Abstract
In the present study, paclitaxel was archaeosomed to reduce side effects and improve its therapeutic index. Carriers have made a big evolution in treatment of many diseases in recent years. Lipid carriers are of special importance among carriers. Archaeosome is one of the lipid carriers. Paclitaxel is one of the drugs used to treat breast cancer which has some unwanted side effects despite its therapeutic effects. Archaeosomes were extracted from methanogenic archi bacteria and synthesized with a certain ratio of paclitaxel in PBS. The mean diameter of archaeosomal paclitaxel was measured by Zeta sizer instrument, Drug releasing of archaeosomal paclitaxel was examined within 26 h which results showed that the most drug releasing occurs during first 3 h. The cytotoxicity effect of archaeosomal paclitaxel on breast cancer’s cell line was evaluated by MTT assay which results showed that the cytotoxicity effect of archaeosomal paclitaxel on breast cancer’s cell line is more than that of the standard paclitaxel formulation. The results indicated that new drug delivery of paclitaxel using archaeosome, increases the therapeutic index of the drug.
Keywords: Paclitaxel, Archaeosome, Drug delivery, Breast cancer
Introduction
The issue of controlled drug delivery to body is one of the key issues in drug delivery industry. Hence, carriers have been studied as anti-cancer drug delivery agents. In addition to improve the treatment of prevalent diseases, they extend the economic life of drugs [1]. Carriers enhance drug solubility, control drug release, reduce side effects and improve drug biodistribution [2]. Over the last decades, drug delivery based on lipids has attracted more attentions among these carriers [3]. Archaeosomes are lipid carriers whose structure is made of archaeo bacterial membrane lipids including di-ether or tetra-ether [4]. They are biocompatible and biodegradable and non-toxic in in vivo conditions [5].
Paclitaxel is one of the drugs used to treat breast cancer. This anti-cancer agent is used to treat breast, ovarian, head and neck, and non-small cell lung cancers [6].
Present work intends to study archaeosome paclitaxel in order to improve its therapeutic index and reduce side effects.
Materials and Methods
Materials
Paclitaxel and MTT solution (0.5 mg/ml) were purchased from Sigma Chemical Co., USA. Ethanol, isopropanol, DMSO, Pepton, Yeast extract, HCl, KCl, Mg2Cl2·5H2O, MgSO4·7H2O and CaCl2·2H2O as well as RPMI 1640 medium were purchased from Merch Co. and Invitrogen Co., respectively. Also, MCF-7 cell was obtained from Pasteur Institute, Iran.
Archaeal Strains and Growth
In order to prepare, growth media, certain ratios of Pepton, Yeast extract, HCl, KCl, Mg2Cl2·5H2O, MgSO4·7H2O, CaCl2·2H2O were dissolved in 8 ml of PBS pH 7.4. After autoclaving at 121 °C for 15 Archi bacterium was added to medium and placed in shaker incubator for 48 h at 30 °C and 150 rpm. Proper growth of bacteria in culture medium after 48 h was ensured by counting. Afterward, culture medium containing bacteria was centrifuged (4,000 rpm, 15 min). The clear supernatant was removed and precipitated phase containing cells was dissolved in 20 ml of PBS. Archi bacteria morphology was determined using an optical microscope (Fig. 1).
Fig. 1.
Archi bacteria morphology was determined using an optical microscope
Extraction of Archaeosome from Cells and Preparation of Archaeosomal Paclitaxel
Archaeosomes were extracted according to Bligh and Dyer method. In this method after washing cells with cold PBS the solution was homogenized with a mixture of chloroform and methanol in such proportions that a miscible system was formed with the water in the cells. Diluting system with water and chloroform resulted in phase partitioning and formation of two separate layers. In this case, chloroform layer contains lipids [7]. Total polar lipids (TPL) were precipitated using cold acetone and resuspended and stored in chloroform/methanol [8].
