Abstract
Metabolic dysfunctions are often linked to reproductive abnormalities. Adiponectin (ADP), a peripheral hormone secreted by white adipose tissue, is important in energy homeostasis and appetite regulation. GnRH neurons are integral components of the reproductive axis, controlling synthesis, and release of gonadotropins. This report examined whether ADP can directly act on GnRH neurons. Double-label immunofluorescence on brain sections from adult female revealed that a subpopulation of GnRH neurons express ADP receptor (AdipoR)2. GnRH/AdipoR2+ cells were distributed throughout the forebrain. To determine the influence of ADP on GnRH neuronal activity and the signal transduction pathway of AdipoR2, GnRH neurons maintained in explants were assayed using whole-cell patch clamping and calcium imaging. This mouse model system circumvents the dispersed distribution of GnRH neurons within the forebrain, making analysis of large numbers of GnRH cells possible. Single-cell PCR analysis and immunocytochemistry confirmed the presence of AdipoR2 in GnRH neurons in explants. Functional analysis revealed 20% of the total GnRH population responded to ADP, exhibiting hyperpolarization or decreased calcium oscillations. Perturbation studies revealed that ADP activates AMP kinase via the protein kinase Cζ/liver kinase B1 pathway. The modulation of GnRH neuronal activity by ADP demonstrated in this report directly links energy balance to neurons controlling reproduction.
GnRH neurons are a key gatekeeper of the hypothalamic-pituitary-gonadal axis and thus reproduction. Abnormal weight decrease or increase can have profound effects on the reproductive axis, suppressing reproductive function and ovarian cyclicity (1, 2) or reducing fertility and causing anovulation (3, 4), respectively. Adiponectin (ADP), an adipose tissue-specific hormone, plays an important role in the regulation of food intake (reviewed in Refs. 5, 6). Although many of the actions of ADP are peripherally mediated, centrally mediated effects have been proposed (7). ADP trimer and globular forms are found in brain and can pass the blood brain barrier (8, 9). Direct application of ADP depolarizes neurons in the area postrema and paraventricular nucleus (PVN), neurons with access to factors in blood via circumventricular organs (10). A subpopulation of GnRH neurons surrounds the organum vasculosa lamina terminalis (also a circumventricular organ) (11). Whether ADP can directly influence GnRH cells and thus reproductive function is unclear.
ADP has 2 main receptors, ADP receptor (AdipoR)1 and AdipoR2. These receptors share 67% amino acid identity and contain 7-transmembrane domains, but contrary to GPCRs, their N terminus is intracellular and the C terminus is extracellular (12, 13). Activation of either AdipoR in the periphery leads to downstream events via phosphorylation of AMP kinase (AMPK) (14). Both receptors have been detected in brain (10, 15–17). ADP was shown to enhance AMPK activity in the arcuate nucleus of the hypothalamus (Arc) via AdipoR1 to stimulate food intake (18). Certainly, metabolic signals relay indirect information to GnRH cells via neurons in the Arc (19). However, a direct action of ADP on GnRH neurons is also plausible due to the fact that: 1) AdipoR1 and AdipoR2 mRNA is found in hypothalamic regions (reviewed in Refs. 17, 20) containing GnRH neurons; 2) ADP inhibited GnRH secretion from an immortalized GnRH-expressing cell line (21); and 3) in rodents, the level of ADP, like GnRH, is tightly controlled during sexual differentiation, puberty, gestation, and lactation (22). Thus, ADP via hypothalamic GnRH cells may directly link food intake to reproductive function.
To address this issue, this study characterized the expression of AdipoRs in GnRH neurons and assayed whether ADP can directly alter GnRH neuronal activity. We show that 1) GnRH neurons express AdipoR2, 2) exogenous application of ADP directly decreased GnRH neuronal activity in a subpopulation of GnRH cells, and 3) the effect occurred via protein kinase C (PKC)ζ/liver kinase B (LKB)1 activation of AMPK.
Materials and Methods
Animals
All procedures were approved by National Institute of Neurological Disorder and Stroke, Animal Care and Use Committee and performed in accordance with National Institutes of Health (NIH) guidelines. GnRH-green fluorescent protein (GFP) mice were a generous gift from Dr D. Spergel and have been described previously (23). Tissue was taken from both GFP+/+ mice and from NIH Swiss mice.
In vivo
Adult female GnRH-GFP mice and NIH Swiss mice were anesthetized in a CO2 chamber, followed by cervical dislocation. Brains were removed, trimmed (cerebellum and lateral temporal lobes removed), and either immersed in 4% formaldehyde/PBS for 2–4 hours, rinsed in PBS, and stored at 4°C or frozen on dry ice and stored at −80°C until cutting. Sections were cut on a vibratome 3000 sectioning system (25–30 μm) (Ted Pella, Inc) or on a cryostat (16 μm) (Leica CM 3050S cyrostat; Leica Biosystems) and stored at −80°C.
In vitro
Timed-pregnant mice were euthanized in a CO2 chamber, followed by cervical dislocation and removal of embryos. Nasal regions were cultured as described in Klenke and Taylor-Burds (24). Briefly, nasal pits of embryonic day 11.5 NIH Swiss mice without regard to sex were isolated under aseptic conditions in Gey's Balanced Salt Solution (Gibco BRL) enriched with glucose (Sigma Chemical Co). Explants were adhered onto coverslips by a plasma (Cocalico Biologicals)/thrombin (Sigma) clot and maintained at 37°C in a defined serum-free medium (SFM) in a humidified atmosphere with 5% CO2. On culture day 3, fresh media containing fluorodeoxyuridine (8 × 10−5M; Sigma) were applied for 3 days to inhibit proliferation of dividing olfactory neurons and nonneuronal explant tissue. On culture day 6, and every 2 days afterward, the medium was changed with fresh SFM. Explants were used between 6–10 days in vitro (div).
PCR on single GnRH cells and explant cDNA
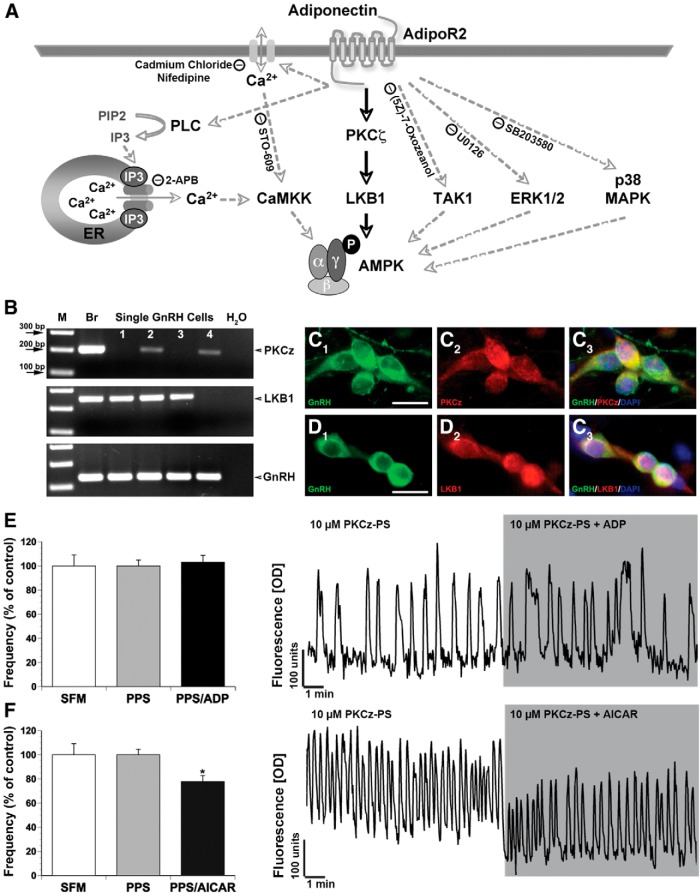
To determine the expression of AdipoRs, AMPK subunits, PKCζ, and LKB1, PCR was performed on single GnRH cell cDNAs (7 div, n = 10/15) and brain/liver cDNAs (positive control) as previously described (25, 26). Due to the technique used to generate the cDNA pools, 3′ untranslated region-biased primers were generated with the forward primers less than 500 bases from the polyA site. All primers were screened using BLAST to ensure specificity of binding. For each reaction, 30.5-μL H2O, 5-μL 10× PCR buffer (Applied Biosystems), 4-μL 25mM MgCl2 (Applied Biosystems), 5-μL deoxyribonucleotide mix (10mM each), 2-μL 6.25μM forward and reverse primer each, and 0.5-μL AmpliTaq Gold polymerase (Applied Biosystems) were added to 1-μL template cDNA. PCR was performed according to conditions as shown in Table 1. Amplified products were run on a 1.5% agarose gel. Specific bands of the predicted size were observed in the control lane (brain, liver), whereas no bands were detected in the negative control lane (water). cDNAs from single cells were initially screened by PCR for GnRH to assure the cell phenotype, and β-tubulin and L19 (microtubule and ribosomal, respectively), 2 housekeeping genes (see primer sequences in Ref. 27). Only cells positive for all 3 transcripts were used in this study (25, 27, 28).
