Abstract
Explant culture allows manipulation of developing organs at specific time points and is therefore an important method for the developmental biologist. For many organs it is difficult to access developing tissue to allow monitoring during ex vivo culture. The slice culture method allows access to tissue so that morphogenetic movements can be followed and specific cell populations can be targeted for manipulation or lineage tracing.
In this paper we describe a method of slice culture that has been very successful for culture of tooth germs in a range of species. The method provides excellent access to the tooth germs, which develop at a similar rate to that observed in vivo, surrounded by the other jaw tissues. This allows tissue interactions between the tooth and surrounding tissue to be monitored. Although this paper concentrates on tooth germs, the same protocol can be applied to follow development of a number of other organs, such as salivary glands, Meckel's cartilage, nasal glands, tongue, and ear.
Keywords: Anatomy, Issue 81, Tooth, Culture Techniques, Embryo Culture Techniques, Organ Culture Techniques, Developmental Biology, animal biology, animal models, Tooth germ, live slice, development, tissue chopper, lineage tracing, molar, incisor, gland
Introduction
For a number of experiments it is important to be able to culture tissue ex vivo to follow development. Culture of developing tissue provides access at defined periods of development and allows manipulation of genes by the addition of factors to the culture medium, or on loaded beads, and by the use of transfection and electroporation1. For many experiments it is important to be able to visualize the tissue as it grows, for example, to follow the fate of lineage labeled cells as the tissue undergoes morphogenesis. This can be particularly problematic for tissues that develop deep within the embryo, which are not obvious when a block of tissue from the embryo is cultured. Teeth are good examples of this, as they develop within the mandible, maxilla and frontal nasal process. When the whole mandible is cultured the superficial structures of the tooth can be viewed but changes in morphology can only be analyzed after sectioning of fixed tissue2. We have adapted a live slice culture technique allowing us to follow tooth germ development and providing access to the different parts of the tooth during development. The technique cultures the tooth germ slices at the gas-liquid interface, using a modified Trowell method3. These slice cultures have been very useful in directly following morphogenesis of the tooth, and allowing lineage tracing of distinct components, such as the enamel knot and the dental papilla and follicle1,4-7. The technique is not limited to mouse embryos and has successfully been used to culture live slices of pig and snake dental tissue8,9. In addition to the benefit of being able to visualize tooth development, the slice method also has the advantage that the thin slices of tissue have increased access to nutrients from the medium and air from the incubator. This results in improved growth of the tooth germs, which match development in vivo, and show invasion of endothelial cells into the papilla7. In contrast, tooth germs in whole mandible cultures develop slower than those in vivo and the center of the culture is often necrotic in long-term cultures. In slice culture, the tooth develops within a slice of the jaw, and its interaction with the surrounding developing bone and other tissues can be monitored. In our method the tissue is chopped straight after dissection with no need to embed in a support medium10,11 and no need for any system to attach the tissue to the chopping block. The method is therefore noninvasive and rapid, allowing many mandibles to be sectioned in one session.
Protocol
1. Set Up
Sharpen the dissection instruments (using a sharpening stone lubricated with mineral oil) and sterilize prior to use for organ culture using a 70% ethanol spray and dry-heat sterilization. Sharpen the dissection needles using electrolysis in 2 M sodium hydroxide using a 12 V supply.
Prepare the culture medium used for the dissection and culture of embryonic organs, consisting of Advanced Dulbecco's Modified Eagle Medium F12 (DMEM F12) supplemented with 1% GlutaMAX and 1% penicillin-streptomycin.
2. Embryo Dissection
Cull a timed mated pregnant female using a schedule one method as approved by the Home Office or other governing body. Dissect away the skin around the lower abdomen, and cut open the abdomen using scissors. Locate the two uterine horns, which connect together at the lower midline.
Dissect out the uterus and place in a tube filled with medium on ice. When on ice, the tissue can be left for several hours.
