Abstract
Reference intervals (RIs) of serum thyroid stimulating hormone (TSH) and free thyroxine (fT4) were determined in 402 healthy pregnant women by enzyme-linked immunosorbent assay (ELISA) technique after partitioning them into three trimesters. The reference population was chosen from a study population of 610 pregnant females by applying strict inclusion and exclusion criteria. The assays were done using proper quality control measures. RIs were calculated from the central 95 % of the distribution of TSH and fT4 values located between the lower reference limit of 2.5 percentile and upper reference limit of 97.5 percentile value 0.90 confidence intervals for the upper and lower reference limits were also determined. The reference intervals for TSH were 0.25–3.35 μIU/ml for the first trimester; 0.78–4.96 μIU/ml for the second trimester and 0.89–4.6 μIU/ml for the third trimester. Similarly, the reference intervals for fT4 for first, second and third trimesters were 0.64–2.0, 0.53–2.12 and 0.64–1.98 ng/dl respectively. The values thus obtained varied from those provided by the kit literature. In comparison to our derived reference intervals, the reference data from kit manufacturer under-diagnosed both subclinical hypo- and hyper-thyroidism within our pregnant reference population.
Electronic supplementary material
The online version of this article (doi:10.1007/s12291-013-0332-1) contains supplementary material, which is available to authorized users.
Keywords: Reference interval, Reference range, Thyroid hormones, Trimester-specific, Pregnancy, ELISA
Introduction
Proper maternal thyroid function during pregnancy is vital for the health of the mother as well as the developing child. It also plays a critical role in the neonatal and child neuro-development [1]. Any thyroid disorder during gestation, whether hypo- or hyperthyroidism, may lead to varied obstetric complications with or without irreversible damage to the foetus [2, 3]. So, all pregnant women should ideally be screened with a valid biomarker of thyroid function having a distinct reference range. To ensure 100 % optimal maternal health it is also important to understand the maternal thyroid physiology during pregnancy. During gestation a number of hormonal and metabolic alterations occur, namely, rise in thyroxine binding globulins (TBG), surge of human chorionic gonadotropin (βHCG), altered iodide metabolism, haemodilution etc. At different trimesters a different set of factors predominate. These factors may act either synergistically or antagonistically to influence the thyroid hormone levels in blood [4]. So, the reference intervals (RI) for thyroid hormones in pregnant population naturally may not be the same as that provided by the kit literature. This may lead to improper assessment of thyroid function during pregnancy.
A reference interval, according to IS015189, is the interval between, and including, 2 reference limits. Most frequently the central 95 % of the distribution located between the 2.5 and 97.5 percentiles are used for determining RI of a parameter [5–7]. For a valid comparison of an observed value with the reference, the subject must sufficiently resemble the reference individual in all respects [8]. As the physiology of thyroid hormones in a pregnant woman differs from one trimester to the other, trimester-specific reference intervals provide the best practical framework for clinical decision making regarding thyroid functions in pregnancy. This also ensures the adequacy of thyroid hormones throughout pregnancy [9].
There are reports of a few studies done worldwide on this subject. But these results cannot be extrapolated due to differences in ethnicity, iodine intake status, method of immunometric assay and statistical methodology used to derive the RIs in individual studies. Thus, a population based, trimester-specific RI is essential in everyday clinical practice for the correct interpretation of thyroid hormone values and accurate classification of thyroid disorders in pregnancy.
With this idea, the purpose of our study was to determine trimester-specific reference intervals of serum thyroid stimulating hormone (TSH) and free thyroxine (fT4) in pregnant women of Indian origin at North Kolkata.
Materials and Methods
This study was conducted in the Department of Biochemistry in collaboration with the Antenatal Clinic of Department of Gynaecology and Obstetrics, R.G.Kar Medical College and Hospital, Kolkata from February 2012 to July 2012. It is a cross-sectional, descriptive and observational study in the reference population of pregnant women. Approval from the institutional Ethical Committee was obtained prior to undertaking the study.