Table 1.
Primer Sequences and PCR Conditions for ADP Receptors, AMPK Subunits, PKCζ, and LKB1 Analysis
| Gene | Primer | Sequence | Product size (bp) | Conditions 94°C/10 min, × ([/30 sec], [/30 sec], [/1 min]), 72°C/5 min |
|---|---|---|---|---|
| AdipoR1 | 1550F | 5′-caactgctgctccttcacag-3′ | 184 | 40× [94°C, 60°C, 72°C] |
| NM_028320.3 | 1733R | 5′-cccgttatcagccaggttac-3′ | ||
| AdipoR2 | 3466F | 5′-agcagggagactctggttca-3′ | 172 | 40× [94°C, 60°C, 72°C] |
| NM_197985.3 | 3637R | 5′-gaggcaccacaaggtcaagt-3′ | ||
| AMPKα1 | F1 | 5′-ttctaccaagtcgggagacg-3′ | 265 | 40× [94°C, 61°C, 72°C] |
| NM_001013367.3 | R1 | 5′-tgtgtggcattccattcatc-3′ | ||
| AMPKα2 | F2 | 5′-cccatcctcagtgtcaatcc-3′ | 162 | 40× [94°C, 61°C, 72°C] |
| NM_178143.2 | R2 | 5′-aaggcagcttcagacaacac-3′ | ||
| AMPKβ1 | F1 | 5′-cccaccctccttttagttcc-3′ | 233 | 40× [94°C, 61°C, 72°C] |
| NM_031869.2 | R1 | 5′-tgacaggtgacaggaccttg-3′ | ||
| AMPKβ2 | F1 | 5′-gtgatgtgacgtggaagtgg-3′ | 234 | 40× [94°C, 62°C, 72°C] |
| NM_182997.2 | R1 | 5′-cgctgacagaagacagcaag-3′ | ||
| AMPKγ1 | F1 | 5′-ctggaagctctgggaatcag-3′ | 220 | 40× [94°C, 62°C, 72°C] |
| NM_016781.2 | R1 | 5′-tctacccgaggagcacaaag-3′ | ||
| AMPKγ2 | F1 | 5′-tggatctggaaaggctatgc-3′ | 218 | 40× [94°C, 60°C, 72°C] |
| NM_145401.2 | R1 | 5′-gtttcccttcaactgcatgac-3′ | ||
| AMPKγ3 | F1 | 5′-gtctgcaggaaacaccactg-3′ | 163 | 40× [94°C, 60°C, 72°C] |
| NM_153744.3 | R1 | 5′-tcattggaccactggatctg-3′ | ||
| PKCζ | P2_F | 5′-ttagcctagtctgggggtcc-3′ | 204 | 40× [94°C, 60°C, 72°C] |
| NM_008860.3 | P2_R | 5′-caatccagggttccagtgc-3′ | ||
| LKB1 | 205F | 5′-gtgctccagcaacaagatcc-3′ | 248 | 40× [94°C, 64°C, 72°C] |
| NM_011492.3 | 452R | 5′-gaaaccaacccaaagacagc-3′ |
Immunocytochemistry (ICC)
Antibodies
Primary antibodies were rabbit polyclonal unless otherwise indicated: GnRH (SW-1, 1:3000) (29), mouse monoclonal anti-GnRH (F1D3C5, 1:4000; gift from Dr A. Karande, Department of Biochemistry, Indian Institute of Science, Bangalore, India) (30), chicken anti-GFP (1:1000; Abcam), AdipoR1 (in vivo 1:80 and in vitro 1:160; Abcam) and AdipoR2 (in vivo 1:875 and in vitro 1:800; Alpha Diagnostic), and PKCζ (1:1800; Novus Biologicals) and LKB1 (1:2000; Novus Biologicals). Antibody specificity was validated by 1) omission of the primary antibodies, 2) preabsorption of the AdipoR2 antibody with its immunogen (20 μg/mL; Alpha Diagnostic; see Supplemental Figure 1), and 3) examination of areas known to express AdipoR1 (31, 32) and AdipoR2 (17) (see Figure 1). Female mouse brain sections containing the Arc were used as positive controls (33) for staining of explants.
Figure 1.
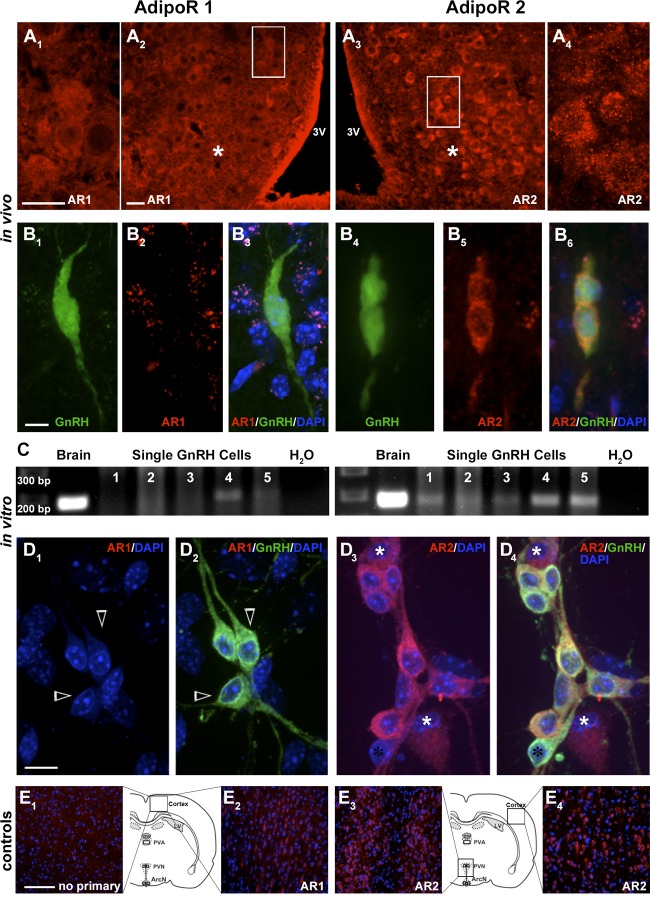
AdipoR2 is expressed in GnRH neurons. Mouse brain sections at the level of the Arc (asterisk) show cells immunopositive for AdipoR1 (AR1, A1 and A2, high magnification of boxed area [A2 shown in A1]) and AdipoR2 (AR2, A3 and A4, high magnification of boxed area [A3 shown in A4]). 3V, third ventricle. GnRH neurons in female adult GnRH-GFP mice do not express AdipoR1 (B1–B3). However, AdipoR2 is located on the cell membrane of GnRH cells (B4–B6). PCR on cDNAs from 5 single-cell GnRH neurons maintained in explants shows bands of appropriate size for AdipoR1 (C, left) in a small number of GnRH cells, whereas all neurons are positive for AdipoR2 (C, right). Primary GnRH neurons in explants do not stain for AdipoR1 (D1 and D2, open arrows), whereas most GnRH cells express AdipoR2 (D3 and D4, black asterisk single labeled GnRH positive cell; white asterisks single labeled AdipoR2 positive cells). Control staining for AdipoR1 and AdipoR2: omission of primary antibody (E1) and neurons positive for AdipoR1 in the cortex (E2), as well as for AdipoR2 in the PVN (E3) and cortex (E4). Scale bars: 50 μm (A1 and A2), 5 μm (B), 10 μm (D), and 100 μm (E).
