Abstract
A study of iron, zinc, copper and selenium concentration levels was carried out in three compartments namely, maternal serum (MS), colostrums and cord blood serum (CS) of healthy Indian mothers (n = 42) who delivered healthy normal neonates without any congenital anomalies at Bhabha Atomic Research Centre hospital, Mumbai. Fe, Zn, Cu in maternal serum, cord blood and colostrums were estimated by flame atomic absorption spectrometry while Se was determined by graphite furnace absorption spectrometry. It was seen that there was a significant difference in the level of trace elements in the three compartments. The average levels of Fe in the three compartments were 1,132 ± 519, 2,312 ± 789 and 1,183 ± 602 μg/L while Zn was 514 ± 149, 819 ± 224 and 7,148 ± 2,316 μg/L respectively. Mean Cu values were 1,614 ± 295, 301 ± 77 and 392 ± 174 μg/L respectively while Se values were 70 ± 15, 36 ± 10 and 23 ± 8 μg/L respectively. The results indicated a positive correlation of Fe and Zn concentrations in MS versus CS which were (r = 0.386), (r = 0.572) respectively and Fe levels in MS and colostrums (r = 0.235). A few inter element correlations were found within compartments. Zn and Se showed a negative correlation in both MS (r = −0.489) and colostrums (r = −0.258) while a positive inter correlation of Fe and Zn was seen in MS (r = 0.44) and in CS (r = 0.54). This study gave us an overview of the serum and colostrum values of mother and neonates in Indian population, data of which are scarce.
Keywords: Maternal serum, Cord serum, Colostrum, Iron, Zinc, Copper, Selenium, Atomic absorption spectrometry
Introduction
Human milk is the first food human encounter and is the sole source of all nutrients required for the biological functions and growth during early stages of life [1]. Micronutrients such as iron, zinc, copper and selenium are extremely vital for fetal cell growth and decreased levels of these have been shown to be associated with many complications related to pregnancy and neonatal outcome. These metals are mostly bound to proteins, forming metalloproteins which are part of enzymatic systems which have structural and storage functions, or use the protein to be transported to their target site in the organism.
Fe has received the most attention in paediatric nutrition. Most of the Fe is in protein-bound complexes, and 60–70 % of it is present as part of the haemoglobin molecule and in serum most of it is attached to transport protein transferrin. Pregnancy imposes additional heavy iron requirements on the female, especially in the third trimester at which time daily iron needs increase from pre-pregnancy requirements of ≈1–1.5 to ≥6 mg/day [2–4].
Zinc participates in the synthesis and degradation of carbohydrates, proteins, lipids and nucleic acids. As it is required for cell division and differentiation it is an essential trace element administered during pregnancy to improve fetal growth. During pregnancy the requirements for zinc increases and many studies have shown that pregnant women are at risk of zinc deficiency [5–8] .
Copper is known to be a component of a number of copper metallo enzymes such as catalase, superoxide dismutase, and cytochrome oxidase and its deficiency can lead to variety of nutritional and vascular disorders [9, 10].
Selenium (Se) is one of the essential trace elements in humans and is an essential component of more than 10 selenoproteins [11] with multiple biochemical functions. Selenium is a major cofactor of some important enzymes such as glutathione peroxidase (GSH-Px) which acts as a primary antioxidant enzyme and iodothyronine 5-deiodinase on which the deiodination reactions are dependent [12]. It is important for its role in regulating growth and development of the fetus and newborn and concentration levels are critical as both low and high levels have harmful manifestations.
The neonatal period is one of the most critical with respect to nutrition, therefore a study was carried out to estimate and correlate the concentration of trace elements iron, zinc, copper and selenium in three compartments namely maternal serum, cord serum and colostrums (3rd day postpartum). The correlation of these trace elements within the individual compartments and each element in every other compartment namely maternal serum, cord serum and colostrums were evaluated.
Materials and Methods
A randomized cross sectional study was done in the Department of Pediatrics, Bhabha Atomic Research Centre Hospital. This is a tertiary care multi-specialty hospital which caters to the needs of the employees and the families of Department of Atomic energy. The study was conducted over a period of 1 year on mothers and the newborns who are delivered at BARC Hospital. Mothers with age less than 40 years, with gestational age of more than 36 completed weeks who delivered normally as singleton live born healthy neonate without any visible congenital abnormalities were enrolled in the study. Also those mothers with multiple gestations; antenatal illness like toxaemia of pregnancy, diabetes mellitus, hypothyroidism and those who received nutritional supplementation other than iron, calcium and folic acid were excluded from the study. All mothers were healthy without any major antenatal illness.
