Abstract
Cancer cells generally exhibit increased glycolysis for ATP generation (the Warburg effect). Compounds that inhibit glycolysis have potential applications in cancer treatment. dl-glyceraldehyde (DLG) and 2-Deoxyglucose (2-DG) have been proven effective in the inhibition of glucose metabolism. Ehrlich ascites carcinoma (EAC) cells were injected intraperitoneally (i.p) in 10–12 weeks old Swiss albino mice, weighing between 20 and 30 g. The anticancer activity of DLG and 2-DG were determined by tumor volume, tumor weight, viable and nonviable tumor cell count, average survival time, percentage increase in life span and tumor inhibition ratio. The blood samples were obtained for biochemical analysis after 9 days of treatment to study the effect on liver, kidney and haematological parameters. Histopathological examination of liver and kidney was also performed. One-way ANOVA test and Dunnett’s test were used for comparisons of parameters in study groups. Both DLG and 2-DG individually decreased the tumor weight, tumor volume, viable tumor cell count and significantly increased the life span of treated mice, however the combination was found to be better. The biochemical parameters of liver and kidney functions and haematological parameters were restored close to control group as compared with the EAC bearing mice. Histopathological examination of liver and kidney in EAC control group showed large areas of necrosis, congestion and mononuclear cell infiltration but such changes were not observed in liver and kidney sections observed after i.p injection of DLG and 2-DG for 9 days. Improvement was much better in the group where combination of these two drugs were used.
Keywords: dl-Glyceraldehyde, 2-Deoxyglucose, Ehrlich ascites carcinoma, Anticancer, Glycolysis
Introduction
Cancer cells in general have higher rates of glycolysis than normal cells. This property, initially observed by Cori [1] and Warburg [2], probably developed through adaptation to limited oxygen supply inside tumor mass due to poor vascularisation. Moreover, it has been suggested that increased aerobic glycolysis is required for increased synthetic activity, required for cell multiplication. This suggests that tumor cells consume more glucose, and therefore, are more vulnerable to low level glucose or its metabolism.
For the design and development of anticancer drugs it is important to understand the differences between cancer cells and normal cells in their biochemical metabolism.
As the metabolic alteration in glycolysis is frequently seen in cancer cells of various tissue origins, targeting the glycolytic pathway may preferentially kill the malignant cells and likely to have broad therapeutic implications.
In 1929 Mendel discovered that dl-Glyceraldehyde (DLG) in concentrations of 10−3 M almost completely inhibited the anaerobic formation of lactic acid from glucose in rat sarcoma [3]. Glyceraldehyde, even in much higher concentrations, did not appreciably affect the respiration of normal liver, kidney and brain. The inhibition of glycolysis by glyceraldehyde was demonstrated by Ashford [4] and Holmes [5] in brain tissue and by Needham and Nowinski [6] in chick embryos.
It has been suggested that l-glyceraldehyde condenses with dihydroxyacetone phosphate to form l-sorbose-1-phosphate which inhibits hexokinase inhibiting glycolysis [7]. The reduction in rate of glycolysis could also be due to decreased levels of fructose-2,6-phosphate by DLG [8]. Another mechanism for anticancer effect could be inhibition of protein synthesis by DLG, as demonstrated by reduced incorporation of labelled amino acids into proteins.
The glucose antimetabolite, 2-DG, a competitive inhibitor of glucose transport, inhibits glucose uptake [9] and glucose phosphorylation by hexokinase and selectively inhibits glycolytic energy (ATP) production [10] and also energy-dependent DNA repair and cellular recovery processes [11].
Additionally it may inhibit the tumour growth by inhibiting the glycolysis at the phospho glucoisomerase level [12] or be incorporated into glycoprotein components, resulting in cell surface changes and growth modulation.
Here we report the combined effect of the two antiglycolytic agents DLG and 2-DG as anticancer drugs and their effect on liver, kidney and haematological parameters.
Materials and Methods
The study design chosen was case control study. Healthy 10–12 weeks old Swiss albino mice of either sex, weighing between 20 and 30 g were used as experimental animals. They were kept under standardized animal house conditions (12 h light and 12 h dark condition) in clean polypropylene cages at controlled temperature (22 ± 1 °C) and relative humidity (60–70 %) in institutional registered animal house which was approved by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). They were maintained with standard pellet diet and water ad libitum. The experimental protocol was approved by the institutional animal ethics committee. CPCSEA guidelines were adhered to during the maintenance of animals and experiments.