AdipoR1, AdiopR2, PKCζ, and LKB1 immunofluorescence
Staining of the first antigen-antibody complex (AdipoR1, AdipoR2, PKCζ, or LKB1) was performed with donkey antirabbit Alexa Fluor 568 (1:1000; Molecular Probes). The second antigen-antibody complex (GnRH or GFP) was visualized with donkey antimouse Alexa Fluor 488 (1:1000; Molecular Probes) or donkey antichicken Alexa Fluor 488 (1:1000; Molecular Probes), depending on the species of the primary used. For analysis, pictures were taken on a spinning disk confocal system (CSU10; Yocogawa) mounted on an Eclipse TSE200 microscope (Nikon) using an EMCCD ImageM digital camera with I-Vision software (Biovision), or on a Nikon Eclipse E800 with a Retiga SRV camera using QCapture software (QImaging). Images were further analyzed using NIH ImageJ software (W. Rasband; NIH), and figures assembled in Adobe Photoshop CS.
Functional assays
Whole-cell patch clamp
Whole-cell patch clamp recordings were performed using a Multiclamp 700B amplifier (Molecular Devices) with a 1440A digidata (Molecular Devices) and analyzed in pClamp 10 (Molecular Devices). Electrodes were made from thick walled borosilicate glass pulled to a resistance of 4–8 MΩ on a Flaming/Brown Micropipette Puller (Sutter Instrument). After pipette liquid junctions were canceled, tight (>2 GΩ) seals were obtained, and pipette capacitance was neutralized before rupturing the patch. pClamp 10 Membrane Test was used to monitor cell quality with a 10-mV step. Recordings were made in I = 0 mode. Cells were accepted if after seal rupture access resistance was less than 20MΩ, and input resistance was greater than 800MΩ. Cell activity was monitored in I = 0 mode for 1–3 minutes before acute application of ADP (5 μg/mL) was applied with a Picospritzer III (Parker Hannifin Corp). ADP was applied for 4 seconds per 8–10 psi, approximately 20–30 μm from the cell soma. Electrodes used for drug application were the same as those used for cell recordings. Cells were classified as “responders” if they exhibited the same response to a second application of ADP, and hyperpolarizing responses had to be more than or equal to 2× the noise of the baseline.
Solutions
A modified artificial cerebrospinal fluid (aCSF) contained: 125 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 5 mM HEPES (free acid), and 20 mM glucose (pH 7.35–7.4) when bubbled with 95% CO2/5% O2, osmolarity 310–320 mOsm/L. Intracellular solution contained: 105 mM KCl, 30 mM K gluconate with 1 mM CaCl2, 10 mM HEPES-free acid, 5 mM EGTA, 4 mM MgATP, and 0.4 mM NaGTP. Intracellular solution was pH adjusted to 7.3 with KOH, osmolarity was 290–300 mOsm/L.
Calcium imaging
To determine the role of AdipoRs across multiple GnRH neurons, and the signal transduction pathways employed, experiments were performed on GnRH cells maintained in explants between 6 and 10 div as previously described (34). Briefly, Calcium Green-1 AM probe (Molecular Probes) was diluted to 2.7 mM in 80% dimethyl sulfoxide and 20% pluronic F-127 solution (Molecular Probes). This solution was diluted 1:200 with SFM to a final concentration of 13.5 μM. Explants were placed in loading solution at 37°C in a 5% CO2 humidified incubator for 20 minutes, washed twice with fresh SFM (10 minutes each), and mounted in a perfusion chamber and bathed in 400 μL of medium. Using a peristaltic pump (Spectra Hardware, Inc), medium was removed, and treatment solutions (400 μL) were applied consecutively. Calcium Green-1 was visualized using an inverted Nikon microscope, through a 20× fluorescence objective and a CCD camera (Retiga; QImaging) connected to a computer. Experiments were piloted by imaging software (IPVision Spectrum; Scanalytics, Inc) and pictures acquired every 2 seconds for 33 minutes. Excitation wavelengths were provided via a medium-width excitation bandpass filter at 465–495 nm, and emission was monitored through a 40-nm bandpass centered on 535 nm. Fluctuations in [Ca2+]i were analyzed a posteriori with IPVision software. Individual GnRH neurons were identified, and the region of interest was marked. Calcium Green-1 fluorescence intensity was plotted and analyzed with MATLAB (Mathworks). The recordings were divided into 2–3 periods for analysis; ie, for 2 periods, SFM control period (10 min) (Figure 2C) and treatment period (10 min); for 3 periods, SFM control period (10 min) (Figure 3D), pretreatment period (10 min), and treatment period (10 min). All recordings were terminated by a 40 mM KCl stimulation to ensure the viability of the recorded cells. The identity of the recorded cells was confirmed by staining explants with GnRH antiserum as previously described (35). Briefly, explants were fixed in 4% formaldehyde (1 h), rinsed in PBS, and placed in cryoprotectant (36) until staining for GnRH using standard Alexa Fluor 568 (1:1000; Molecular Probes) cytochemistry (Figure 2A).
Figure 2.
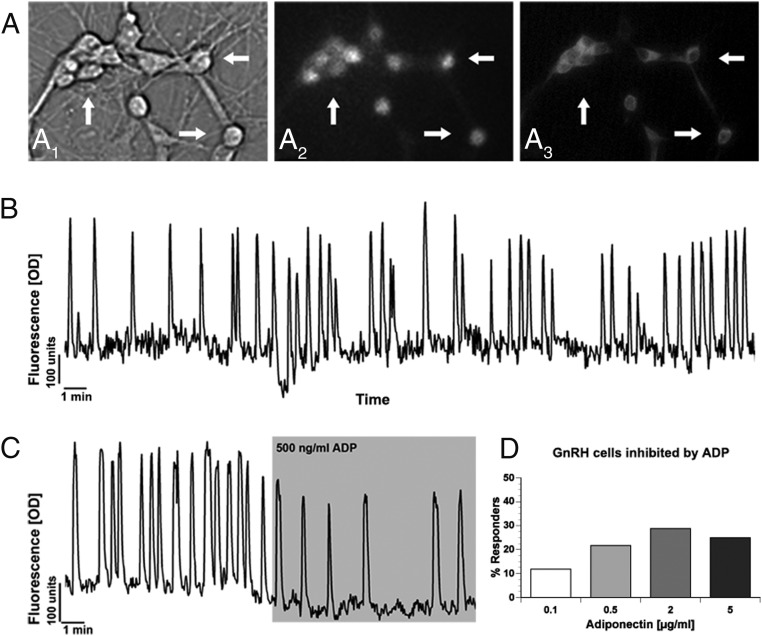
ADP inhibits GnRH neuronal activity. A, Calcium Green-1 imaging of GnRH neurons. Cells were identified by their bipolar morphology (A1, bright field), loaded with fluorescent calcium-sensitive dye (A2, fluorescence), and their identity was verified after imaging by ICC (A3, immunofluorescence). Arrows indicate identical cells in all fields. B, Representative recording showing spontaneous baseline oscillations in intracellular calcium levels in a single GnRH neuron during 30 minutes in SFMs (Y-scale, OD units). C, Representative recording showing spontaneous baseline oscillations in intracellular calcium levels in a GnRH neuron during 10 minutes in SFM and subsequent decrease in activity during a 10-minute superfusion of ADP (500 ng/mL) (Y-scale, OD units). D, Concentration of ADP (0.1–5 μg/mL) vs the percent of GnRH cells that were inhibited.
Figure 3.
