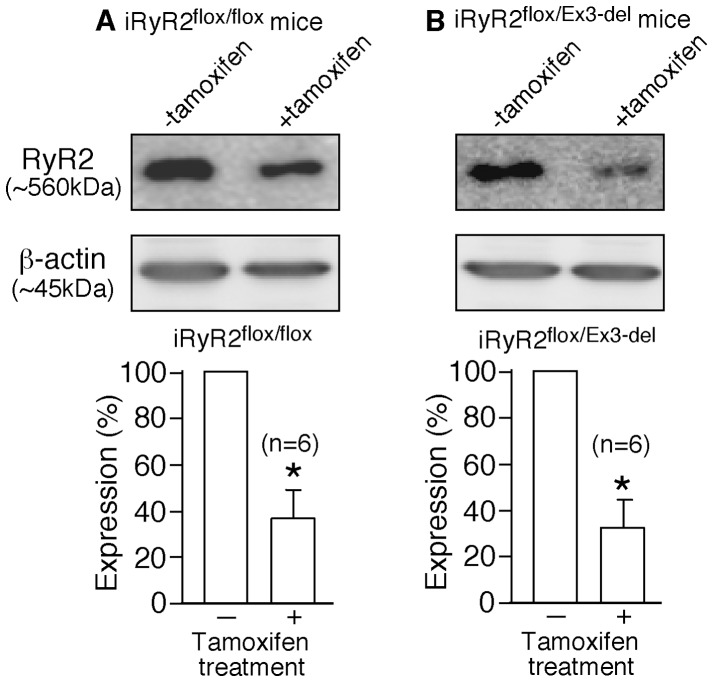
To confirm that tamoxifen treatment did reduce RyR2 expression, we carried out immunoblotting analysis of whole heart homogenates from iRyR2flox/flox and iRyR2flox/Ex3-del mice before and after tamoxifen treatment. Indeed, we found that the expression level of RyR2 in iRyR2flox/flox mouse hearts was significantly reduced to 37±12% of (n = 6, P<0.001) ∼12 days post tamoxifen treatment (as compared to the level before treatment) (Fig. 8). Similarly, the expression level of RyR2 in iRyR2flox/Ex3-del mouse hearts was decreased to 33±8% (n = 6, P<0.001) ∼12 days post tamoxifen treatment (as compared to the level before treatment) (Fig. 8). Taken together, these observations support the notion that the absence of CPVT phenotype in the RyR2 Ex3-del+/− mutant mice is most likely due to the predominant expression of the WT allele as a result of the markedly reduced expression of the Ex3-del mutant RyR2 allele.
Figure 8. Cardiac specific, conditional knockout of the WT RyR2 allele results in markedly reduced RyR2 expression.
Whole heart homogenates were prepared from iRyR2flox/flox (A) and iRyR2flox/Ex3-del (B) mice before and after tamoxifen treatment, and used for immunoblotting analysis using antibodies against RyR2 or β-actin. Note that the expression of RyR2 in iRyR2flox/flox hearts (n = 6) or iRyR2flox/Ex3-del hearts (n = 6) was significantly reduced 12 days post tamoxifen treatment (*P<0.001).
Discussion
Of more than 150 disease-associated RyR2 mutations, exon-3 deletion (Ex3-del) is the only large genomic deletion found in the human RYR2 gene to date [2], [11]–[14]. Analysis of the genomic DNA sequence reveals that exon-3 in human RYR2 is flanked by two Alu elements. Recombination between these two Alu elements due to DNA polymerase slippage during chromosomal replication results in a deletion of 1.1 kb genomic DNA sequence encompassing exon-3 [11]. Similar genomic deletions around exon-3 have been detected in a number of unrelated families, suggesting that the area around exon-3 of the human RYR2 gene is highly susceptible to Alu-mediated genomic rearrangements [11]–[14]. These findings also suggest that the deletion of exon-3 in human RyR2 may represent a common genetic defect and a significant cause of RyR2-associated cardiac abnormalities.