Procurement and Maintenance of EAC Cells
Ehrlich ascites carcinoma (EAC) cells were procured from Amala Cancer Research Centre, Thrissur, Kerala, India.
The EAC cell line was maintained in vivo in Swiss albino mice by serial intraperitoneal passage at 7–10 day intervals as described by Geran et al. [13].
Induction of EAC in Mice
Ascites developed within 7–8 days after EAC cells inoculation. The ascitic fluid was obtained under aseptic conditions from these mice and washed three times with normal saline by centrifugation at 1,000 rpm. EAC cells after washing were tested for viability using trypan blue. The cells were examined microscopically using a haemocytometer and suspended in normal saline containing 2 × 106 cells/0.1 mL.
Sixty overnight fasted Swiss-albino mice were injected intraperitoneally with 0.1 mL cell suspension containing 2 × 106 cells on day zero. The next day, these mice were randomized and divided into four groups (15 mice in each group) and one group of 15 mice with same age and weight was kept under same conditions as normal control without EAC cells.
Group I and II were normal and EAC control respectively. Group III, IV and V received intraperitoneally DLG (0.5 g/kg), 2-DG (2 g/kg) and a combination of these two drugs or vehicle phosphate buffer-saline (PBS) according to body weight as follows:
Treatments given were as follows:
- Group I
Normal control; mice without EAC cells on day zero and 0.1 mL PBS from day 1 to 9
- Group II
EAC control; EAC cells on day zero and 0.1 mL PBS from day 1 to 9
- Group III
EAC cells on day zero and DLG from day 1 to 9
- Group IV
EAC cells on day zero and 2-DG from day 1 to 9
- Group V
EAC cells on day zero and both DLG and 2-DG from day 1 to 9
On 10th day, 10 mice from each group were sacrificed to obtain the blood sample for analysis of haematological and biochemical parameters and liver, kidney for histopathological examination. Out of the 10 sacrificed mice in each group, blood samples were collected from two mice in equal amount and were pooled to obtain five samples in each group to get the sufficient quantity of serum for analysis. Ascitic fluid was also obtained for the estimation of anticancer effect by determining the tumor volume, tumor weight, viable and non viable cell count while remaining five mice from each group were studied for survival.
Selection of Optimum Dose
2-Deoxyglucose
The optimum dose for 2-DG was selected as 2 g/kg per mice after reviewing the literature [14, 15]. It was dissolved in PBS so that total volume of PBS injected intraperitoneally per mice was ≤0.2 mL to avoid volume overload.
dl-Glyceraldehyde
LD50 for DLG in mice is reported to be 3.0 g/kg of body weight [16]. The optimum dose of DLG was selected as 0.5 g/kg (1/6th of LD50 per mice). It was given by dissolving in PBS such that the total volume of PBS injected intraperitoneally per mice per day was ≤0.2 mL to avoid volume overload.
Determination of Tumor Weight
The mice were dissected and the ascitic fluid was collected from the peritoneal cavity. The tumor weight was measured by taking the weight of the mice before and after the collection of ascitic fluid from the peritoneal cavity [17].
Tumor inhibition ratio (T/C): it was calculated by the formula [18].
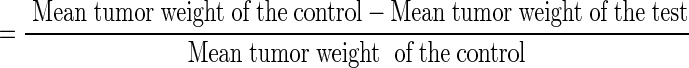 |
Estimation of Viable and Non-viable Tumor Cell Count
The viability and non viability of the cells was checked by trypan blue assay. The ascitic fluid was taken in a WBC pipette and diluted 100 times with phosphate buffer saline (PBS).Then a drop of the diluted suspension was placed on the Neubauer counting chamber and the cells were stained with trypan blue (0.4 % in normal saline). The cells that did not take up the dye were viable and those that took the stain were non viable. These viable and non viable cells were counted [17].
 |
Evaluation of Survival and Average Survival Time
After 9 days of administration of drugs, five mice were randomly taken from each group to observe their survival. These animals were observed for their mortality daily until their death or up to a maximum of 75 days.
The mice of all the experimental groups were monitored regularly for alteration in body weight, signs of toxicity and mortality.
