Abstract
Diabetes mellitus is one of the most common endocrine metabolic disorders. Dual endocrine deficits of impaired insulin action (insulin resistance) and inadequate insulin secretion create an environment of chronic hyperglycemia and general metabolic disarray. Oxidative stress plays an important role in diabetic pathogenesis. Oxidative stress induced by streptozotocin (STZ) has been shown to damage pancreatic beta cell and produce hyperglycemia in rats. The present study was made to evaluate the antioxidant activity of ethanolic extract of the Evolvulus alsinoides in STZ induced rats. The antioxidant activities were done by using standard protocols. For histopathological analysis, the pancreatic tissues of all experimental groups were fixed with 10 % formalin for 24 h then the samples were stained with hematoxylin–eosin for the microscopic observation. Our results showed the significant decrease in lipid peroxidation and increases in the antioxidant (both enzymatic and nonenzymatic) levels after treatment with standard as well as the E. alsinoides. There is no significant difference between control and plant alone group rats. The histopathology reports also revealed non-toxic effect and protective effect of E. alsinoides in the kidney of STZ induced diabetic rats. Our result indicated that the E. alsinoides extract effectively increased the antioxidant level thereby it prevents oxidative stress during diabetes mellitus and also it showed the protective effect on kidney of STZ induced rats. Hence it can be used to maintain the antioxidant level during diabetes mellitus.
Keywords: Enzymatic antioxidants, Non-enzymatic antioxidant, LPO, Streptozotocin, Evolvulus alsinoides
Introduction
Diabetes mellitus is considered as one of the five leading causes of death in the world. About 150 million people are suffering from diabetes worldwide, which is almost five times more than the estimates 10 years ago and this may double in the year 2030 [1]. It is characterized by chronic hyperglycemia and disturbances of carbohydrate, fat and protein metabolism associated with absolute or relative deficiency in insulin secretion or insulin action [2]. In diabetes mellitus, chronic hyperglycaemia produces multiple biochemical consequence and diabetes-induced oxidative stress could play a role in the symptoms and progression of the disease [3].
Oxidative stress may result in overproduction of oxygen free-radical precursors and/or decreased efficiency of the antioxidant system [4]. The oxygen free-radical generation is associated with auto-oxidation of glucose, impaired glutathione metabolism, alterations in the antioxidant enzymes and formation of lipid peroxides [5–7]. There are various endogenous defense mechanisms against free radicals, such as the enzymes GSH, SOD, GPx and CAT, whose activities eliminate superoxide, hydrogen peroxide and hydroxyl radicals [8]. Oxidative stress is increased in experimental models of streptozotocin (STZ)-induced diabetes mellitus [9, 10]. If the diabetic state is associated with a generalized increase in tissue oxidative stress, it might well be reflected in the changes in tissue antioxidant system. So the present study was aimed to test the antioxidant enzymes and lipid peroxidation profile in the kidney of treated and untreated diabetic rats.
Materials and Methods
Plant Material
The whole plant of Evolvulus alsinoides (L.) L. used for the investigation was obtained from Coimbatore District, Tamilnadu, India. The plant was authenticated by Dr. P. Satyanarayana, Botanical Survey of India, TNAU Campus, Coimbatore. The voucher number is BSI/SRC/5/23/2011-12/Tech.-514. Fresh plant material was washed under running tap water, air dried and powdered.
Sample Extraction
100 g of dried plant powder was extracted in 500 ml of ethanol in an orbital shaker for 72 h. Repeated extraction was done with the same solvent till clear colorless solvent is obtained. Obtained extract was evaporated and stored at 0–4 °C in an air tight container.
Animals
Wistar albino rats weighing about 150–180 g were procured from Karpagam University Animal house, Coimbatore, India. The animals were kept under standard conditions, fed with rodent diet and water. The study was approved by Institutional Animal Ethical Committee constituted for the purpose of CPCSEA.
Induction of Experimental Diabetes
Rats were rendered diabetic by a single intraperitoneal injection of freshly prepared STZ (45 mg/kg body weight) in 0.1 M citrate buffer (pH 4.5) in a volume of 1 ml/kg body weight [11]. Diabetes was identified in rats by moderate polydypsia and marked polyuria. After 48 h of STZ administration, blood glucose levels were estimated and rats with a blood glucose ranging between 200 and 400 mg/dl were considered diabetic and used for the experiments.
Experimental Protocol
The animals were divided into five groups of six animals each. Group I served as a control; group II consisted of STZ-induced diabetic rats; group III consisted of STZ-induced diabetic rats treated with glibenclamide (1.25 mg/kg body weight/day/rat); groups IV consisted of STZ-induced diabetic rats treated ethanolic extract of E. alsinoides (150 mg/kg body weight/day/rat) and group V were normal rats treated with ethanolic extract of E. alsinoides (150 mg/kg body weight/day/rat).
