Abstract
Hypoxia is a condition of low pO2, which creates a unique microenvironment affecting cell phenotype and subsequent immune response generation. Little is known about the impact of hypoxia on the phenotypic expression of NK cell, TREM-1, TLR-4 and inflammatory chemokines. In the present study we have determined the frequency of peripheral blood populations of CD16/CD56 (NK Cells) expressing cells, presence of activation marker CD354 (TREM-1), Toll like receptor (CD 284) on the cell surface and chemokines IL-8 and RANTES in the cellular supernatant of normoxia and hypoxia exposed cells by flow cytometry. GRP-78 expression was determined by reverse transcriptase polymerase chain reaction. The blood was collected from healthy individuals and exposed to normoxic and hypoxic (0.5 %) environment for 24 h. The percentage of NK cells (CD 16/56) was marginally up regulated while TLR-4 expression was diminished in hypoxia exposed cells as compare to the normoxic cells. TREM-1 expression was significantly up-regulated (p < 0.05) in hypoxia as compared to the normoxic control. In addition when monocytic cell line THP-1 was exposed to 0.5 % hypoxia for 24 h, TLR4 expression was significantly decreased in hypoxic cells as compared to normoxic cells. Furthermore, GRP-78 mRNA expression was also upregulated by hypoxia or LPS exposure. These events are paralleled by strengthening up-regulation of the chemokines IL-8 and RANTES an otherwise necessary event for the chemotaxis of the neutrophils and macrophages to the inflammatory site. In conclusion, this study provides a novel insight into the mechanism linking low oxygen tension to the regulation of immune and inflammatory responses, leading to new perspectives of the role of hypoxia in programming immune cell functions.
Keywords: Hypoxia, GRP-78, Chemokine, IL-8, RANTES, Inflammation
Introduction
The inflammatory sites have been known for low oxygen tension (Hypoxia), which leads to extensive infiltration of inflammatory leukocytes [1]. As a consequence, immune effector cells in hypoxic sites have an acute need to respond to these demanding conditions to maintain their viability and activity. Human peripheral blood mononuclear cells (PBMCs) contain many antigen presenting cells which are specialized for the activation of resting T cells and the initiation and regulation of many types of immune responses [2–6]. Because of this, we have investigated the functional changes that accompanying the metabolic adaptation of human PBMCs to hypoxia, as these events are likely to affect the development of both inflammatory and immune functions. For instance, stimulation of chemokines such as IL-8 and RANTES in hypoxic conditions by human PBMCs, expression of toll like receptor-4 (TLR-4), triggering receptor expressed on myeloid cells-1 (TREM-1) and GRP-78 in hypoxic cells. TREM-1 is a recently identified immunoglobulin-like cell surface receptor mainly expressed on neutrophils and a subset of CD14 high monocytes [7]. Activation of TREM-1 by the crosslinking antibody leads to the production of multiple pro-inflammatory cytokines and chemokines, and this response can synergize with innate immune stimuli to amplify inflammatory responses [8–11]. Hypoxic stress also triggers unfolded protein response pathway due to accumulation of misfolded proteins in the endoplasmic reticulum (ER). ER stress-associated gene GRP78, a representative ER chaperone is reported to be involved in the development of inflammation [12].
In this study, we evaluated hypoxia induced simultaneous changes in human PBMCs and monocytic cell line THP-1 in the secretion of proinflammatory chemokines IL-8 and RANTES as a representative of inflammation and in the phenotypic expression of TREM-1, TLR-4, CD16/56. Furthermore, Hypoxia induced stress response was measured by GRP-78 mRNA expression.
Methods
Cells
Blood (10 ml) was taken from healthy volunteers in the presence of heparin or 0.2 % EDTA. Peripheral blood mononuclear cells (PBMCs) were separated on lymphoprep (Nycomed, Oslo, Norway) and were washed three times with phosphate-buffered saline (PBS). PBMCs were counted and plated in six well culture plates at a concentration of 1 × 106 cells/ml. The cells were subjected to one of the following conditions: normoxia and hypoxia for 24 h. All experiments were performed with or without lipopolysaccharide (LPS; 0.1 μg/ml, Escherichia coli 055: B5, Sigma Chemical Co., St. Louis, MO). After exposure to hypoxia, cells were harvested for analysis of surface markers, and supernatants were collected for determination of chemokine levels [14].
In addition, the human monocytic cell line THP-1 was cultured in RPMI-1640 with 10 % FBS and antibiotics and was subjected to the same experimental conditions, with or without LPS.