Remove the uterus and place in a Petri dish in medium under the microscope. Where possible embryo dissections should be performed in a laminar-flow hood to minimize contamination.
Dissect out the embryos from the uterus and free them from their extraembryonic tissues.
Decapitate the embryos using a tungsten needle and transfer the heads into a fresh dish of medium.
Isolate the mandibles from the heads by placing a needle into the oral cavity and cut through towards the back of the head (Figures 1A and 1B). Isolated mandibles can be temporarily stored in medium on ice while waiting for chopping.
For whole mandible slices stages E11.5-E15.5 are ideal for tooth germ culture. After E15.5 the developing bone in the mandible is too hard for chopping. Tooth slices can still be made but the bone (dentary and forming alveolar bone) must first be removed by dissection before chopping9. Remove bone carefully using tungsten needles and forceps.
3. Embryo Slicing
Prepare the mandible tissue slices using a standard table tissue chopper (Figure 1C). Use a fresh blade after approximately 200 mandibles, and make sure it lies flush with the mounting disc when it falls.
Clean the mounting disc and blade with 70% ethanol before use. Care must be taken when using the machine, as the blade is very sharp.
Set the chopper at a cutting distance of between 200-400 μm according to the age of the specimen, and depending on whether the whole tooth germ is required. Set the blade force on maximum.
Transfer a mandible to the plastic mounting disc using a pipette or forceps. Arrange the mandible on the disc making sure the orientation is still clear. The developing tongue can be used as a useful landmark for determining orientation. For molars use a frontal/transverse orientation (see plane of chopping in Figure 1B), while for incisors use a sagittal slice through the mandible to reflect the different orientation of these teeth as they grow (see plane of chopping in Figure 2A).
Remove the excess medium from around the tissue using filter paper in order to slightly dry the mandible on the mounting disc.
Immediately after drying, place the disc on the circular stainless steel table of the chopper. Turn the machine on and press start. The table traverses automatically from left to right while at the same time the chopping arm carrying the blade is raised and dropped at up to 200 strokes/min.
Turn off the machine once the tissue has been completely sliced. Make sure the blade arm is in the raised position and remove the disc. Do not place fingers under the blade arm or leave the machine unattended when chopping.
Take the mounting disc and immediately add medium to the slices on the disc in order to prevent excessive drying. Carefully dislodge the sliced tissue from the bottom of the disc using a tungsten needle and then pipette into a small Petri dish of medium (Figure 2A).
Separate the slices using a needle and then select the tissue slices containing the tissue of interest under a stereomicroscope (Figures 1D, 1E, and 2B). Place selected slices in fresh culture dishes with medium.
4. Slice Culture
Prepare central-well organ culture dishes ready for culture by adding approximately 2 ml of autoclaved water to the outer well to prevent dehydration, and by positioning a metal grid across the center of the dish (Figures 3A and 3B).
Prepare metal grids by cutting strips approximately 4 cm x 1.5 cm from sheets of stainless steel mesh. Use a hole punch to make a hole in the center and bend the sides to create a staggered end. The grid should fit flat across the central well, lying parallel to the bottom of the dish (Figures 3A and 3B). Autoclave the grids to sterilize before use.
Cut a piece of transparent polyethylene terephthalate (PET) membrane (pore size 0.4 μm) large enough to cover the hole in the grid. Place the cut membrane into the dish with the selected slice.
Place the slice on top of the membrane and then carefully lift the membrane out of the medium, with slice flattened in the center.
Place the filter on the grid over the circular hole in the center, and add approximately 1 ml of culture medium to the well, up to the level of the membrane.
For manipulations add proteins or small molecule inhibitors to the medium (for example8).
Photograph the slice for a record of morphology at day 0 of culture.
Place the dishes in a humidified atmosphere of 5% CO2 at 37 °C in an incubator.
Photograph the cultures at regular intervals for on average 7 days. Change the culture medium every 48-72 hr. For photography view the slices with a light source from underneath (Figures 2C-E).