Archaeosomes were prepared by hydrating extracted TPL in PBS containing 10 mg of paclitaxel dissolved in 100 μl of DMSO. In order to uniform the size of archaeosomes and increase drug loading efficiency, the solution was sonicated (Bandelin Sonorex Digitec, 60 Hz) for 10 min.
Size Measurement of Particles
The mean diameter of particles was measured by Zeta sizer device (Malvern Instruments Ltd.).
Loading Efficiency
1 ml (2.5 mg) of prepared formulation was centrifuged (13,000 rpm, 30 min, 4 °C) for studying the amount of loaded paclitaxel. Afterward, the light absorbance of supernatant of archaeosomal paclitaxel formulation was measured at 227 nm by spectrophotometer (UV-1601 PC, Shimadzu Co.) and loading efficiency was calculated using the below formula [9]:
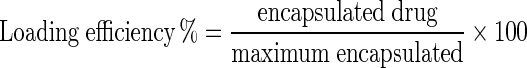 |
1 |
Different concentrations of paclitaxel were prepared as series and absorbance was measured at 227 nm to plot the standard curve.
In Vitro Release Study
Studying the pattern of drug release from archaeosomes was carried out by pouring 1 ml (2.5 mg) of archaeosomal paclitaxel in a dialysis bag in 100 ml of phosphate buffered saline PBS pH 7.4 placed on a magnetic stirrer (26 h, 37 °C). Then, the drug released in PBS was measured at 227 nm in different time intervals within 26 h and the percentage of released paclitaxel was obtained using drug standard curve.
Cytotoxicity Assay
100 μl of culture medium containing 10000 MCF7 cells was decanted in 96-well plates and incubated (5 % CO2 and 37 °C). The supernatant of cells was removed after 24 h and different concentrations of archaeosomal paclitaxel and its control was poured on cells and then incubated for 24 h. Afterward, the supernatant was removed and 100 μl of MTT solution (0.5 mg/ml) was added. 3 hours after incubation, purple color in live cells (due to the formation of formazane) was dissolved in 100 μl of isopropanol and absorbance was measured at 570 nm by the Power Eave XS spectrophotometer. Thereupon IC50 was calculated using Pharm program.
Statistical Analysis
The results are expressed as mean ± standard deviation (SD, n = 3). The data were statistically analyzed by one-way analysis of variance using IBM Statistics SPSS software version 19, and significant difference was set at p < 0.05.
Results
Size Measurement of Particles
The mean diameter of archaeosomes in archaeosomal formulation was 521.4 nm.
Loading Efficiency
Loading efficiency of drug was calculated with according to standard curve of paclitaxel. Considering the formula of loading efficiency (Formula 1) the amount of non-loaded drug was obtained that the percentage of loaded drug was calculated 99.1 ± 3.7 % within 26 h by subtracting it from the initial amount of drug.
In Vitro Release Study
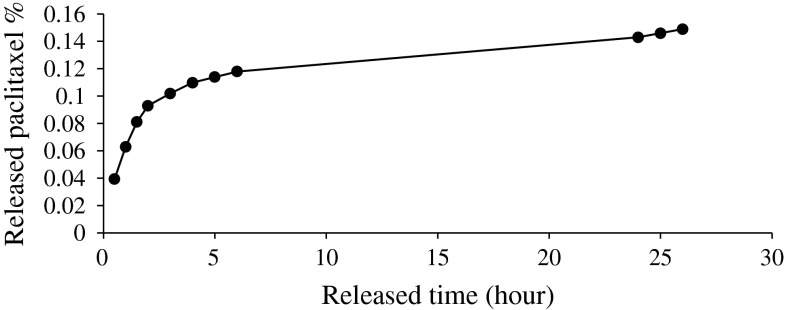
Using the standard curve of paclitaxel, the amount of released paclitaxel of archaeosomal paclitaxel was obtained during 30, 60 and 90 min, 2, 3, 4, 5, 6, 24, 25 and 26 h time intervals. The percentage of released paclitaxel was 0.149 % after 26 h. The releasing pattern of archaeosomal paclitaxel is shown in Fig. 2.