Biochemical Studies
After 45 days of treatment the animals were sacrificed under chloroform anesthesia. Liver and kidneys was quickly excised off, a portion of liver washed with saline and liver homogenate was prepared using 0.1 M phosphate buffer, pH 7.4. The liver homogenate was centrifuged and the supernatant was used for the determination of lipid peroxidation [12], enzymatic antioxidant like superoxide dismutase [13], catalase [14], glutathione peroxidase [15], glutathione reductase [16], glucose-6-phosphate dehydrogenase [17] and non-enzymatic antioxidants like total reduced glutathione [18], vitamin C [19] and vitamin E [20].
Histological Observation
One portion of the kidney in all the experimental groups was fixed in 10 % formalin for histological observation. It was done by Dunn, 1974 [21] method.
Statistical Analysis
The values were expressed as mean ± SD. The statistical analysis was carried out by one way analysis of variance using SPSS (version 10) statistical analysis program. Statistical significance was considered at p < 0.05.
Result and Discussion
Antioxidants are substances or nutrients in our foods which can prevent or slow the oxidative damage to our body. When our body cells use oxygen, they naturally produce free radicals (by-products) which can cause damage. Antioxidants act as “free radical scavengers” and hence prevent and repair damage done by these free radicals. Health problems such as heart disease, muscular degeneration, diabetes mellitus, cancer etc. are all contributed by oxidative damage [22]. Hence the enzymatic and nonenzymatic antioxidant levels were measured in diabetic rats before and after treatment with standard as well as plant extract.
The diabetic state is associated with a generalized in-crease in tissue oxidative stress, which might be reflected in the changes in the tissue antioxidant system. Results of the changes in antioxidant enzymes are presented in Table 1. All enzymatic antioxidants like SOD, catalase, glutathione reductase, GPx and glucose-6-phosphate dehydrogenase levels were decreased in diabetic condition due to the glycation of proteins. After the treatment with plant extract the antioxidant enzymes level reaches near to the normal level as that of standard. There is no significant difference found between control and plant alone group.
Units
- Superoxide dismutase
Enzyme required for 50 % inhibition of NBT reduction/min/mg protein
- Catalase
μmoles of H2O2utilized/min/mg/protein
- Glutathione peroxidase
μmoles of GSH utilized/min/mg/protein
- Glutathione reductase
n moles of NADPH oxidized/min/mg protein
- Glucose-6-phosphate dehydrogenase
min/mg protein
Table 1.
Enzymatic antioxidant levels in kidney of diabetic condition before and after treatment with Evolvulus alsinoides of control and experimental groups
| Groups | SOD | Catalase | Glutathione reductase | GPx | Glucose-6-phosphate dehydrogenase |
| Control | 3.31 ± 0.33b | 0.72 ± 0.057b | 3.18 ± 0.23b | 1.96 ± 0.08d | 3.22 ± 0.05b |
| Diabetic control | 1.10 ± 0.53a | 0.305 ± 0.30a | 1.96 ± 0.92a | 0.76 ± 0.21a | 2.45 ± 0.16a |
| Diabetic + glibenclamide | 2.98 ± 0.20b | 0.66 ± 0.030b | 3.02 ± 0.62b | 1.51 ± 0.10b | 3.19 ± 0.05b |
| Diabetic + Evolvulus alsinoides | 3.27 ± 0.46b | 0.64 ± 0.09b | 3.06 ± 0.28b | 1.59 ± 0.08b | 3.04 ± 0.05b |
| Evolvulus alsinoides alone | 3.35 ± 0.52b | 0.74 ± 0.09b | 3.65 ± 0.38b | 1.86 ± 0.29d | 3.48 ± 0.21d |
Values are expressed as mean ± SD for six animals in each group. Values not sharing common superscript letters (a–d) differ significantly at p < 0.05 (DMRT)
Oxidative stress is the imbalance between production and removal of reactive oxygen species (ROS). Increased oxidative stress contributes substantially to the pathogenesis of diabetic complications which is the consequences of either enhanced ROS production or attenuated ROS scavenging capacity. Several reports have shown the alterations in the antioxidant enzymes during diabetic condition [23, 24]. The antioxidative defense system like SOD and catalase showed lower activities in brain during diabetes and the results agree well with the earlier published data [25]. The decreased activities of SOD and catalase may be a response to increased production of H2O2 and O2− by the auto oxidation of excess glucose and non-enzymatic glycation of proteins e.g. glycation of SOD, catalase [26–28]. Hodgson and Fridovich [29] and Pigleot et al. 1990 [30] have reported the partial inactivation of these enzyme activities by hydroxyl radicals and hydrogen peroxide. GPx catalyses the reaction of hydroperoxides with reduced glutathione to form glutathione disulphide and the reduction product of hydroperoxide [31]. In the present study, decline in the activities of these enzymes in STZ induced animals due to the oxidative stress elicited by STZ and attainment of normalcy in plant treated rats.