The tumor response was assessed on the basis of average survival time (AvST). The AvST was calculated from the animals dying within 75 days from start of therapy. The AvST was calculated as follows [17]:
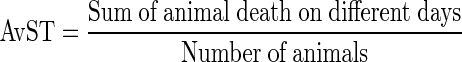 |
Percentage Increase in Average Life Span (% IALS)
The (% IALS) was also calculated using the following formulae [19]:
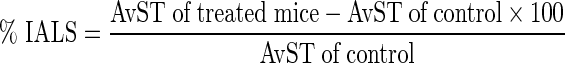 |
Determination of Haemoglobin and Haematological Parameters
Blood was collected from retro–orbital plexus with the help of a capillary in sterile eppendrof tube on 10th day after sacrificing the animals and haemoglobin (Hb) was determined by Cyanmethemoglobin method. RBC count, total leucocyte count (TLC) and differential leucocyte count (DLC) were determined by standard procedures.
Biochemical Analysis of Blood Sample
Glucose, urea, creatinine, total bilirubin, ALT, AST, total protein and albumin was estimated by kinetic measurements using Erba Diagnostics Mannheim GmbH kits manufactured by Transasia Biomedicals Limited.
Statistical Analysis
Statistical Analysis was performed using Graph pad Instat® ver 3.10, 32 bit for windows was used for statistical analyses. All data are expressed in mean ± 2 standard deviation (SD) showing 95% confidence intervals. Thereafter, One-way ANOVA was used for parameters with normal distribution inter-group comparisons. Dunnett’s test was used as a post test for comparisons with control group and obtaining p value for detecting the group/s responsible for the difference. The results were evaluated at a significance level of p < 0.05 and 0.001 which were considered significant and highly significant, respectively.
Observations and Results
Effect of dl-Glyceraldehyde and 2-Deoxyglucose on Serum Glucose and Kidney Functions
The mean serum glucose decreased to 60 mg/dL in the EAC control (group II) as compared to the normal control which had serum glucose of 119 mg/dL. The mean serum glucose was 66 and 76 mg/dL in mice treated with DLG and 2-DG, respectively. Treatment with both these drugs together increased the mean serum glucose to 130 mg/dL, a level close to the normal control group.
EAC control (group II) showed significant increase in the serum urea and creatinine level as compared to the levels in normal control group, in the three treatment groups the values decreased, which are not significantly different from the normal control group (Table 1).
Table 1.
Effect of dl-glyceraldehyde and 2-deoxyglucose on serum urea, creatinine and glucose (mean ± 2 SD) in EAC induced mice (n = 5; each consisting of serum samples pooled from two mice of the same group)
| Parameter (mg/dL) | Group I (control) | Group II (EAC) | Group III (EAC + DLG) | Group IV (EAC + 2-DG) | Group V (EAC + DLG and 2-DG) |
|---|---|---|---|---|---|
| Urea | 45 ± 4 | 68 ± 8b | 47 ± 8ns | 49 ± 11ns | 51 ± 7ns |
| Creatinine | 0.3 ± 0.2 | 0.8 ± 0.2b | 0.3 ± 0.2ns | 0.5 ± 0.1ns | 0.3 ± 0.2ns |
| Glucose | 119 ± 7.5 | 60 ± 7.3b | 66 ± 9.2b | 76 ± 16.6b | 130 ± 38.9ns |
Statistically significant a (p < 0.001), b (p < 0.01), c (p < 0.05) and ns non significant when compared with control group i.e. group I
Effect of dl-Glyceraldehyde and 2-Deoxyglucose on Liver Functions
EAC inoculation in mice significantly increased the levels of serum aspartate transaminase (AST) and serum alanine transaminase (ALT) and bilirubin, whereas levels of the serum total protein and albumin decreased significantly as compared to their levels in control group, suggesting compromised liver function.
The values of ALT, AST, bilirubin, total protein and albumin tend to return to normal in all the three treatment groups, being not significantly different from controls, except ALT which showed an increase in mice treated with 2-DG alone (Table 2).
Table 2.