Data was collected on the first and third Thursday of each month for a period of 6 months. 610 healthy pregnant women with history of consumption of iodised salt and having uncomplicated single intrauterine gestation were consecutively recruited. The gestational age was determined by last menstrual period (LMP) and ultrasonography report. Participants were interviewed, detailed history was taken and the case record form was filled. After an initial general survey, systemic examination was done. Also the records of their regular antenatal check up were noted. The women were properly counselled and provided their written informed consent.
From the study population the reference sample group was designed by applying the following exclusion criteria:-
History of thyroid dysfunction (goitre, hyper or hypothyroidism, thyroid carcinoma).
Signs and symptoms of thyroid disorder like neck swelling, exophthalmos, hand tremors, tachycardia, palpitations cold sensitivity etc.
Past or present history of intake of anti-thyroid drugs.
Family history of thyroid illness.
Women suffering from chronic medical disorder like hypertension, diabetes mellitus, asthma, inflammatory bowel disease, tumours, renal disease and other autoimmune diseases.
Past history of spontaneous abortion.
History of multiple or complicated pregnancy (hyperemesis, gestational diabetes, hypertension, perinatal infection or stillbirths).
Regular use of any medication other than iron and folate.
History of blood borne diseases like HIV, hepatitis B or C, syphilis.
8 ml of fasting venous blood was collected from each participant, serum separated and stored at −20 °C. Analysis for fasting plasma glucose, serum liver function tests, serum renal function tests and serum lipid profile were done. Also serum antithyroperoxidase antibody (Anti-TPO) was measured using commercially available Enzyme-linked immunosorbent assay (ELISA) kits. On the basis of biochemical analysis, some population of pregnant women were excluded:
-
10.
Elevated serum Anti-TPO (>40 IU/ml according to kit literature) [10].
-
11.
Fasting plasma glucose >140 mg/dl on 2 occasions.
-
12.
Impaired renal function tests, liver function tests and lipid profile.
The remaining study population (402) constituted the reference sample group. The reference sample group was then partitioned into 3 trimesters of pregnancy according to gestational age -First trimester: <13 weeks (125), Second: 13–27 weeks (151), and Third: >28 weeks (126).
Laboratory Hormonal Analysis
TSH, fT4 and Anti-TPO antibody assays were done on commercially available ELISA kit namely Accubind (Monobind Inc, USA).TSH was estimated by Immunoenzymometric method type 3 [11, 12] while fT4 with competitive enzyme immunoassay, analog method [13–15] sequential ELISA method was used to estimate anti-TPO antibody [16]. The analytical sensitivity for TSH and fT4 assays were 0.10 μIU/ml and 0.15 ng/dl respectively. The total and intra-assay precision values for TSH assays respectively were 8.2 and 5.6 % (for low serum range); 7.65 and 4.88 % (for medium serum range) and 7.1 and 4.3 % (for high serum range).
Similarly, for fT4 these values were 10.98 and 9.81 % (for low serum range); 6.01 and 4.26 % (for medium serum range) and 7.3 and 3.25 % (for high serum range).
Statistical Analysis
All data were entered into MS Excel and analysed using SPSS 17. The nature of distribution of TSH and fT4 values of each trimester were examined by inspecting histograms. The Anderson–Darling’s test as recommended by International Federation of Clinical Chemistry (I.F.C.C) was applied to evaluate the goodness-of-fit to Gaussian distribution [17, 18]. Extreme (highly deviating) values were identified using box plots. These identified probable outliers were tested by the two stage method of Horn et al. [19]. Where conversion of data to almost Gaussian distribution was not possible, Dixon’s Range test was applied to confirm outliers [7]. The confirmed outliers, if present, are eliminated from the reference sample group.
For data which were normally distributed parametric estimation was done: mean (xm), standard deviation (sx) and median of TSH and fT4 were determined separately for each trimester. Reference intervals were estimated by determining the 2.5 and 97.5 percentile calculated as xm ± 1.96 * sx. 0.90 Confidence Intervals (CI) were calculated by the formula: Percentile ± 2.81 * sx/√n. [5–7].