Given its relative high prevalence and potential importance in the pathogenesis of cardiac arrhythmias and cardiomyopathies, we have attempted to generate a mouse model for the human RyR2 exon-3 deletion. To this end, we produced a mouse line in which the entire exon-3 in mouse RyR2 plus a 15-bp intron sequence on both sides of exon-3 were deleted using the knock-in approach. To our surprise and unlike that seen in patients with RyR2 exon-3 deletion, heterozygous Ex3-del mutant mice are not susceptible to stress-induced cardiac arrhythmias. No homozygous Ex3-del mice were born. Immunoblotting analysis revealed that the expression level of RyR2 protein in heterozygous Ex3-del mouse hearts was reduced to 58% of that in WT hearts. This level (58%) represents the total RyR2 protein expression (i.e. WT and mutant proteins together). The dramatic reduction in RyR2 protein expression observed in heterozygous Ex3-del mutant hearts likely resulted from impaired expression of the Ex3-del mutant RyR2 allele as a consequence of the exon-3 deletion. In line with this view, we found that conditionally and specifically knocking-out only the WT RyR2 allele in the heterozygous Ex3-del mutant heart through the Cre-Lox recombination approach resulted in a dramatically reduced level of RyR2 expression. These observations indicate that a large portion of the RyR2 protein expressed in heterozygous Ex3-del mutant hearts is contributed by the expression of the WT allele. In other words, only a small amount of Ex3-del mutant protein is expressed in heterozygous Ex3-del mutant hearts. Based on these observations, we propose that the lack of CPVT and other phenotypes in heterozygous RyR2 Ex3-del mutant mice is likely due to the predominant expression of the WT RyR2 allele because of a dramatically reduced expression level of the Ex3-del mutant protein. In this regard, the expression of RyR2 in heterozygous Ex3-del mutant mice may resemble that in heterozygous RyR2 KO mice. It is important to know that there are no obvious structural and functional abnormalities detected in heterozygous RyR2 KO mice, while homozygous RyR2 KO is embryonically lethal [36]. Similarly, homozygous RyR2 exon-3 deletion is also embryonically lethal, most likely due to the markedly reduced expression level of the Ex3-del mutant protein and the mutation itself.
Individuals heterozygous for the RyR2 exon-3 deletion display a number of clinical phenotypes. These include sinoatrial node dysfunction, atrial arrhythmias, AV block, atrial standstill, bradycardia, dilated cardiomyopathy, or left ventricular non-compaction in addition to CPVT. However, none of these phenotypes were observed in heterozygous RyR2 Ex3-del mutant mice. The predominant expression of the WT RyR2 allele over the Ex3-del mutant allele may mask the impact of the mutant. In support of this view, we found that upon specifically reducing the expression of the WT RyR2 allele, heterozygous Ex3-del mutant mice exhibited bradycardia and death. The exact cause of bradycardia and death in iRyR2flox/Ex3-del mice after tamoxifen treatment is, however, unclear. Bround et al. [28] have recently shown that tamoxifen-induced, cardiac specific ablation of RyR2 is sufficient to cause bradycardia and death in mice. Since Ex3-del markedly reduces the protein expression of RyR2, removal of the WT RyR2 allele in iRyR2flox/Ex3-del mice after tamoxifen treatment would further decrease the already reduced level of RyR2 protein expression in iRyR2flox/Ex3-del mice. Such a profound reduction in RyR2 protein expression itself would lead to bradycardia and death [28]. Because of the markedly reduced expression of the Ex3-del mutant protein, the functional impact of Ex3-del on the RyR2 channel is unlikely to contribute significantly to bradycardia and death observed in iRyR2flox/Ex3-del mice. Further investigations are clearly needed to understand how deletion of exon-3 in RyR2 causes bradycardia and sudden death in humans.
The exact mechanism by which deletion of exon-3 affects the expression of the RyR2 protein has yet to be determined. Although deletion of exon-3 is rescued by β-strand switching in the context of the NH2-terminal domains of RyR2, it is unclear whether exon-3 deletion affects the stability and turnover of the full-length RyR2 protein in vivo. Furthermore, there may be species differences. The impact of exon-3 deletion on the synthesis and/or degradation of the RyR2 protein in mice and humans may be different. Further studies will be needed to understand how exon-3 deletion leads to a marked reduction in RyR2 expression.
An interesting and important question is whether the deletion of exon-3 affects the expression of the human RyR2 protein in patients. Among the mutant carriers, the clinical phenotypes and severities of patients with the RyR2 exon-3 deletion appear to be variable. For instance, some patients exhibit dilated cardiomyopathies, while others show no consistent structural abnormalities. Further, some patients were characterized with severe sinus bradycardia and atrioventricular block, atrial fibrillation, while others display only bradycardia [11]–[14]. It is possible that variable levels of expression of the RyR2 Ex3-del mutant allele may be attributable, in part, to the various clinical phenotypes and variable severities observed in patients with the RyR2 exon-3 deletion. Analysis of the expression level of the RyR2 protein in heart samples of patients with Ex3-del will be required to test this possibility.
A large number of RyR2 mutations have been associated with cardiac arrhythmias and cardiomyopathies [3]–[5]. The impact of these mutations on the expression of the RyR2 protein is largely unknown. Our present study demonstrates that RyR2 mutations, especially those large genomic rearrangements, may have significant impact on protein expression, thus affecting the ramification of the disease mutations.