Normoxic and Hypoxic Conditions
For normoxic conditions, cells were incubated in a regular incubator (21 % O2, 5 % CO2, 74 % N2). Hypoxic incubation was performed in an incubator where the hypoxic environment (0.5 % O2, 5 % CO2, 94.5 % N2) is kept constant and so are the temperature (37 °C) and humidity (90 %).
MTT Assay for Cell Viability
Cell viability was assessed by mitochondria dependent reduction of a yellow tetrazolium dye 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to insoluble purple formazan by dehydrogenases [13]. This process requires active mitochondria, and even freshly dead cells do not reduce significant amounts of MTT. Human THP-1 cell line was cultured in 96-well flat-bottom plate and treated with or without LPS (100 ng/ml) and kept in hypoxia or normoxia. Thereafter, 20 μl of MTT dye (1 mg/ml in PBS) was added to the culture medium and further incubated for 4 h at 37 °C. The formazan crystals made due to dye reduction by viable cells. Supernatant was aspirated and cells were dissolved using dimethyl Sulphoxide (DMSO). Index of cell viability was calculated by measuring the optical density of color produced by MTT dye reduction at 570 nm.
IL-8 and RANTES Quantification
IL-8 and RANTES expression levels were quantified by means of a cyto-fluorimetry-based ELISA system (flowcytomix, ebiosciences, USA). In brief, human PBMCs were cultured with or without LPS in normoxia or hypoxia. Supernatant was aspirated from each well and kept at −80 °C until used. Chemokines were estimated according to the manufacturer’s instructions. The samples were acquired in BD FACS-Caliber and analyzed using flowcytomix pro software.
Flow Cytometric Analysis for Cell Surface Markers
Cell surface markers expression level was quantified using flow cytometer. In brief, whole blood mixed with incomplete RPMI in 1:1 ratio in normoxia or hypoxia for 24 h. Whole blood wash-lyse technique was used for staining cell surface antigens. Cells were stained with anti CD 16 FITC/56 PE (NK cell marker), anti CD 284 PE (TLR-4), anti CD253 (TREM-1) antibody (BD Pharmingen, San Jose, CA, USA). Samples were acquired using FACS caliber flow cytometer (Becton Dickinson, CA, USA) and analysed using Cell Quest Pro software [15].
RT PCR for GRP-78 mRNA Expression
Human PBMCs were cultured with or without LPS (0.1 μg/ml) and incubated for 24 h in normoxia or hypoxia. RNA was isolated from the treated cells using an RNeasy RNA isolation kit (Qiagen, USA) according to the manufacturer’s instructions. 100 ng of RNA was reverse transcribed using the one step RT-PCR kit (Qiagen, USA). The forward primer 5′-GTT CTT GCC GTT CAA GGT GG-3′, and the reverse primer 5′-TGG TAC AGT AAC AAC TGC ATG-3′, were used to amplify 181 bp of GRP-78 mRNA. β-Actin was amplified using the following primers: forward, 5′-GAG ACC TTC AAC ACC CCA GCC-3′; and reverse, 5′-GGA TCT TCA TGA GGT AGT CAG-3′ to generate a 207 bp product. The PCR conditions for GRP-78 mRNA were: denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, extension at 72 °C for 1 min for 30 cycles. The PCR conditions for β-actin mRNA were: denaturation at 94 °C for 40 s, annealing at 51.1 °C for 40 s, extension at 72 °C for 1 min for 30 cycles.
Statistical Analysis
Values are represented as mean ± SE. Comparison between results of test and control were performed by using ANOVA followed by post hoc analysis with the Dennett’s test. Entire analysis was conducted using SPSS 15 software. The p value of ≤0.05 was considered significant.
Results
Cell Viability Assay
The viability of human PBMCs was measured by MTT assay. The data suggest that there was no significant cytotoxicity observed by hypoxia and LPS treatment to the human PBMCs (Fig. 1).
Fig. 1.