5. Lineage Tracing
If lineage tracing is required, lipophilic dyes such as DiI or DiO can be injected into the tooth germ before step 4, slice culture.
Pull glass capillary needles using a needle puller.
Break the tip of the needle using forceps and place tip down into the dye solution. Leave for a few minutes while the dye fills the tip by capillary action.
Place the filled needle into a holder and attach via tubing to an injector or mouth aspirator.
Position the tip of the needle in the culture medium touching the area of the slice due to be labeled. Apply air pressure to displace a small amount of dye out of the needle and onto the tissue.
Remove needle and check for labeling under fluorescence.
Successfully labeled slices can be transferred to filters and cultured as described in step 4.
Representative Results
In order to follow the movement of the first molar dental follicle, 250 μm frontal slices were taken through a mandible using the method described above. The dental follicle is the layer of mesenchyme that surrounds the outer enamel epithelium (OEE) of the developing tooth, and has previously been shown to take part in the formation of tissue of the periodontium 6. Mandibles were dissected at E14.5, the cap stage of tooth development. In the slice the outline of the dental epithelium was clear, and the condensing dental mesenchyme could be identified as a darker ring around the tooth epithelium (Figure 4A). DiI was injected in the mesenchyme next to the outer enamel epithelium of the tooth slice on the lingual side (Figure 4A). After labeling the slices were cultured for 4 days and photographed at intervals (Figures 4B and 4C). In vivo the tooth germs would have reached the bell stage by E18.5, and a distinct bell stage shape was observed in the slices, indicating a similar progression over this time period. The spot of DiI was seen to extend into a band of cells extending around the outer dental epithelium as the tooth grew (Figures 4B and 4C).
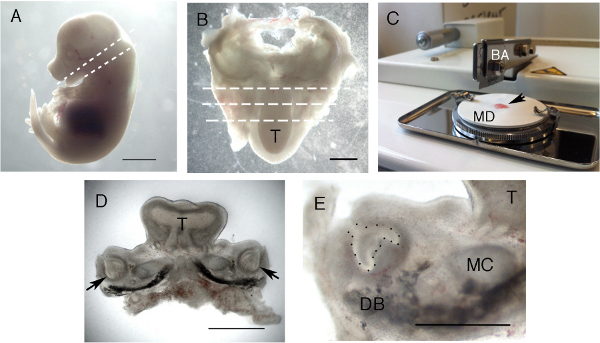 Figure 1. Formation of live slices. (A) E14.5 embryo. Dashed lines indicate the planes cut with a dissecting needle, starting with the lower cut for decapitation. (B) Dissected lower jaw. The tongue is facing downwards. The plane of section for the tissue chopper is shown using dashed lines. (C) Tissue chopper showing blade arm (BA) and white mounting disc (MD) with prepared specimen (arrow). (D) Representative slice through the molar region. Arrows point to molar tooth germs. (E) High power view of a molar slice. The tooth germ epithelium is outlined with black spots. Images sharpened using Photoshop. T = Tongue, DB = Dentary bone, MC = Meckel's cartilage. Scale bar in A = 3 mm. Scale bar in B & D = 1 mm. Scale bar in E = 500 μm. Click here to view larger figure.
Figure 1. Formation of live slices. (A) E14.5 embryo. Dashed lines indicate the planes cut with a dissecting needle, starting with the lower cut for decapitation. (B) Dissected lower jaw. The tongue is facing downwards. The plane of section for the tissue chopper is shown using dashed lines. (C) Tissue chopper showing blade arm (BA) and white mounting disc (MD) with prepared specimen (arrow). (D) Representative slice through the molar region. Arrows point to molar tooth germs. (E) High power view of a molar slice. The tooth germ epithelium is outlined with black spots. Images sharpened using Photoshop. T = Tongue, DB = Dentary bone, MC = Meckel's cartilage. Scale bar in A = 3 mm. Scale bar in B & D = 1 mm. Scale bar in E = 500 μm. Click here to view larger figure.