Funding Statement
This work was supported by research grants from the National Institutes of Health (R01HL75210), Canadian Institutes of Health Research (CIHR), and the Heart and Stroke Foundation of Alberta, N.W.T. and Nunavut to S.R.W.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415: 198–205. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Chen SR (2011) Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 108: 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. George CH, Jundi H, Thomas NL, Fry DL, Lai FA (2007) Ryanodine receptors and ventricular arrhythmias: Emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol 42: 34–50. [DOI] [PubMed] [Google Scholar]
- 4. Dulhunty AF, Beard NA, Hanna AD (2012) Regulation and dysregulation of cardiac ryanodine receptor (RyR2) open probability during diastole in health and disease. J Gen Physiol 140: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venetucci L, Denegri M, Napolitano C, Priori SG (2012) Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol 9: 561–575. [DOI] [PubMed] [Google Scholar]
- 6. Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, et al. (2001) Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum Mol Genet 10: 189–94. [DOI] [PubMed] [Google Scholar]
- 7. Bauce B, Rampazzo A, Basso C, Bagattin A, Daliento L, et al. (2002) Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death: Early diagnosis of asymptomatic carriers. J Am Coll Cardiol 40: 341–349. [DOI] [PubMed] [Google Scholar]
- 8. Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ (2004) Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: A molecular autopsy of 49 medical examiner/coroner's cases. Mayo Clin Proc 79: 1380–1384. [DOI] [PubMed] [Google Scholar]
- 9. d'Amati G, Bagattin A, Bauce B, Rampazzo A, Autore C, et al. (2005) Juvenile sudden death in a family with polymorphic ventricular arrhythmias caused by a novel RyR2 gene mutation: Evidence of specific morphological substrates. Hum Pathol 36: 761–767. [DOI] [PubMed] [Google Scholar]
- 10. Nishio H, Iwata M, Suzuki K (2006) Postmortem molecular screening for cardiac ryanodine receptor type 2 mutations in sudden unexplained death: R420W mutated case with characteristics of status thymico-lymphatics. Circ J 70: 1402–1406. [DOI] [PubMed] [Google Scholar]
- 11. Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, et al. (2007) Expanding spectrum of human RYR2-related disease: New electrocardiographic, structural, and genetic features. Circulation 116: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 12. Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, et al. (2009) The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: A comprehensive open reading frame mutational analysis. J Am Coll Cardiol 54: 2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marjamaa A, Laitinen-Forsblom P, Lahtinen AM, Viitasalo M, Toivonen L, et al. (2009) Search for cardiac calcium cycling gene mutations in familial ventricular arrhythmias resembling catecholaminergic polymorphic ventricular tachycardia. BMC Med Genet 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno S, Omura M, Kawamura M, Kimura H, Itoh H, et al.. (2014) Exon 3 deletion of RYR2 encoding cardiac ryanodine receptor is associated with left ventricular non-compaction. Europace. 2014 Jan 6. [Epub ahead of print]. [DOI] [PubMed]
- 15. Tung CC, Lobo PA, Kimlicka L, Van Petegem F (2010) The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature 468: 585–588. [DOI] [PubMed] [Google Scholar]
- 16. Lobo PA, Kimlicka L, Tung CC, Van Petegem F (2011) The deletion of exon 3 in the cardiac ryanodine receptor is rescued by beta strand switching. Structure 19: 790–798. [DOI] [PubMed] [Google Scholar]
- 17. Amador FJ, Kimlicka L, Stathopulos PB, Gasmi-Seabrook GM, Maclennan DH, et al. (2013) Type 2 ryanodine receptor domain A contains a unique and dynamic alpha-helix that transitions to a beta-strand in a mutant linked with a heritable cardiomyopathy. J Mol Biol 425: 4034–4046. [DOI] [PubMed] [Google Scholar]
- 18. Tang Y, Tian X, Wang R, Fill M, Chen SR (2012) Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies. Circ Res 110: 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, et al. (2005) Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res 96: e77–82. [DOI] [PubMed] [Google Scholar]
- 20. Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, et al. (2006) Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A 103: 12179–12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goddard CA, Ghais NS, Zhang Y, Williams AJ, Colledge WH, et al. (2008) Physiological consequences of the P2328S mutation in the ryanodine receptor (RyR2) gene in genetically modified murine hearts. Acta Physiol (Oxf) 194: 123–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, et al. (2008) Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118: 2230–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, et al. (2010) Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res 106: 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, et al. (2011) Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med 17: 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, et al. (2012) Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res 111: 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loaiza R, Benkusky NA, Powers PP, Hacker T, Noujaim S, et al. (2013) Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res 112: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, et al. (2002) The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci U S A 99: 9225–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bround MJ, Asghari P, Wambolt RB, Bohunek L, Smits C, et al. (2012) Cardiac ryanodine receptors control heart rate and rhythmicity in adult mice. Cardiovasc Res 96: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bround MJ, Wambolt R, Luciani DS, Kulpa JE, Rodrigues B, et al. (2013) Cardiomyocyte ATP production, metabolic flexibility, and survival require calcium flux through cardiac ryanodine receptors in vivo. J Biol Chem 288: 18975–18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, et al. (2001) Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible cre protein. Circ Res 89: 20–25. [DOI] [PubMed] [Google Scholar]
- 31. Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, et al. (2005) Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res 96: 847–855. [DOI] [PubMed] [Google Scholar]
- 32. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–65. [DOI] [PubMed] [Google Scholar]
- 33. Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci U S A 76: 4350–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, et al. (2007) K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J 404: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bai Y, Jones PP, Guo J, Zhong X, Clark RB, et al. (2013) Phospholamban knockout breaks arrhythmogenic Ca2+ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice. Circ Res 113: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, et al. (1998) Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J 17: 3309–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]