Abstract
Empty sella syndrome is a damaged pituitary gland. Either the gland has shrunk or has been crushed and flattened making it look like an empty sella on MRI scan. The reported prevalence of primary empty sella in general population is 8–35 %. The incidence is more in females, the ratio being 5:1. It is generally found in middle aged women who are obese and hypertensive.
Introduction
Empty sella syndrome is a condition in which the pituitary gland shrinks or gets flattened. The pituitary gland sits in sella turcica, which is a saddle like compartment present at the base of the skull. When the pituitary gland shrinks or becomes flattened it cannot be seen on the MRI scan making it look like an empty sella. This is called as empty sella syndrome. Partial empty sella is suggestive that some of the pituitary gland is visible on the MRI scan.
It is of two types, primary and secondary. Primary empty sella occurs when a hole in diaphragmatic sella covering the pituitary allows fluid in, which presses on the pituitary. Secondary empty sella syndrome occurs when the pituitary gland is damaged by a tumor, surgery or radiation therapy [1].
Case History
On our routine delta checking of laboratory investigations, we found abnormal levels of serum prolactin (49.88 ng/ml), TSH (0.32 mIU/l), urine osmolality (87 mOsm/kg) for a 40-year-old female. The case history is as follows. A 40-year-old female patient presented with a history of amenorrhoea of 2 years duration, increased urine output, frequency of micturition and polydipsia of 1 year duration. She was on tablet l-thyroxine since 2 years. There was no history of visual abnormalities or post partum hemorrhage in the past. She had regular menses in the past followed by oligomenorrhea and then amenorrhoea. On examination there was pallor, mild thyromegaly, axillary and pubic hair appeared normal. Her urine osmolality was less than 300 mOsm/kg. Water deprivation test was done and urine osmolality increased to 670 mOsm/kg after the administration of 1 μg of desmopressin.
Laboratory Investigations
| Analyte | Measured value |
|---|---|
| Serum Sodium | 142 meq/l |
| S. potassium | 3.8 meq/l |
| S. chloride | 100 meq/l |
| S. albumin | 4.4 g/dl |
| S. calcium | 9.7 mg/dl |
| S. bicarbonate | 24 mmol/l |
| FT4a | 0.52 ng/dl (normal: 0.8–2.7 ng/dl) |
| TSHa | 0.32 uU/l (0.4–4.2 uU/l) |
| Cortisola | 255.9 nmol/l (138–635 nmol/L) |
| FSHa | 5.38 mIU/ml (2–15 mIU/ml) |
| S.prolactina | 49.88 ng/ml (3.8–23.2 ng/ml) |
aHormone analysis was done by chemiluminescence method
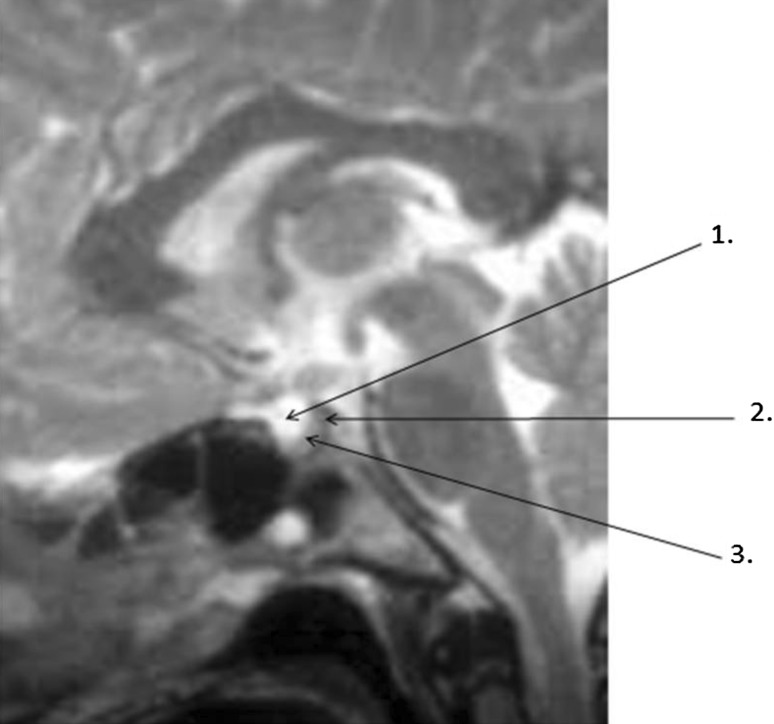
MRI report: MRI brain and sella (plain) (Fig. 1) revealed sella with normal dimensions. Most of the sella was filled with CSF. Pituitary gland appears to be thinned out with concave upper borders, thus confirmed as partial empty sella.