Ethical Issues
Necessary permissions and approvals were obtained from the Hospital Administration and Ethical Committee prior to starting the study. A well informed and valid consent was obtained from those participating in the study.
Sample Collection
The glass tubes used for collection of the blood and colostrum for trace elements estimation were cleaned twice with Suprapur® (Merck) Nitric acid in 1:1 dilution and then rinsed with double distilled water twice before storing them in acid cleaned polypropylene bags. 10 mL sample of venous blood was collected with help of a Teflon catheter from the mother’s during labor and approximately 10 mL of cord blood at time of delivery was collected directly before placental separation. The blood collected was allowed to clot for 30 min at room temperature and then centrifuged at 2,500×g for 10 min. The obtained serum was stored in the trace element free glass tubes cleaned as mentioned previously. The same was further stored at −20 °C until estimation.
Mothers were asked to rinse the breast with deionised water and allow to air dry immediately before taking colostrum samples of 5 mL each on day 3 post partum in trace element free test tubes and stored at −20 °C until analysis.
Cold chain was maintained during the transportation of the blood as well as the milk samples to the Ultra Trace Analytical Facility of Analytical Chemistry Division of Bhabha Atomic Research Centre where the samples were later analyzed. The entire collection procedure was checked meticulously for iron, copper, zinc and selenium estimation and found to be essentially free from contamination.
Sample Analysis
Trace elements Cu, Fe and Zn were determined by Flame Atomic Absorption Spectrometry employing a GBC 906AA AAS unit with deuterium-arc background correction. The elemental hollow cathode lamp of Cu λ 324.7, Fe λ 248.3 and Zn λ 213.8 nm (GBC Australia), air-acetylene flame and nanopure water (18.3 Ω) as diluent were used in this estimation. All measurements were performed using integrated absorbance mode.
Serum
The refrigerated samples were allowed to come to room temperature and suitably diluted with nanopure water to bring the mentioned element in the optimum analytical range. The samples along with calibration standards were aspirated into the air acetylene flame.
Colostrum
One milliliter of sample was treated with Suprapur HNO3 (Merck) and H2O2 to digest and oxidize the organic matter. This procedure was repeated to ensure that all fat had been digested. A few drops of supra pure HClO4 acid were added and fumed off to ensure complete oxidation and elimination of organic matter. 1 % HNO3 in nanopure water was added and sample made up to 5 mL.
The results of Cu, Fe and Zn were expressed as μg/L. The coefficient of variation was from 2 to 5 % and the average accuracy was >95 % when analyzing the reference material.
Trace element Se was determined by Graphite Furnace Atomic Absorption Spectrometer (GFAAS) employing GBC 906AA AAS with GF 3000 electro thermal atomizer and an auto sampler PAL-3000. Pyrolytic graphite coated platform furnace tubes and HCL of Se λ 196 nm (GBC, Australia) were used in this estimation. All measurements were performed using peak height absorbance mode.
Serum and Colostrum
One milliliter sample of serum/colostrums was taken in micro centrifuge tubes and 100 μL of Suprapur HNO3 (Merck) was added to it. The micro centrifuge tubes were heated in water bath at 70 °C for 5 min and centrifuged at 9,000 rpm for 10 min. Supernatant was decanted immediately and selenium was analyzed in supernatant using GFAAS. Palladium nitrate was used as matrix modifier. The results were expressed as μg/L. The detection limit for Se was <5 μg/L and the average accuracy was >95 % when analyzing the reference material.
Quality Assurance
The validation of each batch of serum and colostrums sample was thoroughly checked by Seronorm™ Trace Elements Serum-reference material (Nycomed Pharma AS, Oslo) and Reference Material 1549® (Non-fat milk powder)’ from the National Institute of Standards and Technology (Gaithersburg, MD, USA). (The t test at 95 % CL gave a value less than critical value t0.05 is 2.78 n = 5).
