Abstract
Cisplatinum (Cispt) is an anti-cancer drug with a low level of solubility. One of Cispt’s solvents is dimethyl sulfoxide (DMSO) which can be substituted with chlorine of drug as Cispt’s solvent. Applying such a solvent in biological studies is impossible due to intense reduction in activity. On the other hand, it is specified that Cispt’s stability is increased in aqueous media by increasing sodium chloride (NaCl) concentration up to 0.9 %. Consequently, we intended to study the effect of DMSO on cytotoxicity of Cispt in presence of sodium. MTT assay was employed to study cytotoxicity effect of Cispt + NaCl + DMSO and Cispt + DMSO on G-292 cell line. Cytotoxicity in dilutions of 300 and 9 (p < 0.01) of Cispt in Cispt + NaCl + DMSO formulation was equal to 78 and 7 %. These values were estimated 79 and 18 % for Cispt + DMSO formulation and 79 and 24 % for free drug. IC50 values demonstrated reduction of 45 % in cytotoxicity of Cispt in Cispt + DMSO formulation. Studying chemical structure of Cispt and Cispt dissolved in DMSO showed that NaCl cannot inhibit inactivating effect of DMSO on Cispt and effect of this solvent on Cispt is independent from presence of NaCl. Results represented that using NaCl does not result in stability and keeping cytotoxicity properties of Cispt in DMSO. Findings suggest more studies for using DMSO as a solvent of Cispt.
Keywords: Dimethyl sulfoxide, Sodium chloride, Cisplatinum, Cytotoxicity
Introduction
Cisplatinum is a platinum containing drug with a widespread anti-tumor activity [1]. This drug is an alkylation agent, applied against solid tumors of testicular, ovarian, bladder and epithelial malignancies and cancers of the esophagus, lung, and head and neck. Cisplatinum enters cell via diffusion. The chlorine atom substitutes water and subsequently gives it a positive charge. Positively charged complex can react DNA and form cross bridges within (N atoms of adjacent bases) and between the strings. This process results in an inhibition in DNA replication. Furthermore cisplatinum can connect free sulfhydryl group of tubulin in cell environment which leads to relative depolymerization of microtubules [2]. This can change assembly of microtubules by direct change of tubulin and some alterations in cell skeleton pattern of tumor cells [3]. Although this drug is an effective anti-tumor, side effects such as kidney and liver toxicity, neurological toxicity, nausea and vomiting cause constrain in prescription doses [1, 4–6]. The drug has other side effects like low level of solubility and intravenous injection, too [7]. Solubility of this drug in water at 25 °C can only reach 0.253 g/100 g [8]. To achieve high concentrations, appropriate solvents like DMSO can be used. Cisplatinum can connect DMSO rapidly and produce an additional compound. During this process DMSO substitutes chlorine of cisplatinum [9]. However using DMSO in biological studies is rejected due to sever reduction of cisplatinum’s cytotoxicity activity [10]. On the other hand, it is identified that cisplatinum stability in aqueous solutions is enhanced by increasing sodium chloride concentration (up to concentration of 0.9 %). The reverse procedure occurs in basic solutions like sodium bicarbonate and reduces stability [11]. As a result, it is probable that an increasement in sodium chloride concentration lowers negative effect of this solvent on cisplatinum however no study has been focused on it. In this study, MTT assay was employed to investigate cytotoxicity effect of cisplatinum in DMSO in presence and absence of NaCl on G-292 cell line which is reported for the first time. The results illustrated that sodium chloride not only does not decrease adverse effect of DMSO on sodium chloride, but also lead to reducing cytotoxicity effect of cisplatinum.
Materials and Methods
Materials
Cisplatinum, sodium chloride and DMSO were purchased from Merck (Germany), DMEM culture medium from PAA (Austria) and FBS from Gibco (USA). G-292 cells were supplied by Pasteur institute of Iran. All other materials had an analytical grade and the water was used in distillated form.
Methods
Preparing Different Cisplatinum Compounds
Different compounds in DMEM culture medium containing 10 % bovine serum were prepared according to Table 1. For the mixture of Cispt + NaCl + DMSO, DMSO was the last added component in order to guarantee presence of adequate chlorine. For this compound, as well as Cispt + DMSO, incubation lasted 3 h which was a sufficient time for implementing reaction of cisplatinum and DMSO [10]. Concentration of NaCl for all wells (containing this compound) was 100 mOsmol which is  of normal saline concentration and as a result, totally safe in biological environment. Afterwards, cells were treated for MTT assay.
of normal saline concentration and as a result, totally safe in biological environment. Afterwards, cells were treated for MTT assay.