The non-enzymatic antioxidants activity in kidney of diabetes induced and treated groups were depicted in Table 2. In that all the non-enzymatic antioxidants like reduced glutathione, vitamin C and E showed a reduced level in diabetic control group and the increased level was found after the treatment with plant extract and standard drug. There is no significant difference was originated between control and plant alone group rats. Glutathione plays an important role in the endogenous non-enzymatic antioxidant system. It primarily acts as a reducing agent and detoxifies hydrogen peroxide in the presence of the enzyme glutathione peroxidase [32].Vitamin C, a potent water soluble non-enzymatic antioxidant effectively intercept oxidants in the aqueous phase before they attack and cause detectable oxidative damage [33]. The observed decrease in the levels of vitamin C in the diabetic condition is consistent with previous reports [34]. Vitamin E is the main endogenous antioxidant which reacts with oxygen radicals and prevents free radical chain reaction to protect the membranes. Based on our findings our plant extract protects the organs from oxidative damage.
Table 2.
Non-enzymatic antioxidant levels in kidney of diabetic condition before and after treatment with Evolvulus alsinoides of control and experimental groups
| Groups | Total reduced glutathione | Vitamin C | Vitamin E |
|---|---|---|---|
| Control | 25.54 ± 1.17c | 2.97 ± 0.10d | 3.02 ± 0.27b |
| Diabetic control | 13.82 ± 2.05a | 1.11 ± 0.05a | 1.63 ± 0.68a |
| Diabetic + glibenclamide | 21.83 ± 5.43b | 2.35 ± 0.69b | 2.95 ± 0.17b |
| Diabetic + Evolvulus alsinoides | 22.98 ± 0.65bc | 2.18 ± 0.23b | 2.83 ± 0.74b |
| Evolvulus alsinoides alone | 29.03 ± 1.18d | 2.99 ± 0.11d | 3.02 ± 0.24b |
Values are expressed as mean ± SD for six animals in each group. Values not sharing common superscript letters (a–d) differ significantly at p < 0.05 (DMRT)
The units were expressed as μg/mg protein
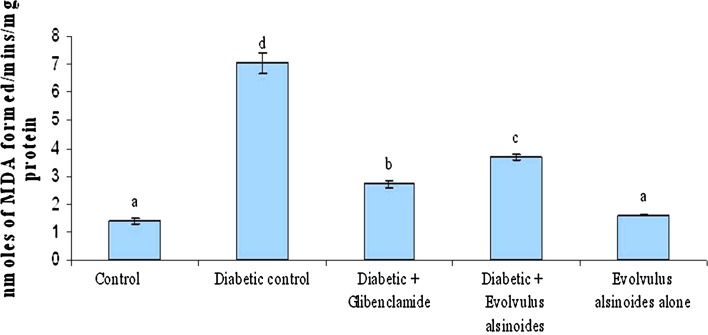
Lipid peroxidation is a characteristic of diabetes mellitus. Lipid peroxide-mediated tissue damage resulted in the development of both type I and II diabetes. In our study the increased levels of lipid peroxidation was found in diabetic control group. After treatment with plant extract the LPO level restored near to control in kidney (Fig. 1). Low levels of lipid peroxides stimulate the secretion of insulin, but when the concentration of endogenous peroxides increases, it may initiate uncontrolled lipid peroxidation, thus leading to cellular infiltration and islet cell damage in type I diabetes [35]. The most commonly used indicators of lipid peroxidation are TBARS products [36]. The increased lipid peroxidation in the tissues of diabetic animals may be due to the observed increase in the concentration of TBARS in the liver and kidney of diabetic rats [10, 37]. Our results showed that the levels of TBARS were high in kidney tissues of diabetic animals and which were restored to normal levels after treatment with E. alsinoides extract.
Fig. 1.