Effect of dl-glyceraldehyde and 2-deoxyglucose on liver function tests (mean ± 2 SD) of EAC induced Swiss albino mice (n = 5; each consisting of serum samples pooled from two mice of the same group)
| Parameter | Group I (control) | Group II (EAC) | Group III (EAC + DLG) | Group IV (EAC + 2-DG) | Group V (EAC + DLG and 2-DG) |
|---|---|---|---|---|---|
| Total protein (g/dL) | 6.0 ± 0.5 | 4.1 ± 0.5b | 6.0 ± 0.9ns | 5.4 ± 0.7ns | 5.6 ± 0.5ns |
| Albumin (g/dL) | 3.7 ± 0.1 | 2.1 ± 0.2a | 2.9 ± 0.5b | 2.7 ± 0.4a | 2.9 ± 0.2b |
| Total bilirubin (mg/dL) | 0.5 ± 0.2 | 0.8 ± 0.1ns | 0.8 ± 0.4ns | 0.3 ± 0.1ns | 0.7 ± 0.3ns |
| AST (SGOT) (IU/L) | 81 ± 8 | 239 ± 57b | 183 ± 90ns | 436 ± 69a | 162 ± 40ns |
| ALT (SGPT) (IU/L) | 41 ± 14 | 75 ± 10b | 47 ± 9ns | 48 ± 14ns | 53 ± 8ns |
Statistically significant a (p < 0.001), b (p < 0.01), c (p < 0.05) and ns non significant when compared with control group i.e. group I
Effect of dl-Glyceraldehyde and 2-Deoxyglucose on Haematological Parameters
Hematological parameters of EAC tumor bearing mice (group II) on 10th day were found to be significantly altered as compared to control. The total WBC cell count was increased with a reduction in the RBC count and haemoglobin. The differential count showed that the percentage of neutrophils was increased while that of lymphocytes was decreased. Group III, IV, V mice treated with DLG, 2-DG and combination of DLG and 2-DG, showed improvement in hemoglobin, RBC count, total WBC count as compared to EAC group (Table 3). The DLG group had values very close to control group for haemoglobin and RBC count.
Table 3.
Effect of dl-glyceraldehyde and 2-deoxyglucose on hematological parameters (mean ± 2 SD) of EAC induced Swiss albino mice (n = 5; each consisting of blood samples pooled from two mice of the same group)
| Parameter | Group I (control) | Group II (EAC) | Group III (EAC + DLG) | Group IV (EAC + 2-DG) | Group V (EAC + DLG and 2-DG) |
|---|---|---|---|---|---|
| Haemoglobin (g/dL) | 13.9 ± 0.6 | 8.6 ± 0.6a | 13.6 ± 1.2ns | 11.2 ± 1.3c | 11.9 ± 1.8ns |
| RBC count (cells × 106/mm3) | 5.2 ± 0.8 | 3.1 ± 0.4b | 4.9 ± 0.6ns | 4.5 ± 0.4ns | 4.7 ± 0.6ns |
| Total WBC count (cells × 103/mm3) | 7.2 ± 0.6 | 19.2 ± 2.6b | 10.0 ± 1.3c | 10.7 ± 1.1b | 9.8 ± 1.4ns |
| Lymphocytes (%) | 71 ± 5 | 39 ± 6b | 51 ± 5b | 50 ± 5b | 58 ± 7b |
| Neutrophiles (%) | 30 ± 2 | 59 ± 6b | 48 ± 5b | 49 ± 4b | 41 ± 8c |
| Monocytes (%) | 1.5 ± 0.5 | 2.5 ± 0.5a | 1.5 ± 0.5ns | 1.2 ± 0.4ns | 1.3 ± 0.5ns |
Statistically significant a (p < 0.001), b (p < 0.01), c (p < 0.05) and nsnon significant when compared with control group i.e. group I
Histopathological Findings
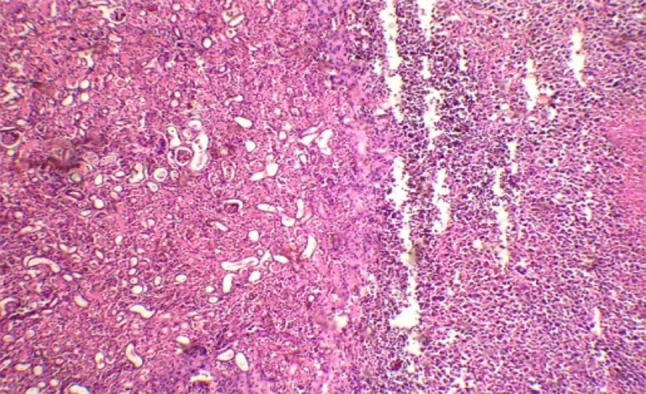
Histopathological examination of liver in group II at the end of the study revealed severe necrosis and mononuclear cell infiltration, portal triaditis and sinusoidal congestion as shown in Fig. 1.
Fig. 1.