Table 1.
Formulation and initial concentration of compounds used for MTT assay
| Formulation | Formulation components | ||
|---|---|---|---|
| Cisplatinum initial concentration (μmol/l) | Dimethyl sulfoxide initial concentration (V/V) | Sodium chloride concentration | |
| Cisplatinum + sodium chloride + dimethyl sulfoxide | 300 | 1.25 | 100 mOsmol |
| Cisplatinum + dimethyl sulfoxide | 300 | 1.25 | – |
MTT Assay
MTT assay was used to evaluate and compare cytotoxicity effect of addressed formulations. G-292 cells—which are fibroblastic cells of human bone tumor—with dilution rate of 1 × 104 for each well of a 96-well plate, were cultivated in DMEM [12]. Culture medium containing 10 % fetal bovine serum and 1 % penicillin/streptomycin was incubated at 37 °C with 10 % CO2. After cultivating cells for 24 h and because of cell adhesion, the supernatant was removed. Identical concentrations of cells and 0, 9, 18, 37, 75, 150 and 300 μmol/l of cisplatinum in both formulations were treated. After 48 h of incubation and removing supernatant, 100 μl of MTT solution (0.5 mg/ml PBS, pH 7.4) was added to each well. After 3 h incubation in 37 °C MTT was removed and 200 μl isopropanol (100 %) was added to each well to dissolve formazan crystals. Then absorbance was measured in 570 nm by Elisa reader (BioTek Instruments, VT, USA). All the experiments were triplex and repeated for three times. Survival percentage and cytotoxicity effect were determined according to formula 1 and 2 and considering portion of treated cells absorption to control cells absorption [13].
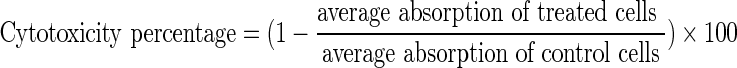 |
1 |
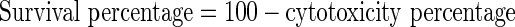 |
2 |
Results
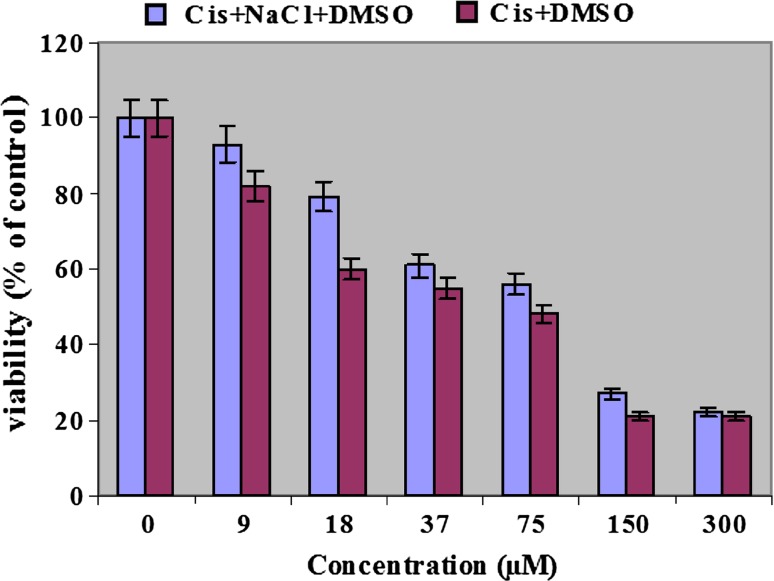
DMSO is an appropriate solvent for cisplatinum. Concentration of 300 μmol/l of cisplatinum in this solvent was prepared easily. It was recognized that cytotoxicity of cisplatinum in both formulations was reduced compared with free drug and NaCl containing drug, represented the highest level of reduction (Fig. 1). NaCl plays a key role in cytotoxicity effect of Cispt + NaCl + DMSO and Cispt + DMSO. In presence of NaCl, cytotoxicity effect of cisplatinum decreases considerably. Such a difference was more evident in lower concentrations of cisplatinum. In concentrations of 9 and 18 μmol/l of cisplatinum in Cispt + NaCl + DMSO formulation, cytotoxicity was estimated 7 and 21 %, respectively while these values identified to be 18 and 40 % for Cispt + DMSO formulation.
Fig. 1.