Effect of Evolvulus alsinoides on lipid peroxidation in kidney of control and experimental rats
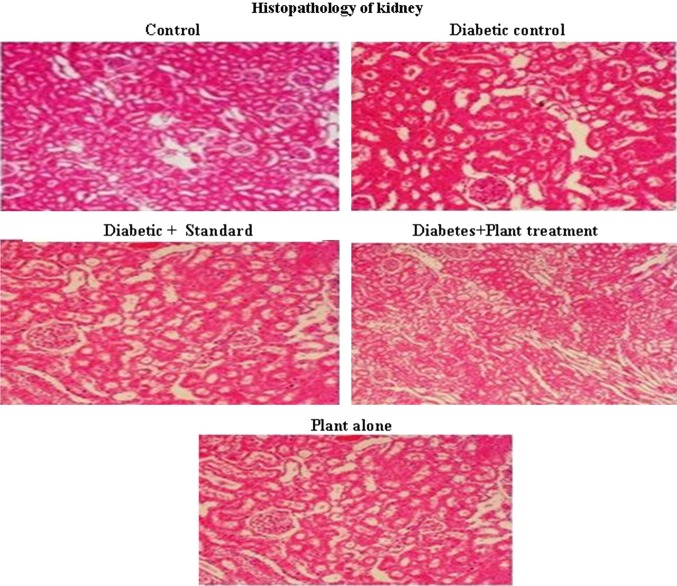
Figure 2 shows the histopathology of rat kidney. The Group II diabetic control rat shows more necrosis, when compared to Group I control (Fig. 2). But this is reversed by the administration of E. alsinoides in Group IV and III. Group V shows that the E. alsinoides did not show any toxicity to the rat kidney.
Fig. 2.
Histopathology of kidney
In conclusion, the results of the present study show that E. alsinoides brings back the antioxidant levels to normal in diabetes-induced rats and protection against tissue lipid peroxidation. In histology also the plant extract showed protective effect of the organ tissues. Future research to refine the extraction procedure of E. alsinoides could lead to improved pharmaceutical products.
Acknowledgments
We, the authors are thankful to our Chancellor, Advisor, Vice Chancellor and Registrar of Karpagam University for providing facilities and encouragement.
References
- 1.Kumar GPS, Arulselvan P, Kumar DS, Subramanian P. Antidiabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J Health Sci. 2006;52(3):283–291. doi: 10.1248/jhs.52.283. [DOI] [Google Scholar]
- 2.Krishnaveni M, Mirunalini S, Karthishwaran K, Dhamodharan G. Antidiabetic and antihyperlipidemic properties of Phyllanthus emblica Linn. (Euphorbiaceae) on streptozotocin induced diabetic rats. Pakistan J Nutrit. 2010;9(1):43–51. doi: 10.3923/pjn.2010.43.51. [DOI] [Google Scholar]
- 3.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 4.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 5.McLennan SV, Heffernan S, Wright L, Rae C, Fisher E, Yue DK, et al. Changes in hepatic glutathione metabolism in diabetes. Diabetes. 1991;40:344–348. doi: 10.2337/diab.40.3.344. [DOI] [PubMed] [Google Scholar]
- 6.Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990;173:932–938. doi: 10.1016/S0006-291X(05)80875-7. [DOI] [PubMed] [Google Scholar]
- 7.Strain JJ. Disturbances of micronutrient and antioxidant status in diabetes. Proc Nutr Soc. 1991;50:591–604. doi: 10.1079/PNS19910073. [DOI] [PubMed] [Google Scholar]
- 8.Soto C, Recoba R, Barron H, Alvarez C, Favari L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp Biochem Physiol C. 2003;136:205–212. doi: 10.1016/S1095-6433(03)00134-X. [DOI] [PubMed] [Google Scholar]
- 9.Szkudelski T. The mechanism of alloxan and streptozotocin action in beta-cells of the rat pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- 10.Chis IC, Ungureanu MI, Marton A, Simedrea R, Muresan A, Postescu ID, et al. Antioxidant effects of a grape seed extract in a rat model of diabetes mellitus. Diab Vasc Dis Res. 2009;6(3):200–204. doi: 10.1177/1479164109336692. [DOI] [PubMed] [Google Scholar]
- 11.Patil SB, Ghadyale VA, Taklikar SS, Kulkarni CR, Arvindekar AU. Insulin secretagogue, alpha-glucosidase and antioxidant activity of some selected spices in streptozotocin-induced diabetic rats. Plant Foods Hum Nutr. 2011;66:85–90. doi: 10.1007/s11130-011-0215-7. [DOI] [PubMed] [Google Scholar]
- 12.Buege JA, Aust SD. Microsomal lipid peroxidation. In: Fleischer S, Packre L, editors. Methods in enzymology. New York: Academic; 1978. pp. 302–330. [DOI] [PubMed] [Google Scholar]
- 13.Das K, Samanta L, Chainy GBN. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind J Biochem Biophys. 2000;37:201–204. [Google Scholar]
- 14.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 15.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione purification and assay. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 16.Beutler E. In: Grune, Stratton, editors. Red cell metabolism—a manual of biochemical methods. 3rd ed. New York: Academic; 1984. p. 67–73.