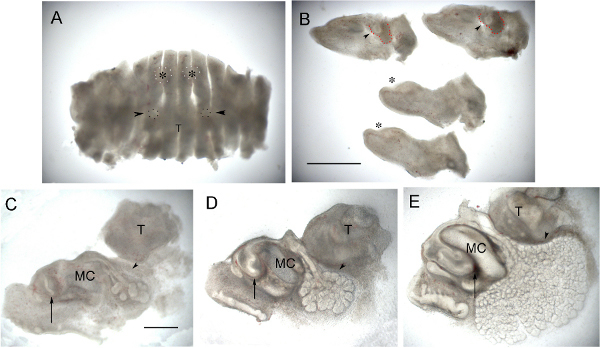 Figure 2. Incisor and gland culture. (A) E12.5 Mandible that has been chopped sagittally. Developing incisors (outlined white spots with asterix) and submandibular glands (outlined with black spots and arrowed) can be just made out. (B) Same mandible showing central 4 slices separated out. The developing submandibular gland can be seen as a bud surrounded by condensed mesenchyme (arrowheads) in the two more lateral sections (top of image). The condensing mesenchyme around the gland is outlined in red. The incisors are at the epithelial thickening stage (asterix) in the two central slices (bottom of image). (C) Sagittal slice through an incisor at E14.0 at Day 0. (D) Same slice 1 day later. (E) Same slice after 3 days in culture. (C-E) Arrow points to incisor and forming labial cervical loop. Arrowhead points to developing submandibular gland. In culture the tooth germ extends backwards as the cervical loops grow, while the salivary gland continues to undergo branching morphogenesis and lumen formation. Images sharpened using Photoshop. T = Tongue, MC = Meckel's cartilage. Scale bar in A & B = 1 mm. Scale bar in C, D, E = 500 μm. Click here to view larger figure.
Figure 2. Incisor and gland culture. (A) E12.5 Mandible that has been chopped sagittally. Developing incisors (outlined white spots with asterix) and submandibular glands (outlined with black spots and arrowed) can be just made out. (B) Same mandible showing central 4 slices separated out. The developing submandibular gland can be seen as a bud surrounded by condensed mesenchyme (arrowheads) in the two more lateral sections (top of image). The condensing mesenchyme around the gland is outlined in red. The incisors are at the epithelial thickening stage (asterix) in the two central slices (bottom of image). (C) Sagittal slice through an incisor at E14.0 at Day 0. (D) Same slice 1 day later. (E) Same slice after 3 days in culture. (C-E) Arrow points to incisor and forming labial cervical loop. Arrowhead points to developing submandibular gland. In culture the tooth germ extends backwards as the cervical loops grow, while the salivary gland continues to undergo branching morphogenesis and lumen formation. Images sharpened using Photoshop. T = Tongue, MC = Meckel's cartilage. Scale bar in A & B = 1 mm. Scale bar in C, D, E = 500 μm. Click here to view larger figure.
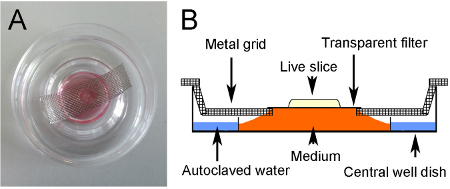 Figure 3. Culture method. (A) Cultured slice. The culture sits on a transparent filter suspended over medium via a metal grid. A hole in the grid allows the slice to be visualized with light from below. (B) Schematic of culture method.
Figure 3. Culture method. (A) Cultured slice. The culture sits on a transparent filter suspended over medium via a metal grid. A hole in the grid allows the slice to be visualized with light from below. (B) Schematic of culture method.