Cytotoxicity effect of cisplatinum in Cispt + DMSO and Cispt + NaCl + DMSO formulation on G-292 cells survival after 48 h incubation. Results are presented in form of average ±5 % error for at least three independent experiments
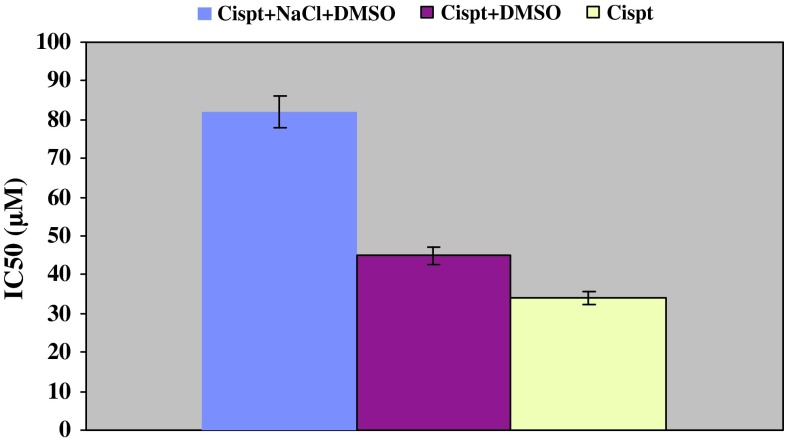
Additionally, IC50 level of both formulations as well as free drug was investigated. Free drug demonstrated the lowest level (34 μM) while NaCl—containing formula demonstrated highest level (82 μM). This value was 45 μM for Cispt + DMSO. It was identified that absence of NaCl results in doubling cytotoxicity effect of cisplatinum (Fig. 2).
Fig. 2.
IC50 level in Cispt + DMSO, Cispt + NaCl + DMSO formulations and free drug. IC50 level for Cispt + DMSO was estimated to be 45 % of the IC50 level of Cispt + NaCl + DMSO which presents negative effect of NaCl on cisplatinum cytotoxicity. Results are presented in form of average ±5 % error for at least three independent experiments
Discussion
Since cisplatinum in not capable to be dissolved in aqueous solvents, it is commonly dissolved in DMSO as a carrier to gain identified concentrations [14, 15]. This standard method is recently developed for studying biological effect of cisplatinum by national institute of cancer [16]. Some studies represent reduction in cytotoxicity effect of the drug on tumor cells in case of combination with DMSO [10]. In this reaction, DMSO substitutes one of the cisplatinum chlorines due to desirability of reaction between platinum of cisplatinum and sulfur of DMSO. This reaction results in formation of DMSO−. This compound readily passes through cell membranes and accumulates in the cell. Formation of this compound inhibits activity of cisplatinum against DNA due to interdiction by DMSO. Findings illustrate that due to the rapid formation of cisplatinum-DMSO, this solvent may not be used for therapeutic applications [10, 17, 18]. Other studies indicate instability of cisplatinum in aqueous media. First form of decomposition for cisplatinum can be related to chloride replacement. Increasing chloride in environment can cause enhancement of cisplatinum stability in aqueous media. In other words, cisplatinum has a high level of stability in 0.9 % sodium chloride solution [11]. Also studies in rodents have shown that using cisplatinum in NaCl solution reduces the main side effect of cisplatinum, kidney damage [19] however in vitro study of cisplatinum in DMSO in absence and presence of NaCl looks essential. According to mentioned points, we tried to increase concentration of chloride using NaCl in order to improve stability of cisplatinum as well as decreasing adverse effects of DMSO on cytotoxicity of cisplatinum. Additionally it is necessary for this formula to be able to avoid kidney damage as an important side effect of using cisplatinum. However investigating cytotoxicity effect of cisplatinum and cisplatinum dissolved in DMSO in the presence of NaCl, represented different results. Cytotoxicity in presence of NaCl not only did not increase, but also decreases significantly. In this study, cytotoxicity of free cisplatinum after 48 h of incubation was estimated which was considerably higher in comparison with both prepared formulas. On the other hand, it was determined that NaCl is safe for cells in concentrations of less than 100 mOsmol which probably comes from a difference in in vitro and in vivo responses. In in vivo environment concentrations of less than 300 mOsmol are safe for cell [20]. Based on results, sodium chloride is not able to inhibit inactivating effect of DMSO on cisplatinum. Conversely, in the presence of this compound cisplatinum showed lower cytotoxicity. It can also be concluded that effect of solvent on cisplatinum is independent from presence of NaCl. These observations suggest further studies for finding improved approaches to use DMSO as a solvent for cisplatinum.
Acknowledgments
This work was carried out as a part of Master’s thesis in nanobiotechnology pilot unit of Pasteur Institute of Iran and it would be necessary to thank all colleagues.