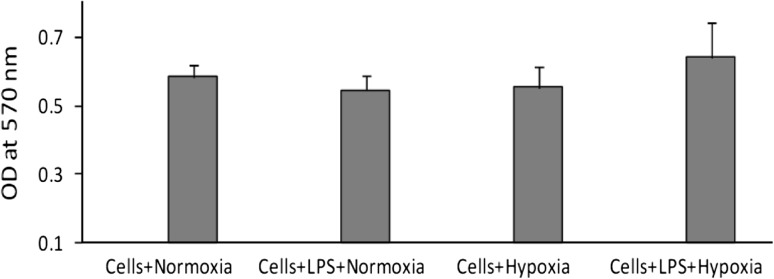
To rule out possible in vitro toxic effects of hypoxia, cells (hPBMCs) with normoxia and LPS-treated or hypoxia and LPS treated were evaluated by MTT assay for cell viability
Evaluation of Cell Surface Markers by Flow Cytometry
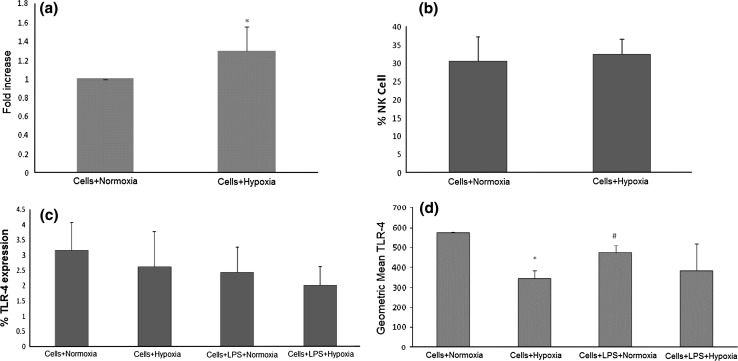
In the present study we have determined the frequency of peripheral blood populations cells expressing CD16/CD56 (NK Cells), CD354 (TREM-1) and TLR-4 (CD 284) by flow cytometry. The blood was collected from healthy individuals and exposed to normoxic and hypoxic environment for 24 h. TREM-1 expression was significantly up-regulated in hypoxia (p < 0.05; Fig. 2a) as compared to the normoxic control while percentage of NK cells (CD 16/56) was marginally up regulated (Fig. 2b). The TLR-4 expression was diminished in hypoxia exposed human PBMCs as compare to the normoxic human PBMCs (Fig. 2c). In addition, when monocytic cell line THP-1 was exposed to hypoxia for 24 h, TLR4 expression was significantly decreased in hypoxic cells as compare to normoxic cells (Fig. 2d).
Fig. 2.
a Expression of TREM-1 in human whole blood (n = 10) under 20 % O2 (Normoxia or control group) or 0.5 % O2 (hypoxia group) for 24 h. The expression significantly increased under hypoxic conditions as compared to the control group p < 0.05. b Expression of CD16/56 in human whole blood (n = 10) under 20 % O2 (normoxia or control group) or 0.5 % O2 (hypoxia group). Significant change was not observed in NK cell percentage by hypoxia exposure. c Expression of TLR-4 in human whole blood (n = 10) under 20 % O2 (normoxia or control group) and LPS-treated or hypoxia (0.5 % O2) and LPS treated were evaluated. Diminished expression of TLR-4 was observed by hypoxia exposure d Expression of TLR-4 in THP-1 cells under 20 % O2 (normoxia or control group) and LPS-treated or hypoxia (0.5 % O2) and LPS treated were evaluated. Significant decrease was observed in TLR4 expression by hypoxia exposure. *p < 0.05 versus cell + normoxia; #p < 0.05 versus cell + normoxia
Increased Production of IL-8 and RANTES Chemokine
Human PBMCs were used for evaluating the effect of hypoxia on the production of IL-8, and RANTES. Increased production of IL-8 and RANTES chemokine was observed in hypoxia and LPS + hypoxia treated cells. Chemokines production was quantified by means of a cytofluorometry-based ELISA system (Table 1).
Table 1.