For data which were not normally distributed, transformation to Gaussianity was done either logarithmically or by square-root transformation and then the mean (ym) and standard deviation (sy) of transformed data were computed. RIs and 0.90 CI were estimated using previous formula. These values were reconverted to original data scale by Inverse ln or Inverse square root function [5, 7].
For data which could not be transformed by either method i.e., ln or square-root function; a nonparametric rank-based method (recommended by I.F.C.C) was used [20].
The Mann–Whitney U test was used to compare the differences between the first and second trimesters values and also the second and third trimesters values, for a level of significance p < 0.05.
Results
The study population comprised of 610 pregnant women out of which 195 (31.96 %) were in the first trimester, 205 (33.6 %) belonged to the second and 210 (34.43 %) in the third trimester. The age of the mothers varied from 16 to 37 years. After implementation of the exclusion criteria, a total of 208 women were excluded from the study, resulting to the final reference sample group of 402 women: first trimester—125 (31 %); second trimester—151 (37.4 %) and third trimester—126 (31.4 %).This final population was used to determine the reference limits and 0.90 confidence intervals of TSH and fT4 for the three trimesters. The measures of central tendency, type of distribution and methodology of RI determination are given in Table 1. The RI of TSH as obtained in our study are 0.25–3.35 μIU/ml for the first trimester; 0.78–4.96 μIU/ml for the second trimester and 0.89–4.6 μIU/ml for the third trimester (Table 2). Similarly, the RI of fT4 for first, second and third trimesters were 0.64–2.0 ng/dl, 0.53–2.12 and 0.64–1.98 ng/dl respectively (Table 3).
Table 1.
Descriptive statistics of reference population partitioned into three trimesters
| Trimester | Parameter | Distribution | Mean ± SD | Median | 2.5 Percentile | 97.5 Percentile |
|---|---|---|---|---|---|---|
| First trimester (N = 125) | TSH (μIU/ml) | Normal (Parametric estimation) | 1.81 ± 0.79 | 1.8 | 0.25 | 3.35 |
| fT4 (ng/dl) | Not normal (Nonparametric rank based estimation) | 1.36 ± 0.35 | 1.4 | 0.64 | 2.0 | |
| Second trimester (N = 151) | TSH (μIU/ml) | Not normal (Nonparametric rank based estimation) | 2.22 ± 1.10 | 1.84 | 0.78 | 4.96 |
| fT4 (ng/dl) | Not normal (Nonparametric rank based estimation) | 1.27 ± 0.38 | 1.28 | 0.53 | 2.02 | |
| Third trimester (N = 126) | TSH (μIU/ml) | Not normal (Ln transformed parametric estimation) | 2.2 ± 0.88 | 2.07 | 0.9 | 4.6 |
| fT4 (ng/dl) | Normal (Parametric estimation) | 1.31 ± 0.34 | 1.33 | 0.64 | 1.99 |
Table 2.
Trimester-wise reference data for TSH according to our study
| Trimester | Reference interval of TSH in μIU/ml | 0.9 CI of lower reference limit of TSH in μIU/ml | 0.9 CI of upper reference limit of TSH in μIU/ml |
|---|---|---|---|
| First | 0.25–3.35 | 0.06–0.44 | 3.15–3.55 |
| Second | 0.78–4.96 | 0.64–0.87 | 4.7–5.07 |
| Third | 0.9–4.6 | 0.8–0.98 | 4.15–5.12 |
Table 3.