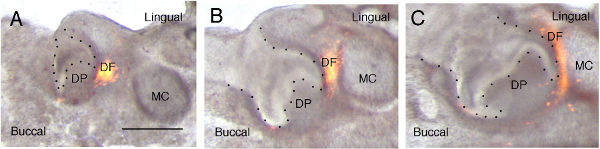 Figure 4. DiI labeling of the dental follicle. (A-C) Merged light and dark field images showing developing molar tooth germ and location of DiI lineage label. (A) Day 0. The tooth germ (arrowhead) is at the cap stage. DiI labels cells of the dental follicle on the lingual side of the tooth. (B) Day 1. (C) Day 4. The tooth germ has reached the bell stage and the DiI labeled cells are seen to spread out in an arc around the developing outer enamel epithelium. Images have been sharpened in Photoshop after merging layers using the screen mode. Epithelium of tooth outlined with black spots. DP = dental papilla lying within the inner enamel epithelium. DF = dental follicle, which runs around the outer enamel epithelium. MC = Meckel's cartilage. Scale bar in A, B, C = 500 μm. Click here to view larger figure.
Figure 4. DiI labeling of the dental follicle. (A-C) Merged light and dark field images showing developing molar tooth germ and location of DiI lineage label. (A) Day 0. The tooth germ (arrowhead) is at the cap stage. DiI labels cells of the dental follicle on the lingual side of the tooth. (B) Day 1. (C) Day 4. The tooth germ has reached the bell stage and the DiI labeled cells are seen to spread out in an arc around the developing outer enamel epithelium. Images have been sharpened in Photoshop after merging layers using the screen mode. Epithelium of tooth outlined with black spots. DP = dental papilla lying within the inner enamel epithelium. DF = dental follicle, which runs around the outer enamel epithelium. MC = Meckel's cartilage. Scale bar in A, B, C = 500 μm. Click here to view larger figure.
Discussion
This method of tooth culture has the advantage that access to the tooth germ is excellent, allowing accurate lineage tracing and placement of beads within the epithelium or the mesenchyme. Defined regions of the developing tooth germ can therefore be specifically targeted. During culture the changing morphology of the tooth germ can be followed, and the effect of manipulations quickly assessed.
The method, however, is only suitable for young tooth germs before substantial formation of hard tissues, such as dentine and enamel, as these cannot be chopped accurately. For mandibles after E15.5 it is necessary to remove the bone before chopping. This has the disadvantage of potentially damaging the tooth germ, which is closely associated with the bone from E16.5. We have successfully sliced dissected tooth germs up to postnatal day 4, after which the tooth becomes too hard for the chopper to cut due to the deposition of enamel. The slice method works well on tooth germs from E13.5, i.e. the bud stage1,6. Before this time-point, when the tooth is at the epithelial thickening stage, the tooth germs do develop but the success rate is reduced and the morphology can be affected over the long term.
One major disadvantage with the method is that the chopping occurs at random through the tooth. In some slices a whole tooth germ will be found within a slice, while in others the chopper may cut the tooth germ through the middle. This means that it may be difficult to obtain large numbers of identical slices. To combat this problem, it is possible to divide a slice down the middle and use the right and left sides as experimental and control. For this, however, the mandible must be carefully placed on the chopping disc to ensure the slice is cut symmetrically. For some experiments it is an advantage to have slices that dissect the tooth so that internal structures, such as the enamel knot, can be accessed for lineage labeling4. The tooth germ appears very robust and such half tooth germs are able to develop well in culture, as previously shown in halved molar tooth germs12,13.
As an alternative to slice culture, tooth germs can be dissected out of the mandible and cultured in isolation14. This removes the problem of poor nutrition and oxygenation associated with culturing large blocks of tissue and leads to good tooth development but as a consequence the tooth develops without interaction with the surrounding tissue. When large amounts of the surrounding mesenchyme are removed the number of tooth germs that form can be altered, highlighting the importance of the surrounding mesenchyme for normal tooth development15. Slice culture is therefore a good method for studying the interactions of tissues, for example how the bone and tooth interact in the formation of the alveolar bone, or how the salivary glands interact with the tongue and oral epithelium, something that is lost when these tissues are cultured in isolation.