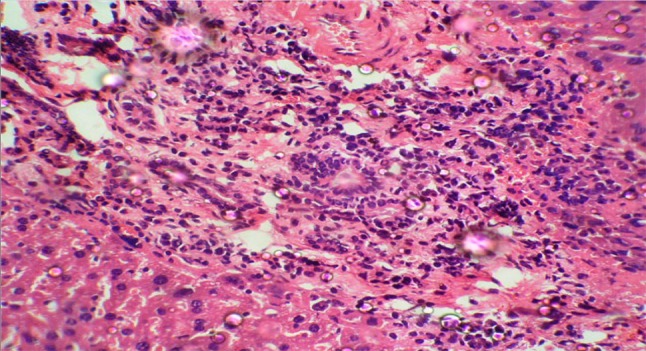
Histopathological section of liver (40×) in EAC control mice (group II)
Histopathological study of liver in group III mice showed sinusoidal congestion and mononuclear cell infiltration but severity was less as compared to EAC group. There was no necrosis, EAC invasion or metastatic foci inside the liver whereas in group IV there was moderate vacuolation, mild sinusoidal congestion and mononuclear cell infiltration, but severity was less as compared to EAC group. In group V (DLG + DG) there was no necrosis, EAC invasion or metastatic foci inside the liver. There were very few areas of congestion and mononuclear cell infiltration (Fig. 2), but no EAC invasion or necrosis as compared to EAC group (Fig. 1). The liver section appeared similar to the control group.
Fig. 2.
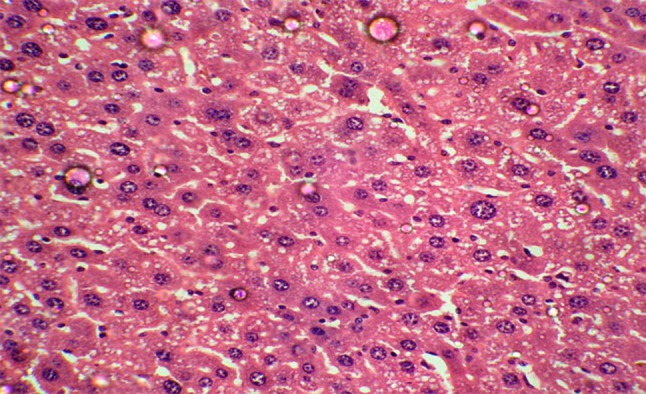
Histopathological section of liver (40×) in DLG and 2-DG treated mice (group V)
Histopathological study of kidney section in group II showed severe congestion, necrosis and mononuclear cell infiltration (Fig. 3). Tumor was seen adherent to the capsule of kidney but there was no evidence of invasion into parenchyma.
Fig. 3.
Histopathological section of kidney (40×) in EAC control mice (group II)
Group III mice showed minimal congestion, no necrosis or mononuclear cells infiltration inside the glomeruli and renal tubules. There was no invasion of EAC cells or metastatic foci in kidney section, whereas in group IV there was minimal congestion, no necrosis or mononuclear cells infiltration inside the glomeruli and renal tubules. Also, there was no EAC cell invasion as seen in group II. The kidney in group V had no congestion, necrosis or infiltration by EAC cells or mononuclear cells inside the glomeruli and renal tubules as illustrated in Fig. 4, which was undistinguishable from the control group.
Fig. 4.
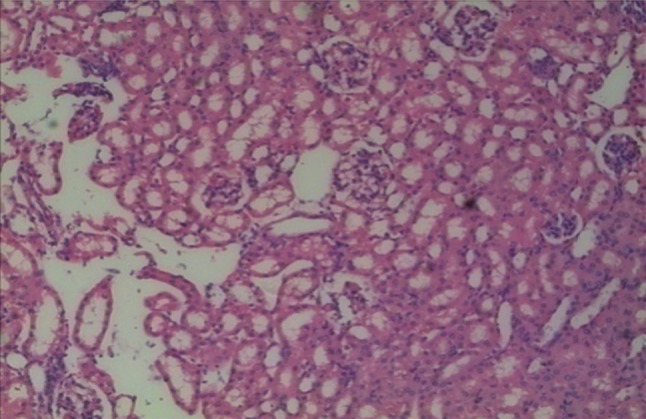
Histopathological section of Kidney (40×) in DLG and 2 DG treated mice (group V)
Anticancer Effect of dl-Glyceraldehyde and 2-Deoxyglucose
The tumor weight and viable cell count decreased significantly in all the three groups. Mean tumor weight was 5.10 g in the EAC group while it was 3.47 g and 3.99 g in mice treated with DLG and 2-DG, respectively. Treatment with both these drugs together decreased the tumor weight to 2.36 g. This was associated with a mean tumor inhibition ratio of 0.32, 0.22 and 0.54 for the group treated with 2-DG, DLG and both 2-DG and DLG together, respectively. Viable cell count decreased with increase in non-viable cell count along with per cent increase in life span and average survival time on treatment in all the groups, most significant change being in group on combination of DLG and 2-DG (Table 4).