Trimester-wise reference data for fT4 according to our study
| Trimester | Reference interval of fT4 in ng/dl | 0.9 CI of lower reference limit of fT4 in ng/dl | 0.9 CI of upper reference limit of fT4 in ng/dl |
|---|---|---|---|
| First | 0.64–2.0 | 0.58–0.82 | 1.98–2.04 |
| Second | 0.53–2.02 | 0.44–0.61 | 1.93–2.1 |
| Third | 0.64–1.99 | 0.55–0.73 | 1.9–2.07 |
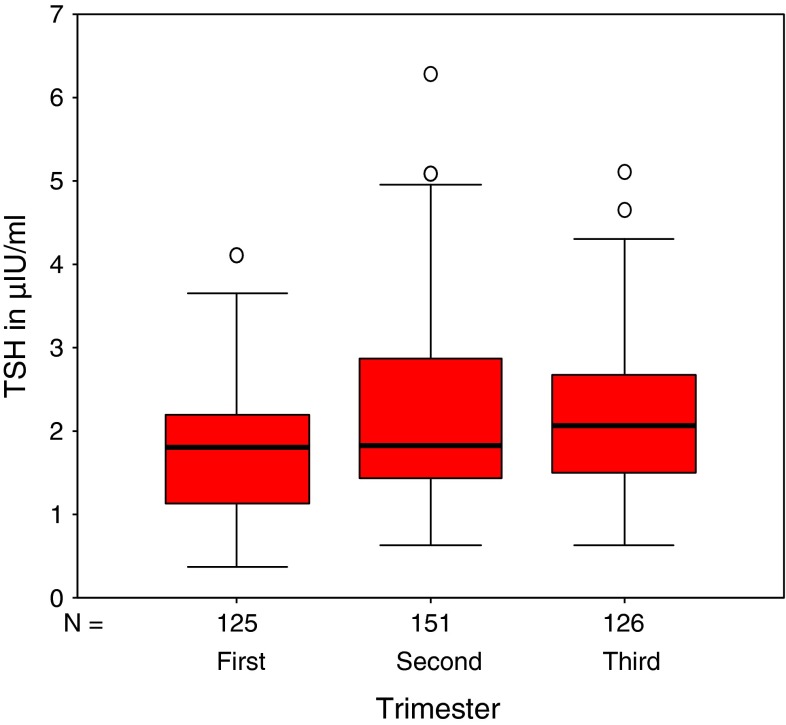
Box plots of TSH and fT4 values for the three trimesters (after outlier assessment),showing the sample minimum, sample maximum, 25th, 50th and 75th percentile along with the extreme values are depicted in Figs. 1 and 2 respectively. Significant difference was observed between first and second trimesters values of both TSH and fT4.
Fig. 1.
Box plots for TSH values of three trimesters after outlier removal. Comparing between first and second trimester values: *p = 0.015 and second and third trimester values: p = 0.417
Fig. 2.
Box plots for fT4 values of three trimesters after outlier removal. Comparing between first and second trimester values: *p = 0.029 and second and third trimester values: p = 0.202
The RIs of TSH and fT4 proposed by the manufacturer were 0.39–6.16 μIU/ml and 0.8–2.0 ng/dl. These values when used to interpret thyroid function tests in our pregnant reference sample group under-diagnosed both subclinical hypothyroidism and subclinical hyperthyroidism (Table 4), when compared to our trimester-wise RIs. No case of either clinical hypo- or hyperthyroidism was found within our reference sample group.
Table 4.
Thyroid clinical entities in the reference sample group using trimester-specific RIs derived from this study and that obtained from the assay kit literature
| Trimester | Source of reference intervals | Subclinical hypothyroidism n (%) | Subclinical hyperthyroidism n (%) |
|---|---|---|---|
| First | Trimester-specific | 4 (3.2) | 0 |
| Kit literature | 0 | 0 | |
| Second | Trimester-specific | 4 (2.6) | 3 (1.98) |
| Kit literature | 0 | 0 | |
| Third | Trimester-specific | 2 (1.58) | 5 (3.96) |
| Kit literature | 0 | 0 |
The different clinical entities are defined as
Normal thyroid function: TSH between reference limits and fT4 between reference limits
Subclinical hypo-thyroidism, TSH over the upper limit of reference limits and fT4 between reference limits
Subclinical hyper-thyroidism, TSH under the lower limit of reference limits and fT4 between reference limits
Clinical hypo-thyroidism, TSH over the lower limit of reference limits and fT4 under the lower limit of reference limits
Clinical hyper-thyroidism, TSH under the lower limit of reference limits and fT4 over the lower limit of reference limits
Discussion
Recent studies have shown that untreated hypothyroidism and subclinical hypothyroidism during pregnancy increases the incidence of maternal anaemia, preeclampsia, postpartum haemorrhage and abruption placenta [21]. Related fetal complications are spontaneous abortion, low birth weight, prematurity, congenital malformation and lower intelligence quotient (IQ) in children. There is also a greater prevalence of subclinical hypothyroidism in women with delivery before 32 weeks [22]. Hyperthyroidism may lead to preeclampsia, still birth, preterm delivery, intra-uterine fetal death and low birth weight [23].