RANTES and IL-8 label in hypoxia and normoxia exposed cells
| Treatment | IL-8 (pg/ml) Mean ± SE | RANTES (pg/ml) Mean ± SE |
|---|---|---|
| Cells + normoxia | 783 ± 373 | 1,196 ± 437 |
| Cells + hypoxia | 3,049 ± 460* | 1,603 ± 409 |
| Cells + LPS + normoxia | 5,153 ± 425 | 5,153 ± 425 |
| Cells + LPS + hypoxia | 32,697 ± 7,304** | 32,697 ± 7,306# |
Effects of hypoxia on chemokine production by human PBMCS. (A) Human PBMCs were cultured under normoxic or hypoxic conditions in the presence or absence of LPS and analyzed for chemokine production in cell supernatant by flowcytomix. Results are Mean ± SE. * p < 0.005 versus cells + normoxia; ** p < 0.01 versus cells + LPS + normoxia; # p < 0.05 versus cells + LPS + normoxia
Effect of Hypoxia on GRP-78 mRNA Expression
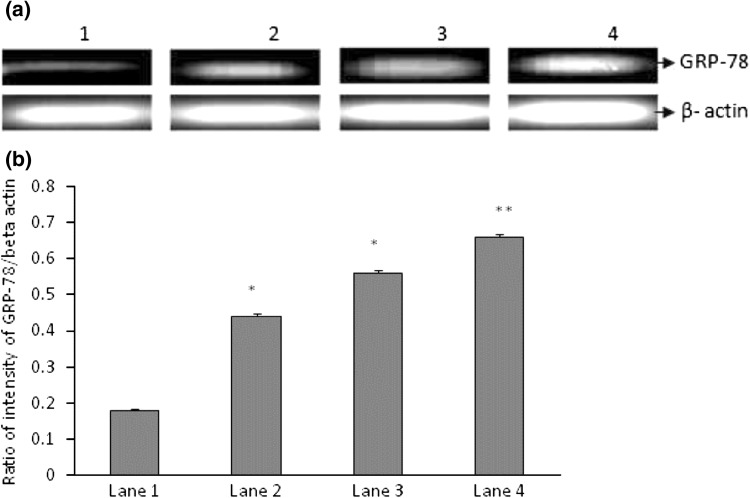
To determine whether hypoxia changed GRP-78 expression, human PBMCs were treated with or without LPS and exposed to normoxia and hypoxia. GRP-78 mRNA expression was measured by semi quantitative RT-PCR. GRP-78 mRNA expression was increased after hypoxia (p < 0.01 vs cells + normoxia) or LPS + hypoxia (p < 0.001 vs cells + hypoxia) treatment for 24 h (Fig. 3a, b).
Fig. 3.
Effects of hypoxia on GRP-78 expression by human PBMCS. a A representative gel of GRP-78 mRNA expression in human PBMCs. Cells were cultured under normoxic or hypoxic conditions with or without LPS and analyzed for GRP-78 mRNA expression by semi quantitative RT-PCR. b Densitogram measured using Image J software for the quantitative expression of GRP-78 (n = 3). Lane 1 cells only, Lane 2 cell + LPS, Lane 3 cells + hypoxia, Lane 4 cells + LPS + hypoxia. *p < 0.01 versus cell + normoxia; **p < 0.001 versus cells + hypoxia
Discussion
Hypoxia activates various transcription factors involved in inflammatory responses including HIF-1 and NF-kB [16]. In the present study, we have evaluated the hypoxic response on human PBMCs and monocytic cell line THP-1 for evaluation of CD16/56 (NK-Cell), CD284 (TLR-4), CD354 (TREM-1), chemokine production (IL-8 and RANTES) and GRP-78 expression. Our findings demonstrated that percentage of NK cells (CD 16/56) was marginally up regulated while TLR-4 expression was diminished in hypoxia exposed hPBMCs as compare to the normoxic hPBMCs while TREM-1 expression was significantly up-regulated in hypoxic hPBMCs (p < 0.05) as compared to the normoxic control. In addition when monocytic cell line THP-1 was exposed to hypoxia, TLR4 expression was significantly decreased in hypoxic cells as compare to normoxic cells. These events are paralleled by strengthening up-regulation of the chemokines IL-8 and RANTES an otherwise necessary event for the chemotaxis of the neutrophils and macrophages to the inflammatory site. Hypoxic stress also up-regulated the GRP-78 expression in human PBMCs as compared to the normoxic control.
TREM-1 is a novel cell surface receptor that has recently been implicated as a key molecule in the development of inflammation. Expression of TREM-1 is detected on a variety of immune cells, such as PMNs, macrophages, and monocytes [17, 18] and strongly elevated after bacterial infection [19]. TREM-1 activation also enhances the production of TNF-α, IL-6, and MIP-2, as well as PMN infiltration [7]. However, nothing is known regarding association of TREM-1 with hypoxia and hypoxia induced inflammation. Studies described herein demonstrated that TREM-1 expression was significantly enhanced in hypoxia induced whole blood versus normoxic control whole blood. It should be noted that these hypoxia exposed samples were obtained from both male and female subjects. Despite the sex variations, our results indicate that the TREM-1 induction level is associated largely with the hypoxia exposure. Furthermore, these events are paralleled by strengthening up-regulation of the chemokines IL-8 and RANTES, an otherwise necessary event for the chemotaxis of the neutrophils and macrophages to the inflammatory site.