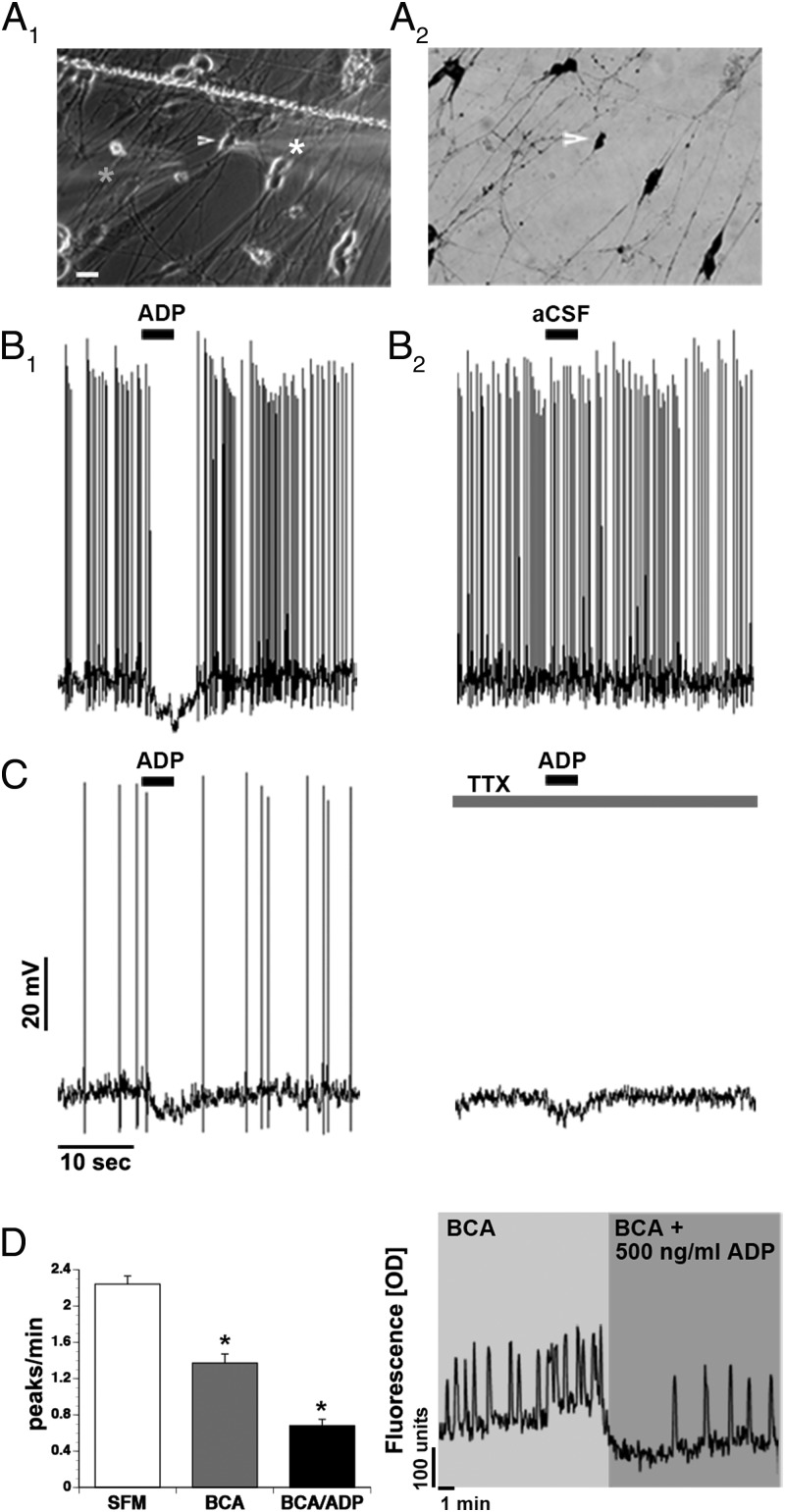
ADP inhibits GnRH neurons directly. A, Whole-cell recordings of GnRH neurons (A1 arrow/white star) showed an acute response to 5-μg/mL ADP applied with a pipette (A1 gray star). Explants were fixed and stained for GnRH after recordings were completed (A2). B, Example of spontaneously active GnRH neuron inhibited by a 4-second application of ADP (B1), replacing the ADP in the electrode with aCSF (B2). C, Example of GnRH neuron inhibited by a 4-second application of ADP ± TTX. D, The combined application of BIC/CNQX/AP5 (BAC) decreased the frequency of calcium oscillations in GnRH neurons. Coapplication with ADP (500 ng/mL) further decreased the neuronal activity. A representative calcium imaging recording showing spontaneous baseline oscillations in intracellular calcium levels of a GnRH neuron during 10-minute periods of BAC and BAC/ADP. Line in A1, scratch in coverslip. Scale bar: 10μM (A1 and A2).
Drugs
The globular form of ADP (Acrp30) was purchased from ProSpec. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) (an AMPK agonist), Compound C (CC) (an AMPK antagonist), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (an AMPA/kainite receptor antagonist), D-(-)-2-amino-5-phosphonopentanoic acid (AP5) (a N-methyl-D-aspartate [NMDA] receptor antagonist), Nifedipine (an L-type calcium channel blocker), thapsigargin (an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases), 2-aminoethoxydiphenylborane (an inositol trisphosphate receptor and transient receptor potential channel antagonist), dantrolene (a ryanodine receptor blocker), 7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate (STO-609) (a Ca2+/calmodulin-dependent kinase kinase [CaMKK]-β inhibitor), (5Z)-7-Oxozeanol (a transforming growth factor-β-activating kinase [TAK]1 inhibitor), pertussis toxin (a Gi-coupled protein inhibitor), 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene (U0126) (an ERK1/2 inhibitor), and 4-[5-(4-fluorophenyl)-2-[4-(methylsulfonyl)phenyl]-1H-imidazol-4-yl]pyri-dine (SB203580) (a p38 inhibitor) were purchased from Tocris. (-)-Bicuculline chloride (BIC) (a gamma-aminobutyric acid [GABA]A receptor antagonist), cadmium chloride (an external calcium blocker) and tetrodotoxin (TTX) (a Na channel blocker) were obtained from Sigma. MYR PKCζ Pseudosubstrate (a PKCζ inhibitor) was obtained from Invitrogen (Grand Island, NY). All stock solutions were stored at −20°C, and working solutions prepared before each experiment by diluting stock solutions (1:500 to 1:2000) into SFM.
Statistical Analysis
Calcium oscillations were monitored as a reflection of neuronal activity (32). A calcium elevation was first identified when a value was greater than the 5 previous and 5 subsequent points. The calcium elevation had to be greater than the mean of the 5 previous and 5 next points plus a minimal value (which represented small fluctuations in baseline) to be considered as a calcium oscillation or peak. The frequency of calcium oscillations was calculated as the number of detected calcium peaks per minute. Statistical analysis was performed using a paired t test to identify a drug effect on the peak frequency among a pool of cells. P ≤ .05 was chosen for significance. After detection of a statistical significant effect between treatment and control period, population analysis of GnRH neurons was performed. Cells were classified into responding and nonresponding. The peaks per minute value of each cell during the treatment period was subtracted from the average peaks per minute value for the same cell during the control period to produce a Δ peaks per minute (ΔPPM) value. The ΔPPM for each cell was then compared with a previously determined value indicating average fluctuations in calcium response between 2 SFM control periods plus SD (ΔPPM = ±0.6). GnRH neurons whose ΔPPM fell outside the ΔPPM range were grouped into a responder subpopulation and analyzed separately. Where indicated, post hoc analyses were performed to evaluate subpopulation dynamics. For the post hoc analyses, an unpaired, two-tailed t test was performed on individual cells in a group using Prism 5. In Results, n and N represent the number of cells and explants recorded, respectively, and error bars ±SEM.
Results
AdipoR2 is expressed by GnRH neurons
The presence of AdipoRs (AdipoR1 and AdipoR2) in GnRH neurons in vivo has not been demonstrated. AdipoR positive cells have been identified in vivo in cells of the Arc (33). Thus, staining conditions were established using sections containing the Arc. Because both antibodies being tested were against the extracellular C terminus, staining protocols ± Triton X-100 were tested. Staining of cells in the Arc for each receptor was clearly detected when Triton X-100 was omitted from the protocol (Figure 1A). Double-label immunofluorescence was performed on hypothalami of female adult GnRH-GFP mice. GnRH cells were detected that coexpressed AdipoR2 protein but not AdipoR1 (Figure 1B). Cell counts revealed that approximately 50% of the GnRH cells were AdipoR2+ (n = 125, N = 2) and that AdipoR2+/GnRH cells were distributed throughout the GnRH rostral-to-caudal continuum, eg, not confined to a specific anatomical area. Previous work has shown that GnRH neurons in nasal explants express a similar suite of receptors as GnRH neurons in the adult brain (26, 27, 37), including expression of receptors to metabolic modulators, such as neuropeptide Y (35). To determine whether this model could be employed to investigate how ADP alters GnRH neuronal activity, the expression of AdipoR1 and AdipoR2 transcripts was examined using cDNA obtained from single GnRH cells. AdipoR1 was expressed in some of the GnRH cells (4/10), whereas most single GnRH neurons tested positive for AdipoR2 transcript (9/10) (Figure 1C). ICC was then performed (with no detergent). Double-label immunofluorescence demonstrated that GnRH cells were negative for AdipoR1 but expressed protein for AdipoR2 (>95%) (Figure 1D), consistent with expression in GnRH cells in vivo.