Hence, thyroid function assessment during pregnancy is very vital for the wellbeing of both the mother and the foetus. This study is the first of its kind in eastern India and the second in our country. Marwaha et al. determined the reference ranges of fT4, free triiodothyronine (fT3), and TSH in pregnant women in a primary care set up at Delhi by Electrochemiluminescence technique, and used the 5th and 95th percentile to define the reference ranges in the disease-free subjects [24].
Our study provides the reference intervals of TSH and fT4 in the first, second and third trimesters (Tables 2, 3). On comparing reference data of one trimester to the other by the Mann–Whitney U test, it was found that the first trimester TSH and fT4 values varied significantly from those of the second but the change was not significant from second to the third. This justifies the partitioning of the TSH and fT4 values into three trimesters while determining the reference intervals.
The RIs of TSH and fT4 as supplied by the kit manufacturer was 0.39–6.16 μIU/ml and 0.8–2.0 ng/dl. Before our study, these values were considered for the determination of thyroid disorders in pregnancy in our laboratory. On applying these RIs to diagnose thyroid disorders in our reference sample group, an appreciable number of pregnant women escaped the diagnosis of subclinical hypothyroidism using the manufacturer’s RI; 3.2 % in the first, 2.6 % in second and 1.58 % in the third trimesters. Also, 1.98 and 3.96 % of pregnant women identified as subclinical hyperthyroidism (by our trimester-specific RIs) in second and third trimester respectively, failed to be diagnosed (Table 4). The fraction of population misdiagnosed would be much greater and would also include the spectrum of clinical hypo- or hyper-thyroidism, if these RIs were applied to the general population; acknowledging the fact that only the reference sample group has been taken into consideration here. This variation is because the RI provided by the reagent manufacturer has been determined on a non-pregnant population and cannot be applied for the interpretation of thyroid function tests of a pregnant woman.
During pregnancy the best laboratory assessment of thyroid function to diagnose hypothyroidism is a TSH combined with fT4 and for hyperthyroidism is fT4, fT3 combined with TSH, as the total hormone estimations are flawed due to the high TBG concentration during this period [25]. In our study fT4 and TSH was estimated. The incidence of hyperthyroidism in pregnancy is quite uncommon (0.2 %) in contrast to hypothyroidism and most hyperthyroid cases are determined by fT4 and TSH values [26]. Only in some hyperthyroid cases fT3 is raised. So, fT3 estimation was excluded from our study. According to I.F.C.C. expert panel on theory of reference values, the well-defined healthy population for reference interval determination must be based on specific exclusion criteria [27, 28]. So, we selected our reference population using a stringent criteria. To begin with, all mothers with any kind of thyroid abnormality were excluded since women positive for thyroid autoantibodies typically have higher TSH values and therefore affect and skew the upper reference limit [29, 30]. Of the antibodies, only anti-thyroperoxidase antibody was estimated. Anti-thyroglobulin antibody has little diagnostic value over TPO antibody measurement for autoimmune thyroid disease as it has been found in individuals without apparent thyroid disease. Also, women with twin pregnancies or with hyperemesis gravidarum were also removed from the reference population due to their potential for low TSH values (higher serum βHCG) and interference with the lower limit of TSH reference range [31]. We also excluded mothers with positive history of autoimmune diseases like diabetes mellitus type1, based on the association between autoimmunity and thyroid dysfunction [32]. Exclusion of women with clinical as well as laboratory signs of medical disorders was also done. The final strictly defined reference population of 402 women was considered adequate for the estimation of reference intervals fulfilling the sample size requirements of National Committee for Clinical Laboratory Standards (NCCLS) [28].