- 17.Balinsky D, Bernstein RE. The purification and properties of glucose-6-phosphate dehydrogenase from human erythrocytes. Biochem Biophys Acta. 1963;67:313–315. doi: 10.1016/0926-6569(63)90239-6. [DOI] [PubMed] [Google Scholar]
- 18.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 19.Omaye ST, Turbull TP, Sauberchich HC. Selected methods for determination of ascorbic acid in cells, tissues and fluids. Methods Enzymol. 1979;6:3–11. doi: 10.1016/0076-6879(79)62181-X. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg HR. Chemistry and physiology of the vitamins. Inter Science Publishers Inc.: New York; 1992. pp. 452–453. [Google Scholar]
- 21.Dunn WL. Handbook of histopathological and histochemical techniques. 3rd ed. Redwood: Trowbridge and Esher, 1974.
- 22.Nirmala A, Saroja S, Devi GG. Antidiabetic activity of Basella rubra and its relationship with the antioxidant property. Brit Biotechnol J. 2011;1(1):1–9. [Google Scholar]
- 23.Genet S, Kale RK, Baquer NZ. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek (Trigonella foenum graecum) Mol Cell Biochem. 2002;236:7–12. doi: 10.1023/A:1016103131408. [DOI] [PubMed] [Google Scholar]
- 24.Preet A, Gupta BL, Yadava PK, Baquer NZ. Efficacy of lower doses of vanadium in restoring altered glucose metabolism and antioxidant status in diabetic rat lenses. J Biosci. 2005;30:221–230. doi: 10.1007/BF02703702. [DOI] [PubMed] [Google Scholar]
- 25.El-Missiry MA, Othman AI, Amer MA. l-Arginine ameliorates oxidative stress in alloxan-induced experimental diabetes mellitus. J Applied Toxicol. 2004;24:93–97. doi: 10.1002/jat.952. [DOI] [PubMed] [Google Scholar]
- 26.Argano M, Brignardello E, Tamagno O, Boccuzzi G. Dehydroepiandrosterone administration prevents the oxidative damage induced by acute hyperglycemia in rats. J Endocrinol. 1997;155:233–240. doi: 10.1677/joe.0.1550233. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura N, Ookawara T, Suzuki K, Konishi K, Mino M, Taniguachi N. Increased glycated Cu, Zn-superoxide dismutase levels in erythrocytes of patients with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1992;74:1352. doi: 10.1210/jcem.74.6.1592880. [DOI] [PubMed] [Google Scholar]
- 28.Yan H, Harding JJ. Glycation induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem J. 1997;328:500. doi: 10.1042/bj3280599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgson EK, Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide inactivation of the enzyme. Biochem. 1975;14:5294–5298. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- 30.Pigeolet E, Corbisier P, Houbion A. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals. Mech Aging Dev. 1990;51:283–297. doi: 10.1016/0047-6374(90)90078-T. [DOI] [PubMed] [Google Scholar]
- 31.Sabu MC, Kuttan R. Antidiabetic and antioxidant activity of Terminalia belerica. Roxb Indian J Exp Biol. 1997;40:270–275. [PubMed] [Google Scholar]
- 32.Arias IM, Jakoby WB. Glutathione: metabolism and functions. New York: Raven Press; 1976. [Google Scholar]
- 33.Beter RE. The role of ascorbate in antioxidant protection of biomembrane: interaction with vitamin E and coenzyme Q. J Bioenerg Biomem. 1994;26:349–358. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- 34.Prince PSM, Menon VP. Antidiabetic activity of Tinospora cordifolia roots in experimental diabetes. J Ethnopharmacol. 1999;65:277–281. doi: 10.1016/S0378-8741(98)00164-0. [DOI] [PubMed] [Google Scholar]
- 35.Metz SA. Oxygenation products of arachidonic acid: third messengers for insulin release. Prostaglandins. 1984;27:147–151. doi: 10.1016/0090-6980(84)90228-4. [DOI] [PubMed] [Google Scholar]
- 36.Lyons TJ. Oxidized low-density lipoproteins, a role in the pathogenesis of atherosclerosis in diabetes. Diabet Med. 1991;8:411–419. doi: 10.1111/j.1464-5491.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 37.Stanely P, Prince M, Menon VP. Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytother Res. 2001;15:213–218. doi: 10.1002/ptr.707. [DOI] [PubMed] [Google Scholar]