All the samples were processed in Laminar Air Flow benches which are equipped with HEPA filters working at an efficiency of 99.7 % for 0.5 microparticles to minimize sample contamination.
Statistical Analysis
The observations were tabulated in a spreadsheet using Microsoft Excel©. Statistical analysis was done using SPSS© version 16.0. All the data was checked for normality using Shapiro–Wilk test. Non-parametric correlations were applied for analysis where the data failed normality. The tests used were Mann–Whitney test, linear regression, Chi square test, unpaired t test, Kruskal–Wallis test and ANOVA. A “p value” of less than 0.05 was considered statistically significant.
Results
Tables 1, 2 and 3 summarize the mean, standard deviation (SD), observed range and IQR of four essential elements namely Fe, Cu, Zn and Se in MS, CS and colostrums (n = 42) respectively. The literature values for these elements in the different compartments are presented in these tables. The values for colostrums are of milk collected on 3 day post partum. Box and whiskers plots were drawn for easier comparison of the concentration levels in the three compartments.
Table 1.
Results of analysis of maternal serum
| Descriptive statistics | Element (μg/L) | |||
|---|---|---|---|---|
| Fe | Cu | Zn | Se | |
| Mean | 1,132 | 1,614 | 514 | 70 |
| Median | 1,165 | 1,550 | 520 | 72 |
| SD | 519 | 295 | 149 | 15 |
| Min value | 370 | 1,040 | 100.0 | 40 |
| Max value | 2,220 | 2,140 | 800 | 106 |
| IQR | 895 | 495 | 197.5 | 20.75 |
| Literature values | ||||
| Ref. [17] | 1,484–2,952 | 413–1,038 | 19.6–93.7 | |
| Ref. [14], [26]* | 740 ± 210 | 2,360 ± 360 | 680 ± 100 | 62 ± 10* |
| Ref. [13], [18]* | 1,021 ± 81* | 2,042.2 ± 418 | 584.0 ± 115.2 | 85.5 ± 12.8 |
| Ref. [19] | 2,030 ± 520 | 650 ± 180 | ||
| Ref. [5] | 1,210.0 ± 610.0 | 520.0 ± 220 | ||
| Ref. [15] | 727.0 ± 73 | 1,873 ± 199 | 705 ± 112 | |
| Ref. [25] | 1,687 ± 353 | 68.3 ± 8.5 | ||
| Ref. [16] | 680 ± 260 | 850 ± 160 | ||
| Ref. [20] | 1,715 ± 268 | 625 ± 183 | ||
* refers to Elem. Conc.
Table 2.
Results of analysis of cord serum
| Descriptive statistics | Element (μg/L) | |||
|---|---|---|---|---|
| Fe | Cu | Zn | Se | |
| Mean | 2,312 | 301 | 819 | 36 |
| Median | 2,145 | 310 | 800 | 34 |
| SD | 789 | 77 | 224 | 10 |
| Min value | 830 | 150 | 350 | 22 |
| Max value | 3,710 | 450 | 1,340 | 60 |
| IQR | 1,115 | 100 | 290 | 13.75 |
| Literature values | ||||
| Ref. [5] | 210 ± 110 | 830 ± 390 | ||
| Ref. [14] | 1,160 ± 170 | 490 ± 240 | 1,140 ± 23 | |
| Ref. [15] | 949 ± 72 | 1,016 ± 119 | 887 ± 98 | |
| Ref. [13], [25*] | 1,907 ± 130 | 449 ± 87* | 37.02 ± 8.9* | |
| Ref. [19], [26*] | 400 ± 130 | 930 ± 220 | 34 ± 7* | |
| Ref. [20] | 870 ± 95 | |||
* refers to Elem. Conc.
Table 3.
Results of analysis of colostrums
| Descriptive statistics | Element (μg/L) | |||
|---|---|---|---|---|
| Fe | Cu | Zn | Se | |
| Mean | 1,183 | 392 | 7,148 | 23 |
| Median | 1,080 | 383 | 7,667 | 23 |
| SD | 602 | 174 | 2,316 | 8 |
| Min value | 320 | 50 | 1,300 | 11 |
| Max value | 2,410 | 740 | 10,900 | 42 |
| IQR | 802.5 | 210.5 | 3,236.5 | 10 |
| Literature values | ||||
| Ref. [17] | 186–2,628 | 1,869–22,050 | 34.2–187.6 | |
| Ref. [32] | 110–990 | 2,960–23,090 | 22.9–54.2 | |
| Ref. [13], [33]* | 849 ± 154 | 590 ± 50* | 3,760 ± 510* | 15.2 ± 2.3* |
| Ref. [19], [26]* | 390 ± 220 | 10,120 ± 5,080 | 29 ± 10* | |
| Ref. [31] | 570 ± 336 | 6,040 ± 3,590 | ||
* refers to Elem. Conc.