Functional analysis of ADP signals on GnRH neurons
ADP inhibits GnRH neuronal activity
To determine the response of GnRH cells to ADP, calcium imaging was used to monitor individual GnRH neuronal responses, as well as GnRH neuronal population dynamics (Figure 2, A and B). GnRH cells were challenged with a single dose of globular ADP from 100 ng/mL (6.25 nM) to 5 μg/mL (312.5 nM) (Figure 2C). GnRH activity was significantly reduced at each concentration tested (Table 2). However, analysis of the GnRH population revealed that the percentage of responding cells (see Materials and Methods) changed from 12% (100 ng/mL) to 22% (500 ng/mL) and thereafter remained similar (29% at 2 μg/mL and 25% at 5 μg/mL) (Figure 2D). These data indicate that ADP reduced GnRH neuronal activity in a subpopulation of GnRH neurons.
Table 2.
Changes of Calcium Oscillations in GnRH Neurons in Response to ADP or AICAR
| Dose | Peaks/min | Peaks/min | Inhibition (Whole Population, %) | GnRH Responders (%) | Inhibition (GnRH Responders, %) | n | N |
|---|---|---|---|---|---|---|---|
| SFM | ADP | ||||||
| 0.1 μg/mL | 1.3 ± 0.08 | 1.15 ± 0.08a | 12 ± 6 | 12 | 18 ± 8 | 85 | 3 |
| 0.5 μg/mL | 1.58 ± 0.05 | 1.26 ± 0.06b | 20 ± 3 | 22 | 51 ± 5 | 157 | 4 |
| 2 μg/mL | 1.76 ± 0.07 | 1.32 ± 0.06b | 25 ± 4 | 29 | 48 ± 5 | 226 | 6 |
| 5 μg/mL | 1.31 ± 0.1 | 0.8 ± 0.07b | 38 ± 6 | 25 | 55 ± 9 | 60 | 2 |
| AICAR | |||||||
| 0.2 mM | 1.29 ± 0.06 | 1.31 ± 0.07 (NS) | 0 | 8 | 50 ± 8 | 74 | 2 |
| 0.5 mM | 1.45 ± 0.06 | 1.06 ± 0.06b | 26 ± 4 | 26 | 58 ± 7 | 128 | 4 |
| 1 mM | 0.98 ± 0.05 | 0.77 ± 0.05b | 22 ± 5 | 12 | 63 ± 8 | 116 | 4 |
t test: NS, P = .410.
P = .0008.
P < .0001.
GABAergic and glutamatergic signals are present in nasal explants and stimulate GnRH neuronal activity (38–41). Therefore, to confirm the specificity of the response, whole-cell patch clamp was employed (Figure 3). Cells were patched in the presence of the GABAA receptor antagonist BIC (20 μM), together with a cocktail of non-NMDA (CNQX; 10 μM) and NMDA (AP5; 10 μM) glutamatergic blockers (cocktail of BIC, CNQX, and AP5 [BCA]) to block ionotropic input. Forty-eight cells (N = 18) were recorded in I = 0 mode, and spontaneous activity was measured before and after a 4-second acute application of ADP (5 μg/mL) through a pipette located 20–30 μm from the cell soma and delivered with a Picospritzer. Forty-seven cells were spontaneously active. Baseline activity was monitored for 1–2 minutes (31/35) and showed a mean firing frequency of 2.2 ± 0.28 Hz. BCA was applied before ADP. Measurements were made during a 10-second window before and during the ADP application. Of the cells tested, 34 cells (70.8%) showed no response, 7 cells (14.6%) showed a more than or equal to 2-fold increase in firing rate, 1 cell had a more than or equal to 2-fold decrease in firing, and 6 (12.5%) were hyperpolarized (−7.7 ± 0.48 mV) (Figure 3B1). To confirm the specificity of the response, 1 hyperpolarized cell had the ADP containing pipette replaced and repositioned with 1 containing aCSF; as shown in Figure 3B2, no effect occurred with aCSF alone. In 6 of the responder cells (2 hyperpolarizing and 4 excitatory), 1 μM TTX was added and ADP reapplied. In the cells with an excitatory response, no change in membrane potential was observed in the presence of TTX, whereas the 2 hyperpolarizing responses were maintained, although they decreased in amplitude slightly (−5.8 to 5.0 mV and −7.6 to −4 mV) (Figure 3C).
Consistent with the patch clamp data, application of 500-ng/mL ADP in the presence of BCA decreased GnRH peaks/min, as measured by calcium imaging (SFM: 2.24 ± 0.09 peaks/min; BCA: 1.37 ± 0.10 peaks/min; BCA/ADP: 0.68 ± 0.07 peaks/min, n = 72, N = 2, P < .0001) (Figure 3D). Population analysis showed that BCA alone inhibited approximately 60% of the GnRH cells and that application of ADP (in the presence of BCA) further inhibited approximately 48% of these cells, eg, 26% of the GnRH neuronal population.
AdipoR2 signaling pathway
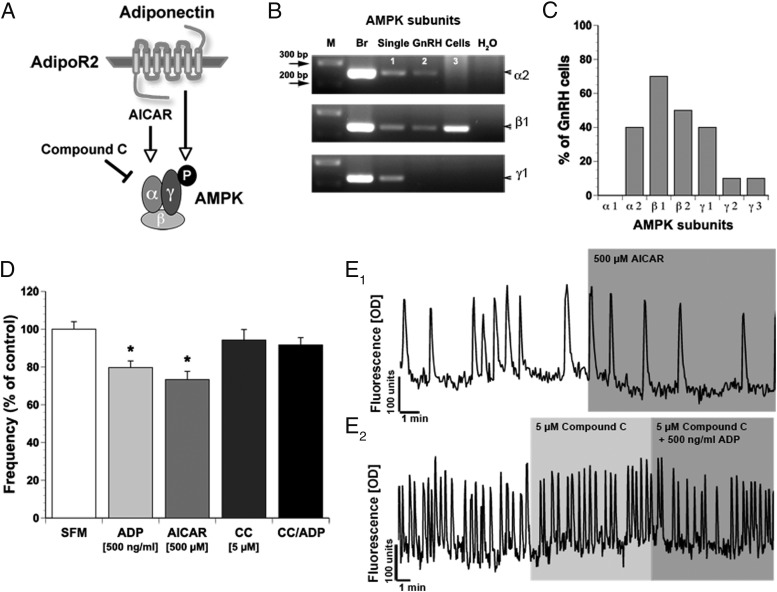
In the periphery, the main signaling pathway of AdipoR2 involves AMPK (Figure 4A). AMPK exists as an obligate heterotrimer, containing a catalytic subunit (α) and 2 regulatory subunits (β and γ). The presence of AMPK subunits in GnRH neurons was examined using single-cell PCR. All subunits except AMPKα1 were detected (Figure 4, B and C). AMPKα2, AMPKβ1, AMPKβ2, and AMPKγ1 were expressed in more than or equal to 40% of the cells (α2, 4/10; β1, 7/10; β2, 5/10; and γ1, 4/10). AMPKγ2 and AMPKγ3 transcripts were detected in only a small percentage of GnRH cells (both 1/10). All 3 subunits together were found in 20% of the cells examined. A specific AMPK agonist (AICAR) (42) was used (200 μM to 1 mM) (Table 2) to determine whether AdipoR2 in GnRH neurons potentially used this signaling pathway. The lowest dose of AICAR had no effect on GnRH neuronal activity, whereas higher doses decreased the frequency of calcium oscillations in a similar manner as ADP (AICAR 500 μM by 26% and 1 mM by 22%) (Table 2 and Figure 4, D and E1). Further, as observed with ADP, AICAR inhibited only a subpopulation of GnRH neurons (Table 2). The expression of AMPK subunits, together with the action of AICAR, is consistent with ADP signaling in GnRH neurons occurring via activation of AMPK.