Our laboratory estimations were done by validated, precise and accurate methods. The assays were done under proper quality control measures. The statistical strengths included examination of distributions, respective application of parametric or non-parametric methods and assessment of outliers.
Pregnancy is a very important stage in the life of a woman. Throughout the period of 280 days the mother harbours the growing child in her uterus. A lot of alterations occur in various biochemical parameters (within physiological limits) during this period. The normal development of the foetus and a smooth obstetric journey depends on these factors. Hence, along with routine antenatal checkups, an ultrasound imaging of the foetus and an array of maternal blood investigations are done to ensure the wellbeing of the child. Fasting plasma glucose, complete haemogram, platelet count, liver and renal function tests are commonly requested biochemical investigations. In the recent years many studies advocate the use of routine thyroid function tests for screening of thyroid disease in pregnancy [33, 34].
During gestation several hormonal changes and metabolic alterations occur resulting in complex effects on thyroid function. βHCG secretion from the placenta is enhanced during the first trimester. This thyrotropic effect of rising βHCG exerts negative feedback on TSH secretion [35]. As the pregnancy progresses from first to next, there is gradual rise of TBG (due to oestrogen stimulated hepatic synthesis). This results in an increase in total T4 and total T3 with simultaneous fall of free hormone concentration which causes feedback enhancement of TSH release from the pituitary [36, 37]. Additionally, due to hyperdynamic circulation during pregnancy, the iodine clearance by the kidney rises. This stimulates the thyroid gland to increase iodine uptake via increased TSH release from pituitary. With the onset of second trimester βHCG surge diminishes while the effect of rising TBG continues till the second and third trimester. The third factor, which intervenes later in gestation, is related to modifications in the peripheral metabolism of the thyroid hormones, particularly at the placenta level. All these factors lead to physiological adaptation in healthy women who are iodine-sufficient. Inadequate iodine intake and pathological conditions like autoimmune diseases alters this physiological balance which is reflected in the serum levels of thyroid hormones [38].
A number of cross-sectional studies have reported trimester-specific reference ranges for fT4 and TSH among pregnant women [39–41]. But these reported ranges vary and these variations are due to differences in method of immunometric assay, ethnic disparities [42] along with variations in iodine nutrition characteristics, inclusion criteria for determination of reference population, sample size and assessment of outliers.
To conclude, it is important that each laboratory determines its own population and method-based reference interval for diagnosing thyroid disorders in pregnancy. Moreover, these RI must preferentially be trimester-specific. To boost this concept we have determined our own laboratory-specific trimester-wise RI for TSH and fT4 and intend to use them for screening of pregnant mothers for thyroid disorders, at any stage of gestation.
Electronic supplementary material
References
- 1.Haddow JE, Palomaki GE, Allan WC, William JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 2.Davis LE, Leveno KJ, Cunningham FG. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988;72:108–112. [PubMed] [Google Scholar]
- 3.Glinoer D, Soto MF, Bourdoux P, Lejeune PB, Delange F, Lemone M, et al. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421–427. doi: 10.1210/jcem-73-2-421. [DOI] [PubMed] [Google Scholar]
- 4.Larsen PR, Davies TF, Schlumberger MJ, Hay ID. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams textbook of endocrinology, Chap. 10. 5. Philadelphia: Saunders Elsevier; 2011. [Google Scholar]
- 5.Solberg HE. International Federation of Clinical Chemistry. Scientific committee, Clinical Section. Expert Panel on Therapy of Reference Values and International Committee for Standardization in Haematology Standing Committee on Reference Values. Approved recommendations (1986) on the theory of reference values. Part I. The concept of reference values. Clin Chim Acta. 1987;165:111–118. doi: 10.1016/0009-8981(87)90224-5. [DOI] [PubMed] [Google Scholar]
- 6.International Organisation for Standardization. 15189 I. Medical laboratories—Particular requirements for quality and competence. 2003.