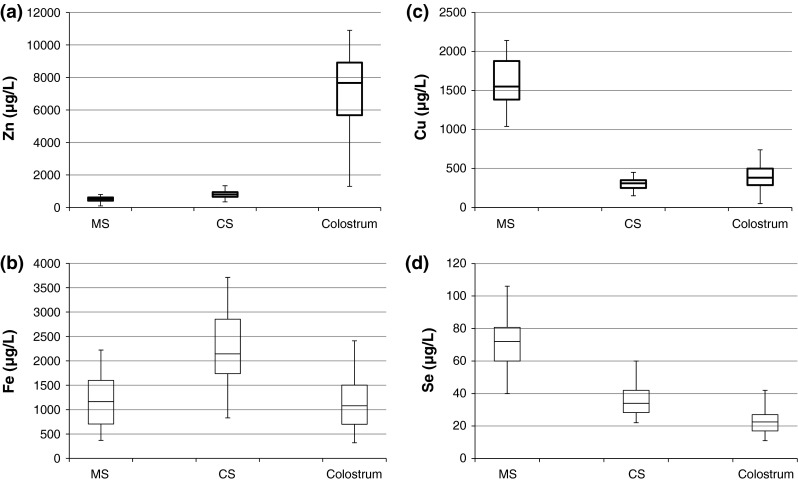
A statistically significant difference was noted between Fe levels in MS and CS levels (p ≤ 0.05) however no significant difference was seen in MS and colostrums levels (Fig. 1a).
Fig. 1.
Boxplots (median at center) of the a Fe, b Zn, c Cu and d Se concentration in maternal serum, cord serum and colostrums
Zn, Cu and Se levels showed significant difference in all compartments, (Fig. 1b–d), MS and CS (p < 0.001, p < 0.001 and p < 0.05), MS and colostrums (p < 0.05, p < 0.05, p < 0.05) and CS and colostrums (p < 0.05, p < 0.05, p < 0.05) respectively. The concentration levels of Cu and Se in colostrums are significantly lower while Zn level is higher than the levels in MS.
The CS have significantly higher concentration of Fe and Zn, than the MS and based on the median values the concentrations are 1.8 and 1.5 times higher, while the Cu and Se values are 0.21 and 0.48 times lower than the MS. There was no significant difference in Fe levels in colostrums and MS but Zn levels were 14 times higher and Cu and Se levels were lower by a factor of 0.25 and 0.32 respectively.
Discussion
Trace Elements in Maternal Serum
The mean serum iron level in the mother was 1,132 ± 519 μg L−1 which were in comparison with the values noted in the non-anemic Indian mothers [13], but were higher when compared to those reported by other workers [14–16]. This was attributed to good socio-economic factors, proper supplementation with iron and good compliance of the mothers who follow up in this hospital during the antenatal period.
The mean maternal serum zinc levels reported in our study are 514 ± 149 μg/L and are higher than some reported in literature [5, 17, 18] but lower than the values denoted by others [14–16, 19, 20]. Review of literature [18] has indicated that pregnant women have lower serum Zn levels than non pregnant healthy women. This is a natural consequence due to an increased zinc uptake by the fetus and placenta and also due to the expanded plasma volume of the mother [21, 22].
Literature review [14, 17, 18] suggests that the serum copper levels of the mothers are significantly higher than the normal non pregnant reference range [23, 24]. Our mean Cu value is 1,614 ± 295 μg/L (Table 1), and is in agreement with the observations in the literature reports. The higher levels of copper in MS are attributed to the increased mobilization of stored copper in tissues which is triggered by increased estrogen levels during pregnancy.