Hara et al. [20] also demonstrated that the expression of TLR4 mRNA was decreased in the human corneal epithelial cells (HCECs) of soft contact lens wearers. These findings are in agreement with studies reporting that TLR4 expression was reduced by hypoxia in other type of cells and organs, e.g., cultured pulmonary artery endothelial cells [21]. On the other hand, the mRNA and protein levels of TLR4 were up-regulated by hypoxia in a cultured microglia cell line [22]. In murine bone marrow-derived dendritic cells, hypoxia did not change the TLR4 mRNA expression [23]. These different responses of TLR4 expression under hypoxic conditions may be because of the different hypoxic exposure times in different experiments. Ock et al. [22] reported that the up-regulation of TLR4 expression in microglia was observed after 8 h of hypoxic exposure. On the other hand, Ishida et al. [21] reported that long-term (48–72 h) hypoxic exposure caused a down-regulation in the expression of TLR4 in cultured pulmonary artery endothelial cells. Their results were in good agreement with ours, where HCECs were cultured under 2 % O2 for 48 h prior to exposure to LPS. The differences in the types of cells and organs, and in the culture conditions, may account for the different TLR4 expression. However, the down-regulated expressions of TLR4 in HCECs under hypoxic conditions are consistent with some of the in-vivo and in-vitro findings. We found that the Hypoxia and LPS induced expression of IL-8 and RANTES in human PBMCs. In our culture system, LPS exposure caused a significant increase in the IL-8 and RANTES production under hypoxic conditions. In addition, mRNA expression of GRP-78 showed that the hypoxia and LPS increased GRP-78 activation was also induced due to hypoxia. In conclusion, this study provides novel insights into the mechanisms linking low oxygen tension to the regulation of immune and inflammatory responses, leading to the new perspectives of the role of hypoxia in programming immune cell functions.
Acknowledgments
Authors are thankful to Ms Gazal Singla for technical assistance. Defence Research and Development Organization (DRDO), Government of India, is acknowledged for the financial support.
Conflict of interest
The authors report no declarations of interest.
References
- 1.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/S1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 2.Mishra KP, Ganju L. Influence of high altitude exposure on the immune system: a review. Immunol Invest. 2010;39:219–234. doi: 10.3109/08820131003681144. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. doi: 10.1016/S0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 4.Austyn JM. Dendritic cells. Curr Opin Hematol. 1998;5:3–15. doi: 10.1097/00062752-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/S0092-8674(01)00455-X. [DOI] [PubMed] [Google Scholar]
- 7.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 8.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 9.Radsak MP, Salih HR, Rammensee HG, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol. 2004;172:4956–4963. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 10.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 11.Gibot S, Cravoisy A. Soluble form of the triggering receptor expressed on myeloid cells-1 as a marker of microbial infection. Clin Med Res. 2004;2:181–187. doi: 10.3121/cmr.2.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K, et al. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med. 2012;209:871–886. doi: 10.1084/jem.20111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Mishra KP, Chanda S, Karan D, Ganju L, Sawhney RC. Effect of Seabuckthorn (Hippophae rhamnoides) flavone on immune system: an in vitro approach. Phytother Res. 2008;22:1490–1495. doi: 10.1002/ptr.2518. [DOI] [PubMed] [Google Scholar]
- 15.Mishra KP, Ganju L, Chanda S, Karan D, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome stimulates Toll-like receptor 4, granzyme-B and Th1 cytokines in vitro. Immunobiology. 2009;214:27–31. doi: 10.1016/j.imbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 17.Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173:7131–7134. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- 18.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 19.Ferat-Osorio E, Esquivel-Callejas N, Wong-Baeza I, Aduna-Vicente R, Arriaga-Pizano L, Sánchez-Fernández P, et al. The increased expression of TREM-1 on monocytes is associated with infectious and noninfectious inflammatory processes. J Surg Res. 2008;150:110–117. doi: 10.1016/j.jss.2007.12.805. [DOI] [PubMed] [Google Scholar]
- 20.Hara Y, Shiraishi A, Ohashi Y. Hypoxia-altered signaling pathways of toll-like receptor 4 (TLR4) in human corneal epithelial cells. Mol Vis. 2009;15:2515–2520. [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida I, Kubo H, Suzuki S, Suzuki T, Akashi S, Inoue K, et al. Hypoxia diminishes toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169:2069–2075. doi: 10.4049/jimmunol.169.4.2069. [DOI] [PubMed] [Google Scholar]
- 22.Ock J, Jeong J, Choi WS, Lee WH, Kim SH, Kim IK, et al. Regulation of toll-like receptor 4 expression and its signaling by hypoxia in cultured microglia. J Neurosci Res. 2007;85:1989–1995. doi: 10.1002/jnr.21322. [DOI] [PubMed] [Google Scholar]
- 23.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of toll-like receptors TLR2 and TLR6 during hypoxia. PLoS One. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]