Fig. 2.
The releasing pattern of archaeosomal paclitaxel formulation within 26 h
Cytotoxicity Assay
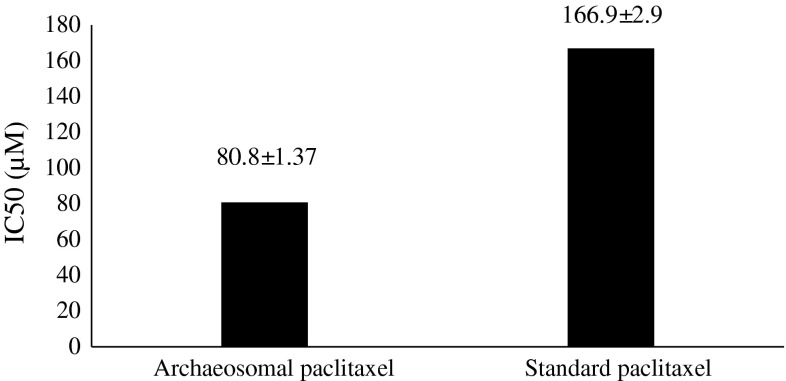
Drug toxicity of archaeosomal paclitaxel was investigated in different concentrations based on MTT assay and results are illustrated in Fig. 3.
Fig. 3.
IC50 (μM) for archaeosomal paclitaxel formulation and the standard paclitaxel
Discussion
Targeted drug delivery is a novel procedure for treatment of various diseases. It has been proved that carriers are capable to deliver drug to targeted cells [1]. By the same token, archaeosomes are a kind of lipid carriers.
Presently, using ordinary liposomes is accompanied with a the major challenge of instability [10, 11]. Archi bacterial lipids have a unique structure in comparison with other phospholipids. These lipids are made of isoprenoid chains attached to glycerol [12, 13]. This structure creates distinctive features such as stability against oxidation, pH increment and dealing with bile salts and lipases [8].
This paper perused the effect of the archaeosomal paclitaxel formulation on breast cancer’s cell line.
Results of the particles’ mean diameter measurement of the archaeosomal paclitaxel formulation, confirmed particles’ size in nano dimensions [14]. In addition, considering the amount of loaded drug illustrated a considerable amount of loaded paclitaxel. The drug releasing pattern in different time intervals was studied by dialysis method. This study represented that drug release is relatively slow and using archaeosomes as drug delivery agents has a significant role in decelerating the rate of drug release, as expected.
As can be seen in Fig. 3, releasing paclitaxel includes fast and slow phases. Furthermore, the amount of drug release is more during first 3 h.
Considering above points, using archaeosomal nanocarriers for Paclitaxel results in a considerable reduction in amount of required drug due to targeted delivery and slow rate of release. Slow rate of release arises from the stability of this carrier due to their physiochemical properties which is also reported by other researchers. Li et al. [15] examined safety potentials of archaeosomes fabricated of PLEE (Polar lipid fraction E) as oral vaccine delivery instruments which results indicated that archaeosomes had remarkable stability in simulated gastrointestinal environments and increasing the lifetime of antigens labeled by fluorescent in digestive system after oral prescription.
Li et al. [15] investigated archaeosomes’ potential as a carrier for oral drug delivery of the peptide drugs (insulin) on diabetic rats [15]. The results of Krishnan et al. [16] research showed that archaeosome has not any toxicity on rats, even in long-term. These reports are in accordance with our records. Gonzalez et al. [17] reported same results for ARC. The cytotoxicity effect of the archaeosomal paclitaxel formulation probed by MTT assay and as a result the archaeosomal formulation without any drug indicated a negligible cytotoxicity effect on MCF-7. Figure 3 indicates that the cytotoxicity effect of the archaeosomal paclitaxel formulation is more than standard paclitaxel. It may come from the fact that the formulation containing archaeosome represents more stability to different conditions (pH, temperature, lipases, etc.) and because of lipid coverage its drug release is less than standard drug.