Table 4.
Effect of dl-glyceraldehyde and 2-deoxyglucose on tumor weight, viable and non viable cell count, tumor inhibition ratio, average survival time (mean ± 2 SD) and percentage increase in life span
| Treatment group | Tumour weight (g) | Tumour inhibition ratio | Viable tumour cell count (107 cells/mL) | Non-viable tumour cell count (107 cells/mL) | Average survival time (d) | Increase in life spanb (%) |
|---|---|---|---|---|---|---|
| Group II (EAC) | 5.10 ± 0.46 | – | 9.97 ± 0.92 | 1.04 ± 0.35 | 25.4 ± 2.40 | – |
| Group III (EAC + DLG) | 3.47 ± 0.25a | 0.32a | 5.18 ± 0.25a | 2.37 ± 0.22a | 49.6 ± 8.80a | 95.30 |
| Group IV (EAC + 2-DG) | 3.99 ± 0.22a | 0.22a | 6.35 ± 0.33a | 1.61 ± 0.17a | 40.2 ± 9.00a | 58.30 |
| Group V (EAC + DLG + 2-DG) | 2.36 ± 0.20* | 0.54a | 4.31 ± 0.2a | 3.50 ± 0.25a | 62.4 ± 7.70a | 145.70 |
Results are mean ± SD; n = 10
aStatistically significant with p < 0.01, and when EAC control (group II) compared with the treated group
bPercentage increase over group II
In mice which were followed up for survival, we observed that certain mice in 2-DG group had developed a subcutaneous mass at the site of injection, probably due to some EAC cells getting lodged subcutaneously during i.p. inoculation. This was not observed in any mice in EAC group, DLG or DLG + 2-DG group, as the mice did not survive long enough to form a subcutaneous mass in EAC group while it seems that DLG in the treatment groups inhibited the formation of the subcutaneous mass.
Discussion
Anticancer effect of DLG and 2-deoxyglucose its effect on glycolysis: we provide the evidence supporting the potential ability of DLG and 2-DG to elicit antitumor effect. Glucose analogues, such as 2-DG and DLG, have been found to profoundly inhibit glucose metabolism in cancer cells in vivo as well as in vitro. 2-DG has been used in different experimental tumour systems, and retardation of local tumor growth has been found [20]. In experimental settings by Kern and Norton [21] in 1987, 2-DG treatment inhibited growth of rat fibrosarcoma, hepatocellular carcinoma, and other carcinoma cells by itself or with other antitumor protocols.
In this study, we observed that there was no spontaneous regression of tumor in EAC transplanted mice group. The tumor bearing mice showed an increase in the tumor volume due to cell multiplication and growth of EAC. There was statistically significant difference between the tumour weights, Average increase in life span between the EAC induced mice and the group treated with DLG, 2-DG and combination of these drugs. However, when both these drugs were given together, the anticancer effect was potentiated as illustrated by percentage increase in average life span and tumor inhibition ratio. The action seems to be synergistic between 2-DG and DLG and therefore each potentiates the effect of other. In addition in groups administered DLG there no subcutaneous mass formation was observed.
Effect of dl-Glyceraldehyde and 2-Deoxyglucose on Serum Glucose, Liver and Kidney Functions
In a study by Shapot VS [22] it was reported that mice with EAC developed severe hypoglycemia that increased with tumour growth. However, in another study it was reported that significant rises in plasma glucose invariably follow 2-DG infusion and sometimes persist for 6–8 h [23]. The protective effect of glucose on 2-DG toxicity, as well as the failure of insulin to protect against 2-DG toxicity, has also been observed in mice [24].
The present study showed that the serum glucose was low in group II, III and IV whereas in group V in which a combination of both the drugs was given, it was restored to approximately similar values as in normal control (group I). This shows that the combined therapy with these two drugs counteracts the effect of the other drug restoring the serum glucose to normal levels as compared to both these drugs given separately; probably they act at two different points and counter act the effect of another agent, this needs to be further studied.