Fig. 1.
MRI brain and sella (plain). 1 Suprasellar cistern filled with CSF. 2 Dorsum sellae. 3 Pituitary gland reduced in size and pushed to one side
Embryology
The pituitary gland is entirely ectodermal in origin and is composed of adenohypophysis and neurohypophysis.
The Rathke’s pouch, from oral ectoderm develops into adenohypophysis. The infundibulum from neural ectoderm develops into neurohypophysis. The oral ectoderm and the neural ectoderm that form the pituitary anlagen are in close contact during early embryogenesis, and this condition is critical for pituitary development [2]. Over several weeks, Rathke’s pouch undergoes constriction at its base until it completely separates from the oral epithelium and near its final position as the adenohypophysis.
Gross Anatomy
The fully developed pituitary gland is peasized and weighs approximately 0.5 g. The gland is enveloped by dura and sits within the sella turcica of sphenoid bone. The superior aspect of the pituitary is covered by the diaphragma sellae, a fold of duramater that separates the CSF filled sub arachnoid space from the pituitary. The infundibulum pierces the diaphragm of sellae to connect the pituitary to the hypothalamus.
The adenohypophysis manufactures peptide hormones. Axons project from neuronal cell bodies in the supraoptic and paraventricular nuclei produce hormones of posterior pituitary. By axonal transport these hormones are released into the systemic circulation [3].
Pathogenesis
An essential prerequisite for the development of the empty sella is an incomplete sellar diaphragm [4]. Other factors are causing increased pressure in the suprasellar subarachnoid space or by reduction in the size of the pituitary gland. Total absence of diaphragma sella has been reported to occur in 20.5 % of normal subjects [5]. PES has also been reported in association with several endocrine autoimmune diseases, and obesity.
Morbid obesity may induce hypercapnia which causes chronic CSF pressure elevation. In subjects with hypoplastic diaphragma sellae, raised CSF pressure leads to the intrasellar herniation of the suprasellar subarachnoid space [6, 7]. Risk factors such as type 2 diabetes, hypertension [8], use of certain drugs [9] and history of pseudotumor cerebri have been proposed in patients with PES. Pregnancy, post partum pituitary necrosis (Sheehan’s syndrome) could promote the onset of PES [10].
In cases of primary end-organ failure (thyroid, adrenal, gonad) pituitary hyperplasia occurs due to loss of feedback control. Replacement of the deficient hormone results in feedback suppression of the pituitary tropic hormone secretion and involution of the hyperplastic pituitary gland resulting in an ‘empty sella’ [11].
Discussion
A high incidence of pituitary dysfunction was documented in patients with the primary empty sella syndrome. These consisted of panhypopituitarism, secondary hypogonadism, hyperprolactinemia, isolated ACTH deficiency and diabetes insipidus (DI) [12].
The present patient had classical presentation of hypothyroidism, was on l-thyroxine; and amenorrhoea of 2 years duration. Hormonal analysis revealed normal cortisol, FSH, LH, and hyperprolactinemia. She had a history of polyuria and polydipsia of 1 year duration. The serum osmolality documented diabetes insipidus and response to AVP confirmed central DI. She has presented with hypothyroidism and hyperprolactinemia indicating that anterior pituitary is involved.
Prolactin (PRL) is unique among the pituitary hormones in that the predominant central control mechanism is inhibitory, reflecting dopamine-mediated suppression of PRL release. This regulatory pathway accounts for the spontaneous PRL hypersecretion that occurs with pituitary stalk section, often a consequence of compressive mass lesions at the skull base [13]. Normally in primary empty sella, the pituitary stalk is compressed, thereby dopamine does not reach the pituitary gland. Hence prolactin levels are increased.
Hyperprolactinemia and intermittent increases in PRL levels have both been associated with the primary empty sella, and as many as 25 % of women with an empty sella have elevated prolactin levels. The degree of hyperprolactinemia found in empty sella syndrome is moderate (usually less than 100 ng/ml) compared to prolactinomas with levels greater than 200 ng/ml) [14].