In a study carried out on a normal population similar to our study group the mean Se value was 100 ± 1.33 μg/L [27]. The mean MS selenium level noted in our study was 70 ± 15 μg/L which is similar to those reported by other workers. [18, 25, 26] where the mothers were in their 3rd trimester. This observation is attributed to the increased demand of Se for fetal growth along with increased blood volume at full term of pregnancy and in accordance with literature report which suggest that there is a diminution in the Se levels decrease as gestation pregnancy progresses and are lowest in the 3rd trimester [18].
Trace Elements in Cord Serum (Table 2)
The mean iron level in the CS was 2,312 ± 789 μg/L which is higher than the values reported by other workers [14, 15]. In our study we found that there was a positive correlation between MS iron and CS iron (p = 0.004, r = 0.386). We also noted that the mean serum iron levels in CS were significantly higher (p < 0.001) to that of the corresponding MS iron levels. The high iron in cord serum compared with maternal serum, suggests that the process of active transfer of iron from the mother to the fetus is adequately maintained.
The mean neonatal CS zinc levels noted were 819 ± 224 μg/L, which were similar to those reported by other workers [5, 14, 15, 19, 20, 28]. The concentrations of zinc in CS are approximately 51 % higher than in the corresponding maternal sera. A positive correlation between the cord serum zinc and maternal serum zinc was seen in our study (p = 0.001, r = 0.572). Thus the level of zinc would be expected to be higher in CS than that in MS.
The mean CS copper levels obtained in our study were 301 ± 77 μg/L which was comparable to values reported by [5, 19, 29] but lower than those reported by others [15, 25]. Copper levels in cord serum are much lower than in maternal serum. We found no statistical correlation between cord serum copper and maternal serum copper, however we found statistically significant difference between the maternal and cord serum copper levels (p < 0.001). Concentrations of copper in umbilical cord sera reached only 28 % of the normal concentration in mother despite the large copper stores of the liver.
The neonatal CS selenium levels noted in our study were 36 ± 10 μg/L which were similar to those given by [25, 26, 28]. The concentrations of selenium in CS are approximately 50 % as compared to corresponding MS which is similar to the finding of [26] who noted cord serum selenium, was 55 % of that of the maternal serum selenium. The exact mechanisms responsible for the maternal–fetal selenium gradients are yet unknown. In our study we noted a significant difference between maternal and umbilical cord selenium levels (p < 0.001). Although it is mentioned in the literature that significant linear correlation between selenium concentrations in MS and CS exist, we noted both the entities to be closely associated though statistically not significant (p = 0.055).
Trace Elements in Colostrums (Table 3)
The colostrum iron levels in our study was 1,183.2 ± 602 μg/L which is in agreement with value reported by [13] but considerably higher as compared to the those mentioned by Food and Nutrition Board [30]. The colostrum iron in our study had a weak positive correlation with the maternal serum iron (p = 0.015, r = 0.235). Also there was a significant difference (p < 0.001) between the colostrum iron and the serum iron of the mother. This linear relationship of maternal iron levels with colostrum iron content shows that iron is transported into colostrum in direct proportion with the levels found in maternal circulation.
In this study the concentrations of Zn in colostrum was 7,148 ± 2,316 μg/L which was approximately 10 times higher than in MS and 15 times higher as compared to the CS. There was a significant difference between MS zinc levels and colostrum zinc level (p < 0.001). There was no significant correlation observed in zinc content between the MS and the colostrum sample (p = 0.068). The reason for the higher concentration of trace elements in colostrum is not known however, it may be to meet requirements since newborn infants require larger amounts of these minerals due to the low volume of colostrum intake in their early life as compared with later days [17].
We found that the concentration of copper in colostrum was 392 ± 174 μg/L which was similar to the concentrations in CS which is in close agreement to the literature report [31]. However there was no correlation found between the copper levels of maternal sera and colostrum (p = 0.17). This finding could be due to the developed mechanism in the mammary glands which could regulate the concentration of elements independently of the maternal status in order to supply the breast fed infant. A statistical significant difference was noted between the maternal serum, colostrum and cord serum copper levels (p < 0.001).
The mean colostrum selenium level obtained in our study was 24.3 ± 9.9 μg/L, which were comparable to those reported by [26, 32] but lower than those reported by [17]. The Se levels in colostrum showed a mean value 35 % of that of MS with a wide range of colostrum selenium value between 11 and 42 μg/L. A statistical significant difference between MS selenium and colostrum selenium levels (p < 0.001) was seen.