- 7.Solberg HE. Approved recommendation (1987) on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. J Clin Chem Clin Biochem. 1987;25:645–656. [PubMed] [Google Scholar]
- 8.Dydkaer R. Observed value related to reference value. In: Graseck R, Alstrom T, editors. Reference values in laboratory medicine. Chichester: John Wiley; 1981. pp. 263–278. [Google Scholar]
- 9.Soldin OP. Thyroid function testing in pregnancy and thyroid disease: trimester-specific reference intervals. Ther Drug Monit. 2006;28(1):8–11. doi: 10.1097/01.ftd.0000194498.32398.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce EN, Oken E, Gillman MW. Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, multivitamin use. Endocr Pract. 2008;14(1):33–39. doi: 10.4158/EP.14.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopton MR, Harrop JS. Immunoradiometric assay of thyrotropin as a “first-line” thyroid-function test in the routine laboratory. Clin Chem. 1986;32(4):691–693. [PubMed] [Google Scholar]
- 12.Spencer CA. Interlaboratory/intermethod differences in functional sensitivity of immunometric assays of thyrotropin (TSH) and impact of reliability of measurement of subnormal concentrations of TSH. Clin Chem. 1995;41:367. [PubMed] [Google Scholar]
- 13.Chopra IJ, Solomon DH, Ho RS. A radioimmunoassay of thyroxine. J Clin Endo. 1971;33:865. doi: 10.1210/jcem-33-5-865. [DOI] [PubMed] [Google Scholar]
- 14.Halpern EP, Brodens RW. Microencapsulated antibodies in radioimmunoassay: determination of free thyroxine. Clin Chem. 1979;25:1561–1563. [PubMed] [Google Scholar]
- 15.Midgeley J. Direct and indirect free thyroxine assay methods in theory and practice. Clin Chem. 2001;47:1353–1363. [PubMed] [Google Scholar]
- 16.Czamocka B, Ruff J, Ferrand M, Carayon P, Lissitzky S. Purification of the human thyroid and its identification as the microsomal antigen involved in the human thyroid disease. FEBS Lett. 1985;190:147–152. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- 17.Anderson TW, Darling DA. Asymptotic theory of certain goodness of fit criteria based on stochastic processes. Ann Math Stat. 1952;23:193–212. [Google Scholar]
- 18.Solberg HE. Statistical treatment of reference values in laboratory medicine: testing the goodness-of-fit of an observed distribution to the Gaussian distribution. Scand J Clin Lab Invest Suppl. 1986;184:125–132. [PubMed] [Google Scholar]
- 19.Horn PS, Feng L, Li Y, Pesce AJ. Effect of outliers and nonhealthy individuals on reference interval estimation. Clin Chem. 2001;47:2137–2145. [PubMed] [Google Scholar]
- 20.Linnet K. Nonparametric estimation of reference intervals by simple and bootstrap-based procedures. Clin Chem. 2000;46:867–869. [PubMed] [Google Scholar]
- 21.Schneuer FJ, Nassar N, Tasevski V, Morris JM, Roberts CL. Association and predictive accuracy of high TSH serum levels in first trimester and adverse pregnancy outcomes. J Clin Endocrinol Metab. 2012;97(9):3115–3122. doi: 10.1210/jc.2012-1193. [DOI] [PubMed] [Google Scholar]
- 22.Lao TT. Thyroid disorders in pregnancy. Curr Opin Obstet Gynecol. 2005;17(2):123–127. doi: 10.1097/01.gco.0000162179.15360.08. [DOI] [PubMed] [Google Scholar]
- 23.Metsman JH. Hyperthyroidism in pregnancy. Best Pract Res. 2004;18(2):267–288. doi: 10.1016/j.beem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Marwaha RK, Chopra S, Gopalakrishnan S. Establishment of reference range for thyroid hormones in normal pregnant Indian women. Int J Obstet Gynaecol. 2008;115(5):602–606. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen UF, Mortensen ASB, Rasmussen AK, Boas M, Hilsted L, Main K. Challenges in interpretation of thyroid function tests in pregnant women with autoimmune thyroid disease. J Thyr Res. 2011; 2011 Article ID 598712. [DOI] [PMC free article] [PubMed]
- 26.Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1991;35(1):41–46. doi: 10.1111/j.1365-2265.1991.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 27.Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta. 2003;334(1–2):5–23. doi: 10.1016/s0009-8981(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 28.NCCLS. How to define and determine reference intervals in the clinical laboratory: approved guideline. NCCLS Document. C28-A and C28-A2. Villanova, PA; 1995.