This paper highlights the use of this method for culturing the tooth germs but the same method is also excellent for culturing developing submandibular and sublingual glands and following the development of structure such as Meckel's cartilage (Figure 2).
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
Sarah A. Alfaqeeh is funded by Kind Saud University College of Dentistry, Ministry of Higher Education, Kingdom of Saudi Arabia.
References
- Rothova M, Peterkova R, Tucker AS. Fate map of the dental mesenchyme: dynamic development of the dental papilla and follicle. Developmental biology. 2012;366:244–254. doi: 10.1016/j.ydbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Ferguson CA, et al. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes & development. 1998;12:2636–2649. doi: 10.1101/gad.12.16.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowell OA. The culture of mature organs in a synthetic medium. Experimental cell research. 1959;16:118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- Matalova E, Antonarakis GS, Sharpe PT, Tucker AS. Cell lineage of primary and secondary enamel knots. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:754–759. doi: 10.1002/dvdy.20396. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Tucker AS, De Bari C, Cobourne MT, Rice DP. A regulatory relationship between Tbx1 and FGF signaling during tooth morphogenesis and ameloblast lineage determination. Developmental biology. 2008;320:39–48. doi: 10.1016/j.ydbio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Diep L, Matalova E, Mitsiadis TA, Tucker AS. Contribution of the tooth bud mesenchyme to alveolar bone. Journal of experimental zoology. Part B, Molecular. 2009;312B:510–517. doi: 10.1002/jez.b.21269. [DOI] [PubMed] [Google Scholar]
- Rothova M, Feng J, Sharpe PT, Peterkova R, Tucker AS. Contribution of mesoderm to the developing dental papilla. The International journal of developmental biology. 2011;55:59–64. doi: 10.1387/ijdb.103083mr. [DOI] [PubMed] [Google Scholar]
- Buchtova M, et al. Initiation and patterning of the snake dentition are dependent on Sonic hedgehog signaling. Developmental biology. 2008;319:132–145. doi: 10.1016/j.ydbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Buchtova M, Stembirek J, Glocova K, Matalova E, Tucker AS. Early regression of the dental lamina underlies the development of diphyodont dentitions. Journal of dental research. 2012;91:491–498. doi: 10.1177/0022034512442896. [DOI] [PubMed] [Google Scholar]
- Cho SW, et al. The primary enamel knot determines the position of the first buccal cusp in developing mice molars. Differentiation; research in biological diversity. 2007;75:441–451. doi: 10.1111/j.1432-0436.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- Sakano M, et al. Cell dynamics in cervical loop epithelium during transition from crown to root: implications for Hertwig's epithelial root sheath formation. Journal of periodontal research. 2012. [DOI] [PubMed]
- Fisher AR. Morphological development in vitro of the whole and halved lower molar tooth germ of the mouse. Archives of oral biology. 1971;16:1481–1496. doi: 10.1016/0003-9969(71)90084-7. [DOI] [PubMed] [Google Scholar]
- Coin R, Schmitt R, Lesot H, Vonesch JL, Ruch JV. Regeneration of halved embryonic lower first mouse molars: correlation with the distribution pattern of non dividing IDE cells, the putative organizers of morphogenetic units, the cusps. The International journal of developmental biology. 2000;44:289–295. [PubMed] [Google Scholar]
- Kavanagh KD, Evans AR, Jernvall J. Predicting evolutionary patterns of mammalian teeth from development. Nature. 2007;449:427–432. doi: 10.1038/nature06153. [DOI] [PubMed] [Google Scholar]
- Munne PM, Tummers M, Jarvinen E, Thesleff I, Jernvall J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development. 2009;136:393–402. doi: 10.1242/dev.025064. [DOI] [PubMed] [Google Scholar]