The general absence of correlation and the difference in the concentration of individual trace elements suggests that homeostasis of trace elements is regulated through molecular processes which transport trace elements through the different compartments.
Inter-Correlation
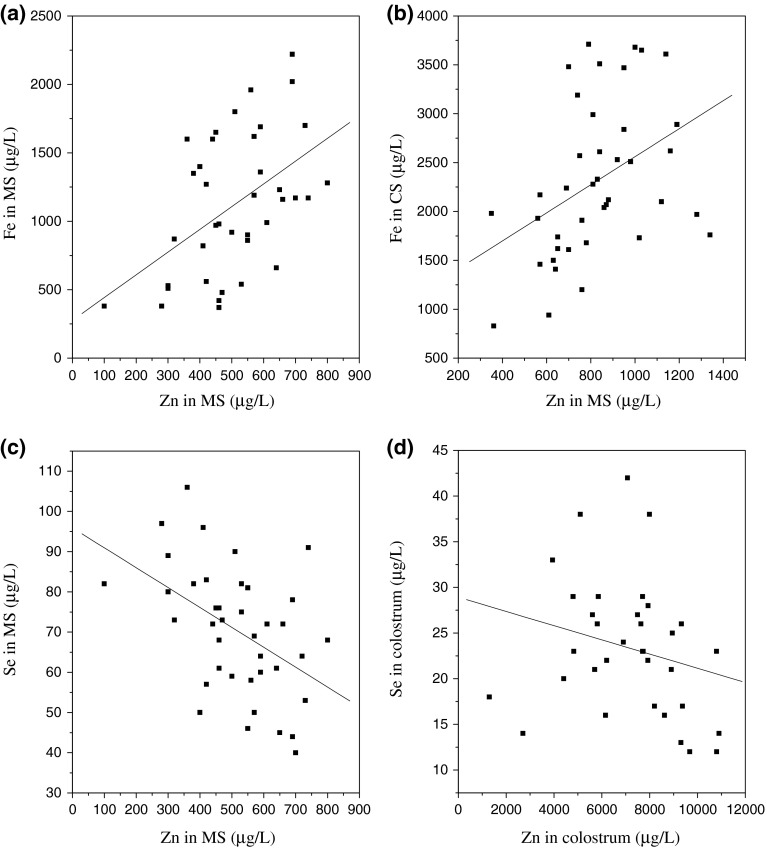
Both zinc and iron contents of MS showed wide variability however, the magnitude of the variation was similar for both elements. We obtained a significant positive correlation (p = 0.003, r = 0.44) (Fig. 2a) between MS iron levels and MS zinc levels. Also positive statistical correlation was obtained between the CS iron levels and CS zinc levels (p = 0.001, r = 0.54) (Fig. 2b). No correlation of colostrum iron with other elements was observed in our study. A significant negative correlation between MS zinc and MS selenium levels (p = 0.001) (r = −0.489) (Fig. 2c). A weak negative correlation was noted between the zinc and the selenium levels in the colostrum (p = 0.035, r = −0.258) (Fig. 2d). The reason for the above interactions however could not be found and further studies would be needed to evaluate the relationship between the two elements.
Fig. 2.
a Correlation Fe versus Zn in MS(r = 0.44), b Fe versus Zn in CS (r = 0.54), c Se versus Zn in MS (r = −0.487), d Se versus Zn in colostrums (r = −0.258)
Conclusions
In this study we have evaluated 42 mothers and their neonates to study the inter-correlations of the trace elements (Fe, Zn, Cu, Se) in the three compartments viz. maternal serum, maternal colostrums and cord serum. The data in our study and trend of trace elements are consistent with earlier literature reports. The data in our study suggested that zinc and selenium levels were lowered in pregnant mothers hence supporting the premise of the need of their supplementation along with iron.
This study gave us an overview in the serum and colostrums values of mother and neonates in Indian population, data of which are scarce. It also highlighted the complex interactions of the trace elements between the maternal and the neonatal organism.
Contributor Information
Mehul Jariwala, Email: mehuljari@gmail.com.
S. Suvarna, Email: ssuvarna@barc.gov.in
G. Kiran Kumar, Email: gkkumar@barc.gov.in
Alpa Amin, Email: alpaamin@barc.gov.in.