Ghatnatti et al. [15] noted endocrine dysfunction in 50 % of PES patients and hyperprolactinemia was the most common endocrine abnormality observed in their study. The patient presented with remarkable polyuria and polydipsia, central diabetes insipidus was diagnosed with a water deprivation test, suggesting either pituitary stalk (or) posterior pituitary compression.
In one of the recently published series, around 29 % of patients had partial central diabetes insipidus and the threshold for thirst was increased in all of them [16]. The fact that involvement of the posterior pituitary is less common than that of the anterior pituitary is partly explained by the vascular supply in the two regions. The inferior hypophysial arteries arising from the cavernous portion of the internal carotid artery divide into medial and lateral arteries. These arteries join with those from the opposite side forming an anastomotic ring around the infundibular process of the neurohypophysis and protect it from excessive damage [17].
Kumar et al. [18] reported a multigravida woman, who developed severe postpartum hemorrhage, disseminated intravascular coagulation followed by Sheehan’s syndrome, postoperatively, she developed polyuria, laboratory evidence of diabetes insipidus and her clinical status improved significantly with intranasal desmopressin supplementation.
Tulandi et al. [19] described the case of a 31-year-old woman who had developed severe bleeding and hypotension after caesarean section. She developed polyuria 7 months later and was diagnosed to have diabetes insipidus, imaging of the brain revealed empty sella.
Weston et al. [20] reported a 35-year-old woman with mild preeclampsia and insulin requiring gestational diabetes who presented with postpartum hemorrhage and hypotension requiring subtotal hysterectomy. Postoperatively, she developed excessive thirst, polyuria, severe headache, and blurred vision. Pituitary function tests revealed central hypothyroidism, hyperprolactinemia, and secondary adrenal failure. Diabetes insipidus was confirmed with electrolyte testing before and after a 10-hour water deprivation test. Imaging showed evidence of ischemic infarction of the pituitary gland.
Dutta et al. [21] reported a 27 year old man who had symptoms suggestive of hypothyroidism, was diagnosed as a case of empty sella on MRI and had resolution of all symptoms with levothyroxine therapy.
In our case study, MRI–brain and sella plain revealed most of the sella filled with CSF. Pituitary gland appeared to be thinned out with concave upper borders. Whether the enlarged sella turcica is due to intrasellar herniation of the suprasellar subarachnoid space with compression of the pituitary gland or whether there was previous enlargement of pituitary gland due to primary hypothyroidism, with subsequent atrophy due to treatment with l-thyroxine, followed by extension of subarachnoid space into the sella turcica is a matter of speculation.
Diagnosis It is based the clinical presentation and radiological evidence
Treatment Based on the type of empty sella. There is no specific treatment if pituitary is normal. If Prolactin levels are high interfering with function of ovaries or testes, medications that lower prolactin levels may be suggested. For secondary empty sella syndrome treatment involves replacing the hormones that are lacking.
Conclusion
This is a rare presentation of empty sella syndrome with both anterior and posterior pituitary involvement. Whether the case is primary/secondary cannot be confirmed as there was no previous MRI done for the patient. As per the clinical presentation and MRI, it could be considered as partial empty sella.
References
- 1.Melmed S, Kleinberg D, Ho Ken . Pitutary physiology and diagnostic evaluation. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams textbook of endocrinology, Chap 8. 12. Philadelphia: Elsevier; 2011. [Google Scholar]
- 2.Sheng HZ, Weatphal H. Early steps in pituitary organogenesis. Trends Genet. 1999;15(6):236–240. doi: 10.1016/S0168-9525(99)01742-4. [DOI] [PubMed] [Google Scholar]
- 3.Solov’ve GS, Bogdanov AV, Panteleev SM, Yanin VL. Embryonic morphogenesis of the human pituitary. Neurosci Behav Physiol. 2008;38(8):829–833. doi: 10.1007/s11055-008-9055-9. [DOI] [PubMed] [Google Scholar]
- 4.Vasudevan DM, SreeKumari S, Vaidyanathan K. Text book of biochemistry for medical students. 6th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2011. p. 528–29.
- 5.Kaufman B. The empty sella tursica, a manifestation of the intrasellar subarachnoid space. Radiology. 1968;90:931–946. doi: 10.1148/90.5.931. [DOI] [PubMed] [Google Scholar]
- 6.Bonnevillie JF, Dietmann JL. Intrasellar pathology. In: Radiology of the Sella turcica. New York: Springer Verlag; 1981. p. 89–126.