References
- 1.Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mol Biol. 2006;39:656–661. doi: 10.5483/BMBRep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Basu A, Krishnamurthy S. Cellular responses to cisplatin-induced DNA damage. J Nucleic Acids. 2010 doi: 10.4061/2010/201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker AR, Petluru PN, Wu M, Zhao M, Kochat H, Hausheer FH. BNP7787-mediated modulation of paclitaxel- and cisplatin-induced aberrant microtubule protein polymerization in vitro. Mol Cancer Ther. 2010;9:2558–2567. doi: 10.1158/1535-7163.MCT-10-0300. [DOI] [PubMed] [Google Scholar]
- 4.Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. doi: 10.1155/2011/843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal N, Sharma SK. Effects of Pluchea lanceolata root extract on cisplatin induced nausea and vomiting in rat pica model. Iran J Pharmacol Ther. 2013;12:19–23. [Google Scholar]
- 6.Astolfi L, Ghiselli S, Guaran V, Chicca M, Simoni E, Olivetto E, et al. Correlation of adverse effects of cisplatin administration in patients affected by solid tumors: a retrospective evaluation. Oncol Rep. 2013;29:1285–1292. doi: 10.3892/or.2013.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 8.Maynard RL. The Merck index. 12. Bethesda: PubMed Central; 1996. p. 2378. [Google Scholar]
- 9.Uribe PM, Mueller MA, Gleichman JS, Kramer MD, Wang Q, Sibrian-Vazquez M, Strongin RM, Steyger PS, Cotanche DA, Matsui JI. Dimethyl sulfoxide (DMSO) exacerbates cisplatin-induced sensory hair cell death in zebrafish (Danio rerio) PLoS ONE. 2013;8:e55359. doi: 10.1371/journal.pone.0055359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer SJ, Benson LM, Fauq A, Naylor S, Windebank AJ. Cisplatin and dimethyl sulfoxide react to form an adducted compound with reduced cytotoxicity and neurotoxicity. Neurotoxicology. 2008;29:444–452. doi: 10.1016/j.neuro.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Pujol Cubells M, Prat Aixela J, Girona Brumos V, Duran Pou S, Villaronga Flaque M. Stability of cisplatin in sodium chloride 0.9 % intravenous solution related to the container’s material. Pharm World Sci. 1993;15:34–36. doi: 10.1007/BF02116167. [DOI] [PubMed] [Google Scholar]
- 12.Tan ML, Choong PF, Dass CR. Direct anti-metastatic efficacy by the DNA enzyme Dz13 and downregulated MMP-2, MMP-9 and MT1-MMP in tumours. Cancer Cell Int. 2010;10:9. doi: 10.1186/1475-2867-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarei M, Norouzian D, Chiani M, Ebrahimi H, Mohammadi M, Akbarzadeh A. Advantages of paclitaxel-loaded nanoniosomes to nanoliposomal formulation: an in vitro study. Int J Life Sci Biotechnol Pharma Res. 2013;2:335–342. [Google Scholar]
- 14.Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23:6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- 15.Axanova L, Morré DJ, Morré DM. Growth of LNCaP cells in monoculture and coculture with osteoblasts and response to tNOX inhibitors. Cancer Lett. 2005;225:35–40. doi: 10.1016/j.canlet.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Stern ST, Potter TM. LLC-PK1 kidney apoptosis assay. Nanotechnol Charact Lab. 2005;1:1–8. [Google Scholar]
- 17.Feng L, De Dille A, Jameson VJ, Smith L, Dernell WS, Manning MC. Improved potency of cisplatin by hydrophobic ion pairing. Cancer Chemother Pharmacol. 2004;54:441–448. doi: 10.1007/s00280-004-0840-z. [DOI] [PubMed] [Google Scholar]
- 18.Fischer SJ, Benson LM, Fauq A, Naylor S, Windebank AJ. Cisplatin and dimethyl sulfoxide react to form an adducted compound with reduced cytotoxicity and neurotoxicity. Neurotoxicology. 2008;29:444–452. doi: 10.1016/j.neuro.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Hanigan MH, Deng M, Zhang L, Taylor PT, Jr, Lapus MG. Stress response inhibits the nephrotoxicity of cisplatin. Am J Physiol Renal Physiol. 2005;288:F125–F132. doi: 10.1152/ajprenal.00041.2003. [DOI] [PubMed] [Google Scholar]
- 20.Prough DS, Bidani A. Hyperchloremic metabolic acidosis is a predictable consequence of intraoperative infusion of 0.9 % saline. Anesthesiology. 1999;90:1247–1249. doi: 10.1097/00000542-199905000-00003. [DOI] [PubMed] [Google Scholar]