Figure 4.
ADP inhibits GnRH neuronal activity via AMPK. A, Schematic representation. AMPK is activated via the AdipoR or by AICAR, an AMPK agonist, whereas CC, an AMPK antagonist, inhibits AMPK phosphorylation. B, AMPK subunits are expressed in single GnRH cells. PCR on cDNAs from single cells shows bands of appropriate size for AMPK α2, β1 and γ1 (M, marker; Br, brain; sGc, single GnRH cell). C, Expression of AMPK subunits varies among GnRH cells. D, AICAR decreases the frequency of calcium oscillations in GnRH neurons similar to ADP, whereas CC prevents the inhibitory ADP effect. E1 and E2 Representative calcium traces. E1, Spontaneous baseline oscillations in intracellular calcium levels during 10 minutes of SFM and subsequent decrease in activity during 10 minutes of AICAR (500μM) application. E2, CC (5μM, 10 min) had no effect on GnRH calcium activity, but coapplication of CC with ADP (500 ng/mL) prevented inhibition.
To verify that the inhibitory effect of ADP on spontaneous calcium oscillations was dependent on AMPK activation, a specific AMPK antagonist, CC (43), was used. Application of 5 μM CC had no effect on GnRH neuronal activity compared with SFM control (1.55 ± 0.06 peaks/min in SFM vs 1.49 ± 0.06 peaks/min in 5μM CC, n = 167, N = 3; P = .087) (Figure 4, D and E2). Coapplication of 5 μM CC with 500-ng/mL ADP resulted in no significant changes between the treatment groups (1.49 ± 0.06 peaks/min in 5 μM CC vs 1.47 ± 0.06 peaks/min in 5 μM CC + 500-ng/mL ADP, n = 167, N = 3; P = .267) (Figure 4, D and E2). These results confirm that AMPK is not constitutively activated in GnRH neurons and are consistent with activation of AMPK by ADP.
A set of experiments was performed to identify the signaling pathway activated after ADP binds to AdipoR2 on GnRH neurons. Multiple upstream kinases have been reported to activate AMPK (Figure 5A). ADP has been shown to increase intracellular Ca2+ levels from external sources through opening of membrane Ca2+ channels and intracellular Ca2+ stores in rat pituitary cells (44). An increase in intracellular Ca2+ causes activation of CaMKK-β (muscle cells [45], T-lymphocytes [46]). CaMKK-β has been shown to play a role in activating AMPK (Figure 5A). The response detected in GnRH cells exposed to ADP was a decrease in calcium signal. However, to abrogate the possibility of a localized calcium signal activating the CaMKK-β pathway, the ADP response was evaluated in the presence of external or internal Ca2+ channel blockers and a CaMKK-β-specific inhibitor, STO-609 (47). Under all conditions, the inhibitory ADP effect remained (Table 3), indicating that the ADP signal does not involve activation of CaMKK-β.
Figure 5.
ADP inhibits GnRH neurons via activation of AdipoR2 and subsequent PCK/LKB1 activation of AMPK. A, Schematic representation of AdipoR pathways that could possibly elicit the inhibitory effect of ADP. B, PCR on single GnRH cell cDNAs revealed expression of PKCζ and LKB1. GnRH neurons were immunopositive for PKCζ (C) and LKB1 (D). E, Coapplication of PPS (10μM, PKCζ pseudosubstrate, a PKCζ antagonist) together with ADP (500 ng/mL) prevented the inhibitory effect of ADP on the frequency of calcium oscillations in GnRH neurons. A representative calcium recording is shown. F, Coapplication of PPS (10μM) together with AICAR (500μM) did not prevent the inhibitory effect of AICAR on the frequency of calcium oscillations in GnRH neurons. A representative calcium recording is shown. Scale bar, 10 μm (C and D).
Table 3.
Signaling Pathways Not Used by ADP in GnRH Neurons
| Drug and Mechanism of Action | Pathway | Change upon ADP Addition (%) | GnRH Responders (Total) (%) | N | n |
|---|---|---|---|---|---|
| Cadmium chloride – external Ca2+ blocker | CaMKK-β | −10 | 15 | 4 | 116 |
| Nifedipine – external Ca2+ blocker | CaMKK-β | −23 | 25 | 3 | 127 |
| Thapsi-gargin – empties internal Ca2+ stores | CaMKK-β | −28 | 20 | 4 | 149 |
| 2-APB – IP3-R and TRP channel blocker | CaMKK-β | −31 | 25 | 2 | 77 |
| Dantrolene – ryanodine receptor blocker | CaMKK-β | −13 | 15 | 2 | 82 |
| STO-609 – CaMKK-β inhibitor | CaMKK-β | −15 | 20 | 5 | 219 |
| (5Z)-7-Oxozeanol – TAK 1 inhibitor | TAK1 | −16 | 20 | 2 | 56 |
| U0126 – ERK1/2 inhibitor | ERK1/2 | −38 | 35 | 4 | 99 |
| SB203580 – p38 inhibitor | p38 | −33 | 20 | 4 | 92 |
| Pertussis toxin – Gi inhibitor | Gi | −15 | 20 | 5 | 144 |
Abbreviation: Ca2+, calcium.
TAK1 has been reported to be a primary activator of AMPK in the rat hippocampus (48) as well as in mouse embryos, embryonic fibroblasts (49), and breast epithelial cells (Figure 5A) (50). TAK1 has also been linked to AdipoR1 and AdipoR2 by work in C2C12 myotubes (51). Therefore, the TAK1 inhibitor (5Z)-7-Oxozeanol (52) was used to investigate whether TAK1 is part of the AdipoR signaling pathway in GnRH neurons. Exposure to ADP in the presence of (5Z)-7-Oxozeanol still caused a decrease in GnRH neuronal activity (Table 3), indicating that TAK1 is not part of the downstream signaling pathway for the AdipoR2 in GnRH neurons.
Work in an immortalized neuronal cell line, suggested that ADP indirectly activates AMPK through phosphorylation of ERK1/2 (53). Application of the ERK1/2 inhibitor U0126 (54) to primary GnRH neurons did not alter the decrease in calcium oscillations seen in response to ADP (Table 3). In human prostate cancer cells, AMPK is also a downstream signaling molecule of p38 MAP kinase (Figure 5A) (55). Thus, we tested whether ADP activates AMPK via p38. However, application of the inhibitor SB203580 (56) did not prevent the effect of ADP. AdipoRs are part of the progestin and AdipoQ receptor family (57), which are characterized by a 7-transmembrane spanning sequence and an intracellular N-terminal and extracellular C-terminal domain. Other members of this family have been reported to signal through Gi-coupled proteins (58). To examine this pathway, explants were preincubated for 6 hours with pertussis toxin to uncouple Gi subunits. Subsequent application of ADP still caused a significant decrease in GnRH activity (Table 3). These results indicate that neither ERK1/2 nor p38 is part of the AdipoR pathway in GnRH neurons and that AdipoR2 does not signal through Gi-coupled proteins to activate AMPK in these cells.
In endothelial cells, the PKCζ/LKB1 pathway is a known activator of AMPK signaling (Figure 5A). Xie et al (49) showed that PKCζ-mediated LKB1 phosphorylation is required for metformin-stimulated LKB1 cytosolic transfer and subsequent AMPK activation. Single-cell PCR was performed and verified the presence of LKB1 in approximately 80% of GnRH cells (8/10) and PKCζ in approximately 40% of GnRH neurons (6/15), (Figure 5B). Double-label ICC confirmed the presence of both proteins in GnRH neurons (Figure 5, C and D). Next, PKCζ-pseudosubstrate (PS), a synthetic peptide that selectively inhibits PKCζ without affecting other PKC isoforms, was used (59). As shown in Figure 5B, application of 10 μM PKCζ-PS prevented the decrease of calcium oscillations in GnRH neurons in response to ADP (0.91 ± 0.04 peaks/min in PKCζ-PS vs 0.94 ± 0.05 peaks/min in PKCζ-PS/ADP (500 ng/mL), n = 107, N = 5; P = .275) (Figure 5B). However, application of AICAR in the presence of PKCζ-PS elicited the inhibitory response previously observed with ADP (1.30 ± 0.06 peaks/min in PKCζ-PS vs 1.01 ± 0.06 peaks/min in AICAR [500 μM], n = 162, N = 6; P = 2 × 10−10) (Figure 5C). Taken together, these results indicate that PKCζ is located upstream of AMPK in the AdipoR2 signaling pathway and is mandatory for the inhibitory effect to occur. To date, PKCζ has not been shown to directly activate AMPK. Therefore, these data imply that LKB1 links PCKζ and AMPK in GnRH neurons.