- 7.Jordan RM, Kendall JW, Kerber CW. The primary empty sella syndrome : analysis of the clinical characteristics, radiographic features, pituitary function and cerebralfluid adeno-hypophysial concentrations. Am J Med. 1977;62:569–580. doi: 10.1016/0002-9343(77)90420-X. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson SF, Rndall RV, Holman CB, MacCarty CS. Empty sella syndrome: report of 10 cases. Med Clin North Am. 1972;56(4):897–907. doi: 10.1016/s0025-7125(16)32355-0. [DOI] [PubMed] [Google Scholar]
- 9.Brisman R, Huges JEO, Mount LA. Endocrine function in nineteen patients with empty sella syndrome. J Clin Endocrinol Metab. 1969;34:570–573. doi: 10.1210/jcem-34-3-570. [DOI] [PubMed] [Google Scholar]
- 10.Degli Uberti EC, Teodori V, Trasforini G, Tomarozzi R, Margotti A, Bianconi M, et al. The empty sella syndrome. clinical, radiological and endocrinologic analysis in 20 cases. Minerva Endocrinol. 1989;14:1–18. [PubMed] [Google Scholar]
- 11.Neelon FA, Goree JA, Lebovitz HE. The primary empty sella: clinical and radiographic characterization and endocrine function. Baltimore: Medicine. 1973;52:73–92. [PubMed] [Google Scholar]
- 12.Brismar K, Efendic S. Pituitary function in the empty sella syndrome. Neuroendocrinology. 1981;32:7. doi: 10.1159/000123133. [DOI] [PubMed] [Google Scholar]
- 13.Disorders of the anterior pituitary and hypothalamus. In: Kasper DL, Braunwald E, Fauci AS, editors. Text book of Harrison’s principles of internal medicine, vol. 2. 16th ed. New York: McGraw-Hill, Medical Publishing Division; 2005. p. 2076–96.
- 14.Gharib Hossein, Frey Harald M, Laws Edward R, Randall Raymond V, Scheithauer Bernd W. Coexistent Primary Empty Sella Syndrome and Hyperprolactinemia. Report of 11 cases. Arch Intern Med. 1983;143(7):1383–1386. doi: 10.1001/archinte.1983.00350070103017. [DOI] [PubMed] [Google Scholar]
- 15.Ghantnatti V, Sarma D, Saikia U. Empty sella syndrome- beyond being an incidental finding. Indian J Endocr Metab. 2012;16(suppl2):321–323. doi: 10.4103/2230-8210.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atmaca H, Tanriverdi F, Gokce C, Unluhizarci K, Kelestimur F. Posterior pituitary function in sheehan’s syndrome. Eur J Endocrinol. 2007;156(5):563–567. doi: 10.1530/EJE-06-0727. [DOI] [PubMed] [Google Scholar]
- 17.Jakubowski J. Blood supply, blood flow and auto regulation in the adenohypophysis and altered pattern in estrogen induced adenomatous hyperplasia. Br J Neurosurg. 1995;9(3):331–346. doi: 10.1080/02688699550041340. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Burrows D, Dang S, Simmons D. Sheehan’s syndrome presenting as central diabetes insipidus: a rare presentation of an uncommon disorder. Endocrine Practice. 2010;1:1–23. doi: 10.4158/EP11385.VVR. [DOI] [PubMed] [Google Scholar]
- 19.Tulandi T, Yusuf N, Posner BI. Diabetes insipidus: a postpartum complication. Obstet Gynecol. 1987;70(3):492–495. [PubMed] [Google Scholar]
- 20.Weston G, Chaves N, Bowditch J. Sheehan’s syndrome presenting post- partum with diabetes insipidus. Aust NZ Obstet Gynecol. 2005;45(3):249–250. doi: 10.1111/j.1479-828X.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 21.Dutta D, Maisnam I, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, Chowdhury S. Panhypopituitarism with empty sella a sequel of pituitary hyperplasia due to chronic primary hypothyroidism. Indian J Endocr Metab. 2012;16(Suppl2):282–284. doi: 10.4103/2230-8210.104060. [DOI] [PMC free article] [PubMed] [Google Scholar]