Total bilirubin, total protein, albumin and ALT enzyme were not significantly raised in group III, IV, V as compared to normal control group which can be explained by prevention of tumour growth after administration of DLG, 2-DG and its combination. The metastasis of cancer cell to liver is also inhibited.
Biochemical parameters such as serum urea and creatinine were significantly deranged in EAC group, whereas these were not significantly raised in group III, IV, V in comparison to normal control group.
Effect of dl-Glyceraldehyde and 2-Deoxyglucose on Haematological Parameters
In tumor-bearing mice, due to the diminution of haemoglobin anaemia occurs, and this may be either due to iron deficiency or due to haemolytic or myelopathic conditions [25].
In this study, the haemoglobin was found to be reduced to 8.6 g% in the EAC group as compared to 13.9 g% in normal control. The haemoglobin in mice on treatment with both these drugs together was 11.9 g%. Administration of DLG and 2-DG by restoring haemoglobin content towards normal in EAC induced mice exhibit their protective role on haematopoietic system.
Conclusion
There was no spontaneous regression of tumour in EAC transplanted mice group. The tumour bearing mice showed a constant weight gain and increase in the tumour volume due to cell multiplication and growth of EAC.
Analyses of the data show that hematological parameters of EAC tumour bearing mice (group II) on 10th day were found to be significantly altered in the form of increased WBC count with a reduction in the RBC count and hemoglobin content as compared to normal control group. Group III, IV, V showed improvement in hemoglobin, RBC count and total WBC count as compared to EAC group.
Similarly, deranged biochemical parameters of liver and renal function tests were observed in EAC group. Histopathological examination of liver and kidney sections in EAC group showed large areas of necrosis, congestion and mononuclear cell infiltration. But such effects on biochemical parameters of liver and renal function tests and histopathological examination were not observed after intraperitoneal (i.p) injection for 9 days of DLG and 2-DG.
Anaerobic glycolysis is an important step in the growth of tumour and DLG by exerting its effect through formation of l-sorbose-1-Phosphate which inhibits Hexokinase or by lowering the levels of Fructose-2,6-bisphosphate and reduced allosteric effect on PFK or by an unidentified mechanism inhibit it.
2-DG inhibits glucose uptake, impairs glycolysis at the phosphoglucoisomerase level and is also known to be incorporated into glycoprotein components, resulting in cell surface changes.
Thus, both these drugs through their inhibitory effect on glycolysis, hampering the main energy providing pathway and other mechanisms exhibit anticancer effect. Combination therapy is found to yield the best anticancer effect and least side effects than given separately. The average survival time may increase further if the treatment cycle is repeated as in the present case only one cycle of 9 days therapy was given. However, more studies with larger sample size are required before these observations can be generalized.
Acknowledgments
Conflict of interest
The study did not receive any specific grant from any funding agency in public, commercial or not-for-profit sector. The authors have no conflict of interest.
References
- 1.Cori CF. The carbohydrate metabolism of tumors: II. changes in the sugar, lactic acid, and CO2-combining power of blood passing through a tumor. J Biol Chem. 1925;65:397–405. [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. [DOI] [PubMed]
- 3.Mendel B, Strelitz F, Mundell D. 1-Glyceric aldehyde and tumor metabolism. Science. 1938;88(2276):149–150. doi: 10.1126/science.88.2276.149. [DOI] [PubMed] [Google Scholar]
- 4.Ashford CA. Effects of hydroxymalonate on the metabolism of brain. Biochem J. 1931;28:2229. [Google Scholar]