A. C. Udas, Email: acudas@barc.gov.in
References
- 1.Prohaska JR. Functions of trace elements in brain metabolism. Physiol Rev. 1989;67:858–901. doi: 10.1152/physrev.1987.67.3.858. [DOI] [PubMed] [Google Scholar]
- 2.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(Suppl):257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 3.Bothwell TH, Charlton RW, Cook JD, Finch CA. Iron metabolism in man. London: Blackwell; 1979. [Google Scholar]
- 4.Viteri FE, Allen L, King J, Lonnerdahl B. The consequences of iron deficiency and anemia in pregnancy. New York: Plenum Press; 1994. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal ASM, Shahidullah Md, Md Islam N, Akhter S, Banu S. Serum zinc and copper levels in the maternal blood and cord blood of neonates. Indian J Pediatr. 2001;68:523–526. doi: 10.1007/BF02723246. [DOI] [PubMed] [Google Scholar]
- 6.Hurley LS, Swenerton H. Congenital malformations resulting from zinc deficiency in rats. Proc Soc Exp Biol Med. 1966;123:692–697. doi: 10.3181/00379727-123-31578. [DOI] [PubMed] [Google Scholar]
- 7.Ortega RM, Quintas ME, Andre’s P, et al. Zinc status of a group of pregnant Spanish women: effects on anthropometric data and Apgar score of neonates. Nutr Res. 1999;19:1423–1427. doi: 10.1016/S0271-5317(99)00099-8. [DOI] [Google Scholar]
- 8.Huddle JM, Gibson RS, Cullinan TR. Is zinc a limiting nutrient in the diets of rural pregnant Malawian women? Br J Nutr. 1998;79:257–259. doi: 10.1079/BJN19980043. [DOI] [PubMed] [Google Scholar]
- 9.Al-Bader A, Hussain T, Al-Mosawi Otaibi M, Abdul H, Khalifa D, et al. Serum zinc and copper concentrations in pregnant women from Kuwait. J Trace Elem Exp Med. 1997;10:5–209. doi: 10.1002/(SICI)1520-670X(1997)10:4<209::AID-JTRA1>3.0.CO;2-4. [DOI] [Google Scholar]
- 10.Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002;6:171–180. doi: 10.1016/S1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- 11.Arthur JR, Beckett GJ. Symposium 2: newer aspects of micronutrients in at risk groups, new metabolic roles for selenium. Proc Nutr Soc. 1994;53:615–624. doi: 10.1079/PNS19940070. [DOI] [PubMed] [Google Scholar]
- 12.Kohrle J, Brigelius-Flohe R, Bock A, Gartner R, Meyer O, Flohe L. Selenium in biology: facts and medical perspectives. Biol Chem. 2000;381:849–864. doi: 10.1515/BC.2000.107. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Rai AK, Basu S, Dash D, Singh JS. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008;121:e673–e677. doi: 10.1542/peds.2007-1986. [DOI] [PubMed] [Google Scholar]
- 14.Awadallah SM, Abu-Elteen KH, Elkarmi AZ, Qaraein SH, Salem NM, Mubarak MS. Maternal and cord blood serum levels of zinc, copper, and iron in healthy pregnant Jordanian women. J Trace Elem Exp Med. 2004;17:1–8. doi: 10.1002/jtra.10032. [DOI] [Google Scholar]
- 15.Upadhyaya C, Ajmera P, Sharma P. Serum iron, copper and zinc status in maternal and cord blood. Indian J Clin Biochem. 2004;19(2):48–52. doi: 10.1007/BF02894257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozkan TB, Durmaz N, Erdemir G, Ilcol YO. Trace element concentrations in breast milk and sera: relations with lactation. J Biol Environ Sci. 2007;1(3):143–147. [Google Scholar]