Discussion
It is well known that ADP has important peripheral functions (7). However, its role(s) in central regulation of reproduction is unclear. In mammals, reproductive function is closely related to energy homeostasis. Metabolic dysregulation, such as obesity and anorexia nervosa, often lead to reproductive abnormalities (reviewed in Ref. 60). GnRH neurons in the forebrain regulate reproductive function. The present study examined whether ADP could directly modulate GnRH activity. We show that GnRH neurons express AdipoR2 and that ADP rapidly decreased GnRH neuronal activity in a subpopulation of GnRH neurons via a PKCζ/LKB1/AMPK signaling cascade. These data indicate that a direct interaction between adipose tissue and GnRH neurons can occur, strengthening a link between metabolic function and reproduction.
Evidence for action of ADP within the brain is growing. Several brain areas express AdipoRs and show physiological or functional changes with ADP exposure (20, 33, 61), including the area postrema (15), Arc (18), and PVN (10), areas important in controlling autonomic function and feeding behavior. In the PVN, ADP has been shown to hyperpolarize oxytocin neurons but excite corticotropin-releasing hormone neurons and thyroid-releasing hormone neurons (62). Although ADP mRNA has been detected in chicken and mouse brain (63, 64), one cannot exclude peripherally derived ADP acting on central nervous system structures (20). In fact, ADP has been shown to reach concentrations in the CSF between 1% and 4% of that found in serum (8).
Cheng et al (65) demonstrated intracerebroventricular infusion of ADP in male rats inhibited the amplitude of LH secretion, a readout of GnRH activity (66). Certainly expression of AdipoR1 and AdipoR2 in cells in the Arc (33) provides an indirect means by which ADP could regulate reproduction. Connections from cells in this nucleus (both neuropeptide Y and β-endorphin neurons) to GnRH neurons have been shown (67). However, we found that GnRH neurons express AdipoR2 and that ADP suppresses GnRH neuronal activity, consistent with the reduction in LH levels seen in vivo by Cheng et al (65).
In addition to ADP, leptin is another key metabolic signal synthesized and secreted by fat cells, which communicates information about nutritional state to the reproductive axis. In a normal metabolic state, leptin increases GnRH activity/secretion (indirectly via afferent forebrain interneurons) (68) and enhances reproductive function in females (69). In cases of metabolic dysfunction, like obesity, leptin resistance can occur (70) eliminating a positive signal to the reproductive axis. Under the same condition, systemic ADP levels are reduced (71), removing an inhibitory signal to the reproductive axis. The combination of these metabolic signals does result in sub- or infertility (72). In this report, we demonstrate a direct action of ADP on GnRH activity, introducing another player to the complex role of metabolic regulation of reproduction. Clearly, more work is needed to understand how changes in metabolic stimulatory and inhibitory signals lead to disruption of the GnRH system under chronic conditions.
In a variety of nonneuronal cells, ADP has been shown to work via AMPK signaling (73–75). The experiments reported here show that GnRH neurons also use the AMPK downstream pathway. Recordings of calcium activity from GnRH neurons showed that AICAR, an AMPK agonist, mimicked the response observed with ADP, decreasing internal calcium oscillations, whereas CC, an AMPK antagonist, prevented the inhibitory effect of ADP. Several kinases have been reported to activate AMPK in nonneuronal cells, including CaMKK-β, TAK1 (45, 46, 48, 49), MAP kinases (like ERK1/2 and p38) (53, 55), and LKB1 (48–50) via PKC (76, 77). When these pathways were tested, only blockade of PKCζ inhibited the ADP response in GnRH neurons. PKCζ promotes AMPK activation by increasing phosphorylation via the AMPK kinase LKB1 (bovine aortic endothelial cells, human retinal pericytes, cultured rat vascular smooth muscle cells, and mouse 3T3-L1 preadipocytes) (49). PKCζ may in fact be one of the major kinases regulating AMPK activation, in the so-called AMP-independent pathways (49), by directly phosphorylating LKB1 (76, 77). GnRH neurons can now be added to the list of cells (endothelial cells, hepatocytes, skeletal muscle cells, and vascular smooth muscle cells) where PKCζ acts as a LKB1 kinase. Thus, the experiments in the present study show that the effect of ADP on GnRH neuronal activity operates via AdipoR2 and the PKCζ/LKB1/AMPK signaling cascade.
Although most GnRH neurons express AdipoR2, only a subpopulation of GnRH neurons showed a reduction in calcium activity. AMPK activation requires the phosphorylation of the α-subunit by the upstream kinases (78). PCR analysis showed that not all GnRH neurons expressed the AMPKα subunit, making the signaling cascade inactive. Whether or not more of the GnRH neurons can be recruited to ADP sensitivity under certain reproductive conditions (such as estrus cycle) or energy homeostasis changes is beyond the scope of this paper but is an interesting possibility. In conclusion, it is well known that ADP has important peripheral functions (7). However, our study adds to the growing evidence that a direct interaction between adipose tissue and the central nervous system also exists, affecting several neuroendocrine systems, including reproduction.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorder and Stroke Grant NS002824-23.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- ADP
- adiponectin
- AdipoR
- ADP receptor
- AICAR
- 5-aminoimidazole-4-carboxamide ribonucleoside
- AMPK
- AMP kinase
- AP5
- D- (-)-2-amino-5-phosphonopentanoic acid
- Arc
- arcuate nucleus of the hypothalamus
- BCA
- cocktail of BIC, CNQX, and AP5
- BIC
- bicuculline chloride
- CaMKK
- Ca2+/calmodulin-dependent kinase kinase
- CC
- Compound C
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- div
- days in vitro
- GABA
- gamma-aminobutyric acid
- GFP
- green fluorescent protein
- ICC
- immunocytochemistry
- LKB
- liver kinase B
- NIH
- National Institutes of Health
- NMDA
- N-methyl-D-aspartate
- PKC
- protein kinase C
- ΔPPM
- Δ peaks per minute
- PS
- pseudosubstrate
- PVN
- paraventricular nucleus
- SB203580
- 4-[5-(4-fluorophenyl)-2-[4-(methylsulfonyl)phenyl]-1H-imidazol-4-yl]pyridine
- SFM
- serum-free medium
- STO-609
- 7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate
- TAK
- transforming growth factor-β-activating kinase
- TTX
- tetrodotoxin
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene.