- 5.Holmes EG. The biochemistry of malignant tissue. Ann Rev Biochem. 1934;3:381. doi: 10.1146/annurev.bi.03.070134.002121. [DOI] [Google Scholar]
- 6.Needham J, Nowiński WW. Intermediary carbohydrate metabolism in embryonic life: general aspects of anaerobic glucolysis. Biochem J. 1937;31(7):1165–1184. doi: 10.1042/bj0311165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lardy HA, Wiebelhaus VD, Mann KM. The mechanism by which glyceraldehyde inhibits glycolysis. J Biol Chem. 1950;187:325–327. [PubMed] [Google Scholar]
- 8.Loiseau AM, Rousseau GG, Hue L. Fructose 2,6-bisphosphate and the control of glycolysis by glucocorticoids and by other agents in rat hepatoma cells. Cancer Res. 1985;45(9):4263–4269. [PubMed] [Google Scholar]
- 9.Woodward GE, Hudson MT. The effect of 2-deoxy-d-glucose on glucolysis and respiration of tumor and normal tissues. Cancer Res. 1954;14:599–605. [PubMed] [Google Scholar]
- 10.Dwarkanath BS, Jain VK. Energy linked modifications of the radiation response in a human cerebral glioma cell line. Int J Radiat Oncol Biol Phys. 1989;17:1033–1040. doi: 10.1016/0360-3016(89)90152-1. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Farooque A, Adhikari JS, Singh S, Dwarkanath BS. Enhancement of radiation and chemotherapeutic drug responses by 2-deoxy-d-glucose in animal tumors. J Can Res Ther. 2009;5:16–20. doi: 10.4103/0973-1482.55135. [DOI] [PubMed] [Google Scholar]
- 12.Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957;224:963–969. [PubMed] [Google Scholar]
- 13.Geran RT, Greenberg NH, McDonald MM, Schumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer chemother Rep. 1972;3:1–7. [Google Scholar]
- 14.Gupta S, Mathur R, Dwarakanath BS. The glycolytic inhibitor 2-deoxy-d-glucose enhances the efficacy of etoposide in ehrlich ascites tumor-bearing mice. Cancer Biol Ther. 2005;4(1):87–94. doi: 10.4161/cbt.4.1.1381. [DOI] [PubMed] [Google Scholar]
- 15.Karczmar GS, Arbeit JM, Toy BJ, Speder A, Weiner MW. Selective depletion of tumor ATP by 2-deoxyglucose and insulin, detected by 31P magnetic resonance spectroscopy. Cancer Res. 1992;52:71–76. [PubMed] [Google Scholar]
- 16.Eng CP, Bhatnagar MK, Morgan JF. Inhibition of mouse ascites tumors by carbohydrate combined with immunization. Can J Physiol Pharmacol. 1972;50:156–163. doi: 10.1139/y72-022. [DOI] [PubMed] [Google Scholar]
- 17.Kundusen S, Gupta M, Mazumder UK, Haldar PK, Saha P, Bala A. Antitumor activity of Citrus maxima (Burm.) Merr. Leaves in Ehrlich’s Ascites Carcinoma cell treated Mice. ISRN Pharmacol. 2011:138737. [DOI] [PMC free article] [PubMed]
- 18.Bissery MC, Guenard D, Gueritte-Vogellien F. Lavelle F experimental anti-tumor activity of taxotera, a total analog. Cancer Res. 1991;51:4845–4850. [PubMed] [Google Scholar]
- 19.Jagetia GC, Rao SK. Evaluation of the antineoplastic activity of guduchi (tinospora cordifolia) in ehrlich ascites carcinoma bearing mice. Biol Pharm Bull. 2006;29(3):460–466. doi: 10.1248/bpb.29.460. [DOI] [PubMed] [Google Scholar]
- 20.Laszlo J, Humphreys SR, Goldin A. Effects of glucose analogues (2-deoxy-d-glucose, 2-deoxy-o-galactose) on experimental tumours. J Nati Cancer Inst. 1960;24:267–281. [PubMed] [Google Scholar]
- 21.Kern KA, Norton JA. Inhibition of established rat fibrosarcoma growth by the glucose antagonist 2-deoxy-d-glucose. Surgery. 1987;102:380–385. [PubMed] [Google Scholar]
- 22.Shapot VS, Blinov VA. Blood glucose levels and gluconeogenesis in animals bearing transplantable tumors. Cancer Res. 1974;34(8):1827–1832. [PubMed] [Google Scholar]
- 23.Bearn AG, Billing BH, Sherlock S. The response of the liver to insulin in normal subjects and in diabetes mellitus: hepatic vein catheterisation studies. Clin Sci. 1952;11:151. [PubMed] [Google Scholar]
- 24.Laszlo J, Humphreys SR, Goldin A. Effects of glucose analogues (2-deoxy-d-glucose, 2-deoxy- D-galactose) on experimental tumors. J nat Cancer Inst. 1960;24:267. [PubMed] [Google Scholar]
- 25.Hoagland HC. Haematologic complications of cancer chemotherapy. Semin Oncol. 1982;9(1):95–102. [PubMed] [Google Scholar]