It can be concluded that archaesome is a convenient lipid carrier for Paclitaxel according to in vitro experiments reported in this paper. Future researches can be focused on modification of archaeosomal carriers to develop their characteristics followed by in vivo studies.
Contributor Information
Azim Akbarzadeh, Phone: +98-21-6696-88-56, FAX: +98-21-6646-51-32, Email: azimakbarzadeh1326@gmail.com.
Mohsen Chiani, Phone: +98-21-6696-88-56, FAX: +98-21-6646-51-32, Email: Chiani110@yahoo.com.
References
- 1.Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today. 2012;17:367–378. doi: 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Dass CR, Choong PF. Carrier-mediated delivery of peptidic drugs for cancer therapy. Peptides. 2006;27:3020–3028. doi: 10.1016/j.peptides.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Woodley JF. Liposomes for oral administration of drugs. Crit Rev Ther Drug Carrier Syst. 1985;2:1–18. [PubMed] [Google Scholar]
- 4.Patel GB, Agnew BJ, Deschatelets L, Perry Fleming L, Dennis Sprott G. In vitro assessment of archaeosome stability for developing oral delivery systems. Int J Pharm. 2000;194:39–49. doi: 10.1016/S0378-5173(99)00331-2. [DOI] [PubMed] [Google Scholar]
- 5.Benvegnu T, Lemiègre L, Cammas-Marion S. New generation of liposomes called archaeosomes based on natural or synthetic archaeal lipids as innovative formulations for drug delivery. Recent Pat Drug Deliv Formul. 2009;3:206–220. doi: 10.2174/187221109789105630. [DOI] [PubMed] [Google Scholar]
- 6.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 7.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamachari Y, Geary SM, Lemke CD, Salem AK. Nanoparticle delivery systems in cancer vaccines. Pharm Res. 2011;28:215–236. doi: 10.1007/s11095-010-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27:4025–4033. doi: 10.1016/j.biomaterials.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Chen J, Sun W, Xu Y. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem Biophys Res Commun. 2010;394:412–417. doi: 10.1016/j.bbrc.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Barbeau J, Cammas-Marion S, Auvray P, Benvegnu T. Preparation and Characterization of Stealth Archaeosomes Based on a Synthetic PEGylated Archaeal Tetraether Lipid. J Drug Deliv. 2011;2011:396068. doi: 10.1155/2011/396068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprott GD. Structures of archaebacterial membrane lipids. J Bioenerg Biomembr. 1992;24:555–566. doi: 10.1007/BF00762348. [DOI] [PubMed] [Google Scholar]
- 13.Kates M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog Chem Fats Other Lipids. 1978;15:301–342. doi: 10.1016/0079-6832(77)90011-8. [DOI] [PubMed] [Google Scholar]
- 14.Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomedicine. 2009;4:299–319. doi: 10.2147/IJN.S4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Zhang L, Sun W, Ding Q, Hou Y, Xu Y. Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine. 2011;29:5260–5266. doi: 10.1016/j.vaccine.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan L, Dicaire CJ, Patel GB, Sprott GD. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect Immun. 2000;68:54–63. doi: 10.1128/IAI.68.1.54-63.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez R, Higa L, Cutrullis R, Bilen M, Morelli I, Roncaglia D, Corral R, Morilla M, Petray P, Romero E. Archaeosomes made of Halorubrum tebenquichense total polar lipids: a new source of adjuvancy. BMC Technology. 2009;9:71–83. doi: 10.1186/1471-2210-9-S1-P71. [DOI] [PMC free article] [PubMed] [Google Scholar]