- 29.Glinoer D, Riahi M, Grun JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab. 1994;79(1):197–204. doi: 10.1210/jcem.79.1.8027226. [DOI] [PubMed] [Google Scholar]
- 30.Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007;92(11):4236–4240. doi: 10.1210/jc.2007-0287. [DOI] [PubMed] [Google Scholar]
- 31.Grün JP, Meuris S, De Nayer P, Glinoer D. The thyrotrophic role of human chorionic gonadotrophin (hCG) in the early stages of twin (versus single) pregnancies. Clin Endocrinol. 1997;46(6):719–725. doi: 10.1046/j.1365-2265.1997.2011011.x. [DOI] [PubMed] [Google Scholar]
- 32.Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123(2):183. doi: 10.1016/j.amjmed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Thung SF, Funai EF, Grobman WA. The cost-effectiveness of universal screening in pregnancy for subclinical hypothyroidism. Am J Obstet Gynecol. 2009;200(3):267. doi: 10.1016/j.ajog.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Dosiou C, Sanders GD, Araki SS, Crapo LM. Screening pregnant women for autoimmune thyroid disease: a cost-effectiveness analysis. Eur J Endocrinol. 2008;158(6):841–851. doi: 10.1530/EJE-07-0882. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura M, Hershman JM. Thyrotropic action of human chorionic gonadotropin. Thyroid. 1995;5:425–434. doi: 10.1089/thy.1995.5.425. [DOI] [PubMed] [Google Scholar]
- 36.Skjöldebrand L, Brundin J, Carlström A, Pettersson T. Thyroid associated components in serum during normal pregnancy. Acta Endocrinol (Copenh) 1982;100:504–511. doi: 10.1530/acta.0.1000504. [DOI] [PubMed] [Google Scholar]
- 37.Sparre LS, Brundin J, Carlström K, Carlström A. Oestrogen and thyroxine-binding globulin levels in early normal pregnancy. Acta Endocrinol (Copenh) 1987;114:298–304. doi: 10.1530/acta.0.1140298. [DOI] [PubMed] [Google Scholar]
- 38.Glinoer D, De Nayer P, Bourdoux P, Lemone M, Robyn C, Van Steirteghem A, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 39.Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38(4):329–332. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 40.Kurioka H, Takahashi K, Miyazaki K. Maternal thyroid function during pregnancy and puerperal period. Endocr J. 2005;52(5):587–591. doi: 10.1507/endocrj.52.587. [DOI] [PubMed] [Google Scholar]
- 41.Soldin OP, Soldin D, Sastoque M. Gestation-specific thyroxine and thyroid stimulating hormone levels in the United States and worldwide. Ther Drug Monit. 2007;29(5):553–559. doi: 10.1097/FTD.0b013e31815709ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price A, Obel O, Cresswell J. Comparison of thyroid function in pregnant and non-pregnant Asian and western Caucasian women. Clin Chim Acta. 2001;308(12):91–98. doi: 10.1016/s0009-8981(01)00470-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.