References
- 1. Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81:289–317 [DOI] [PubMed] [Google Scholar]
- 2. Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1988;84:37–46 [DOI] [PubMed] [Google Scholar]
- 3. Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstural abnormalities in women. Int J Obes. 1979;3:57–73 [PubMed] [Google Scholar]
- 4. Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21:432–438 [DOI] [PubMed] [Google Scholar]
- 5. Rajala MW, Scherer PE. Minireview: the adipocyte–at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773 [DOI] [PubMed] [Google Scholar]
- 6. Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293 [DOI] [PubMed] [Google Scholar]
- 7. Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine. 2010;37:11–32 [DOI] [PubMed] [Google Scholar]
- 8. Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529 [DOI] [PubMed] [Google Scholar]
- 9. Neumeier M, Weigert J, Buettner R, et al. Detection of adiponectin in cerebrospinal fluid in humans. Am J Physiol Endocrinol Metab. 2007;293:E965–E969 [DOI] [PubMed] [Google Scholar]
- 10. Hoyda TD, Fry M, Ahima RS, Ferguson AV. Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol. 2007;585:805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wray S, Hoffman G. A developmental study of the quantitative distribution of LHRH neurons within the central nervous system of postnatal male and female rats. J Comp Neurol. 1986;252:522–531 [DOI] [PubMed] [Google Scholar]
- 12. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769 [DOI] [PubMed] [Google Scholar]
- 13. Deckert CM, Heiker JT, Beck-Sickinger AG. Localization of novel adiponectin receptor constructs. J Recept Signal Transduct Res. 2006;26:647–657 [DOI] [PubMed] [Google Scholar]
- 14. Kola B. Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol. 2008;20:942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fry M, Smith PM, Hoyda TD, et al. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci. 2006;26:9695–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thundyil J, Tang SC, Okun E, et al. Evidence that adiponectin receptor 1 activation exacerbates ischemic neuronal death. Exp Transl Stroke Med. 2010;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Repunte-Canonigo V, Berton F, Cottone P, et al. A potential role for adiponectin receptor 2 (AdipoR2) in the regulation of alcohol intake. Brain Res. 2010;1339:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68 [DOI] [PubMed] [Google Scholar]
- 19. Crown A, Clifton DK, Steiner RA. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology. 2007;86:175–182 [DOI] [PubMed] [Google Scholar]
- 20. Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wen JP, Lv WS, Yang J, et al. Globular adiponectin inhibits GnRH secretion from GT1–7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun. 2008;371:756–761 [DOI] [PubMed] [Google Scholar]
- 22. Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276 [DOI] [PubMed] [Google Scholar]
- 23. Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klenke U, Taylor-Burds C. Culturing embryonic nasal explants for developmental and physiological study. Curr Protoc Neurosci. 2012;Chapter 3:Unit 3.25.1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramer PR, Krishnamurthy R, Mitchell PJ, Wray S. Transcription factor activator protein-2 is required for continued luteinizing hormone-releasing hormone expression in the forebrain of developing mice. Endocrinology. 2000;141:1823–1838 [DOI] [PubMed] [Google Scholar]
- 26. Sharifi N, Reuss AE, Wray S. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor β transcript. Endocrinology. 2002;143:2503–2507 [DOI] [PubMed] [Google Scholar]
- 27. Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci. 2004;24:4737–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Temple JL, Wray S. Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J Neuroendocrinol. 2005;17:591–599 [DOI] [PubMed] [Google Scholar]
- 29. Wray S, Gähwiler BH, Gainer H. Slice cultures of LHRH neurons in the presence and absence of brainstem and pituitary. Peptides. 1988;9:1151–1175 [DOI] [PubMed] [Google Scholar]
- 30. Gangatirkar P, Gangadharan S, Narendranath A, Nagpal S, Salunke DM, Karande AA. Monoclonal antibodies to gonadotropin-releasing hormone (GnRH) inhibit binding of the hormone to its receptor. Hybrid Hybridomics. 2002;21:281–286 [DOI] [PubMed] [Google Scholar]
- 31. Kang KH, Higashino A, Kim HS, Lee YT, Kageyama T. Molecular cloning, gene expression, and tissue distribution of adiponectin and its receptors in the Japanese monkey, Macaca fuscata. J Med Primatol. 2009;38:77–85 [DOI] [PubMed] [Google Scholar]
- 32. Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2009;89:38–47 [DOI] [PubMed] [Google Scholar]
- 33. Guillod-Maximin E, Roy AF, Vacher CM, et al. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol. 2009;200:93–105 [DOI] [PubMed] [Google Scholar]
- 34. Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation. Endocrinology. 2008;149:3500–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151:2736–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watson REJ, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159 [DOI] [PubMed] [Google Scholar]
- 37. Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;150:1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wray S, Fueshko SM, Kusano K, Gainer H. GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol. 1996;180:631–645 [DOI] [PubMed] [Google Scholar]
- 39. Moore JP, Jr, Shang E, Wray S. In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci. 2002;22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci USA. 1995;92:3918–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Constantin S, Klenke U, Wray S. The calcium oscillator of GnRH-1 neurons is developmentally regulated. Endocrinology. 2010;151:3863–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565 [DOI] [PubMed] [Google Scholar]
- 43. Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steyn FJ, Boehme F, Vargas E, et al. Adiponectin regulate growth hormone secretion via adiponectin receptor mediated Ca(2+) signalling in rat somatotrophs in vitro. J Neuroendocrinol. 2009;21:698–704 [DOI] [PubMed] [Google Scholar]
- 45. Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191 [DOI] [PubMed] [Google Scholar]
- 46. Tamás P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J Biol Chem. 2002;277:15813–15818 [DOI] [PubMed] [Google Scholar]
- 48. Park HG, Yi H, Kim SH, et al. The effect of cyclosporine A on the phosphorylation of the AMPK pathway in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1933–1937 [DOI] [PubMed] [Google Scholar]
- 49. Xie Z, Dong Y, Zhang M, et al. Activation of protein kinase C ζ by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375 [DOI] [PubMed] [Google Scholar]
- 50. Herrero-Martín G, Høyer-Hansen M, García-García C, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xin X, Zhou L, Reyes CM, Liu F, Dong LQ. APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3–p38 MAPK pathway. Am J Physiol Endocrinol Metab. 2011;300:E103–E110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ninomiya-Tsuji J, Kajino T, Ono K, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490 [DOI] [PubMed] [Google Scholar]
- 53. Ruscica M, Dozio E, Steffani L, et al. Role of the energy sensor adenosine monophosphate-activated protein kinase in the regulation of immature gonadotropin-releasing hormone neuron migration. J Endocrinol Invest. 2011;34:e362–e368 [DOI] [PubMed] [Google Scholar]
- 54. Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632 [DOI] [PubMed] [Google Scholar]
- 55. Tang CH, Lu ME. Adiponectin increases motility of human prostate cancer cells via adipoR, p38, AMPK, and NF-κB pathways. Prostate. 2009;69:1781–1789 [DOI] [PubMed] [Google Scholar]
- 56. Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746 [DOI] [PubMed] [Google Scholar]
- 57. Tang YT, Hu T, Arterburn M, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380 [DOI] [PubMed] [Google Scholar]
- 58. Thomas P, Pang Y, Dong J, et al. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718 [DOI] [PubMed] [Google Scholar]
- 59. Chen HC, Bandyopadhyay G, Sajan MP, et al. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-β-D-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562 [DOI] [PubMed] [Google Scholar]
- 60. Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism. 2013;62:457–478 [DOI] [PubMed] [Google Scholar]
- 61. Mimee A, Smith PM, Ferguson AV. Circumventricular organs: targets for integration of circulating fluid and energy balance signals? Physiol Behav. 2013;121:96–102 [DOI] [PubMed] [Google Scholar]
- 62. Hoyda TD, Samson WK, Ferguson AV. Adiponectin depolarizes parvocellular paraventricular nucleus neurons controlling neuroendocrine and autonomic function. Endocrinology. 2009;150:832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maddineni S, Metzger S, Ocón O, Hendricks G, 3rd, Ramachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146:4250–4256 [DOI] [PubMed] [Google Scholar]
- 64. Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86:191–209 [DOI] [PubMed] [Google Scholar]
- 65. Cheng XB, Wen JP, Yang J, Yang Y, Ning G, Li XY. GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine. 2011;39:6–12 [DOI] [PubMed] [Google Scholar]
- 66. Levine JE, Duffy MT. Simultaneous measurement of luteinizing hormone (LH)-releasing hormone, LH, and follicle-stimulating hormone release in intact and short-term castrate rats. Endocrinology. 1988;122:2211–2221 [DOI] [PubMed] [Google Scholar]
- 67. Ward DR, Dear FM, Ward IA, et al. Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. PLoS One. 2009;4:e5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hausman GJ, Barb CR, Lents CA. Leptin and reproductive function. Biochimie. 2012;94:2075–2081 [DOI] [PubMed] [Google Scholar]
- 70. Ahima RS, Qi Y, Singhal NS. Adipokines that link obesity and diabetes to the hypothalamus. Prog Brain Res. 2006;153:155–174 [DOI] [PubMed] [Google Scholar]
- 71. Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 72. Bohler H, Jr, Mokshagundam S, Winters SJ. Adipose tissue and reproduction in women. Fertil Steril. 2010;94:795–825 [DOI] [PubMed] [Google Scholar]
- 73. Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 74. Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fang X, Palanivel R, Cresser J, et al. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab. 2010;299:E721–E729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Song P, Xie Z, Wu Y, Xu J, Dong Y, Zou MH. Protein kinase Cζ-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J Biol Chem. 2008;283:12446–12455 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77. Xie Z, Dong Y, Zhang J, Scholz R, Neumann D, Zou MH. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol Cell Biol. 2009;29:3582–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hawley SA, Davison M, Woods A, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887 [DOI] [PubMed] [Google Scholar]