- 17.Almeida A, Lopes CMP, Silva A, Barrado E. Trace elements in human milk: correlation with blood levels, inter-element correlations and changes in concentration during the first month of lactation. J Trace Elem Med Biol. 2008;22:196–205. doi: 10.1016/j.jtemb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez SI, Castanon SG, Calvo Ruata ML, et al. Updating normal levels of copper, zinc and selenium in pregnant women. J Trace Elem Med Biol. 2007;21 S1:49–52. doi: 10.1016/j.jtemb.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Krachler M, Rossipal E, Micetic-Turk D. Trace element transfer from the mother to the newborn-investigations on triplets of colostrum, maternal and umbilical cord sera. Eur J Clin Nutr. 1999;53:486–494. doi: 10.1038/sj.ejcn.1600781. [DOI] [PubMed] [Google Scholar]
- 20.Perveen SAW, Vohra N, Bautista LM, Harper R. Effect of gestational age on cord blood plasma copper, zinc, magnesium and albumin. Early Human Dev. 2002;69:15–23. doi: 10.1016/S0378-3782(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 21.Vir SC, Love AGG, Thompson W. Zinc concentrations in hair and serum of pregnant women in Belfast. Am J Clin Nutr. 1981;34:2800–2807. doi: 10.1093/ajcn/34.12.2800. [DOI] [PubMed] [Google Scholar]
- 22.Aggett PJ, Harris JT. Current status of zinc in health and disease states. Arch Dis Child. 1979;54:909–917. doi: 10.1136/adc.54.12.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SE Minoia C, Apostoli P, Pietra R, Pozzoli L, Gallorini M, Nicolaou G, Allessio L, Capodaglio E. Trace element reference values in tissues from inhabitants of the European community. Sci Total Environ. 1990;95:89–105. doi: 10.1016/0048-9697(90)90055-Y. [DOI] [PubMed] [Google Scholar]
- 24.Caroli SAA, Coni E, Petrucci F, Senofonte O, Violante N. The assessment of reference values for elements in human biological tissues and fluids: a systematic review. Crit Rev Anal Chem. 1994;24:363–398. doi: 10.1080/10408349408048824. [DOI] [Google Scholar]
- 25.Schulpis KH, Karakonstantakis T, Gavrili S, Chronopoulou G, Karikas GA, Vlachos G, Papassotiriou I. Maternal-neonatal serum selenium and copper levels in Greeks and Albanians. Eur J Clin Nutr. 2004;58:1314–8. [DOI] [PubMed]
- 26.Micetic-Turk D, Rossipal E, Krachler M, Li F. Maternal selenium status in Slovenia and its impact on the selenium concentration of umbilical cord serum and colostrum. Eur J Clin Nutr. 2000;54:522–524. doi: 10.1038/sj.ejcn.1601050. [DOI] [PubMed] [Google Scholar]
- 27.Raghunath R, Tripathi RM, Mahapatra S, Sadasivan S. Selenium levels in biological matrices in adult population of Mumbai. Indian J Sci Total Environ. 2002;285:21–27. doi: 10.1016/S0048-9697(01)00892-0. [DOI] [PubMed] [Google Scholar]
- 28.Alimonti A, Petrucci F, Laurenti F, Papoff P, Caroli S. Reference values for selected trace elements in serum of term newborns from the urban area of Rome. Clin Chim Acta. 2000;292:163–173. doi: 10.1016/S0009-8981(99)00217-X. [DOI] [PubMed] [Google Scholar]
- 29.O’ Leary JANG, Vosburgh GJ. Maternal serum copper concentrations in normal and abnormal gestations. Obstet Gynecol. 1966;28:112–116. [PubMed] [Google Scholar]
- 30.Food and Nutrition Board NRC. Recommended dietary allowances. 10th ed. Washington, DC: National Academy of Sciences; 1989.
- 31.Krachler M, Li FS, Rossipal E, Irgolic KJ. Changes of the concentrations of trace elements in human milk during lactation. J Trace Elem Med Biol. 1998;12:159–176. doi: 10.1016/S0946-672X(98)80005-9. [DOI] [PubMed] [Google Scholar]
- 32.Rossipal E, Krachler M. Pattern of trace elements in human milk during the course of lactation. Nutr Res. 1998;18(1):11–24. doi: 10.1016/S0271-5317(97)00196-6. [DOI] [Google Scholar]
- 33.Benemariya H, Robberecht H, Deelstra H. Copper, zinc and selenium concentrations in milk from middle-class women in Burundi (Africa) throughout the first 10 months of lactation. Sci Total Environ. 1995;164:161–174. doi: 10.1016/0048-9697(95)04456-B. [DOI] [Google Scholar]