Abstract
This study investigated potential mechanisms by which age and IGF-I receptor (IGF-Ir) signaling in the neuroendocrine hypothalamus affect estradiol-positive feedback effects on GnRH neuronal activation and on kisspeptin and N-methyl-D-aspartate (NMDA)-induced LH release and on the abundance of NMDA receptor subunits Nr1 and Nr2b and Kiss1r transcript and protein in the hypothalamus of young and middle-aged female rats. We infused vehicle, IGF-I, or JB-1, a selective antagonist of IGF-Ir, into the third ventricle of ovariectomized female rats primed with estradiol or vehicle and injected with vehicle, kisspeptin (3 or 30 nmol/kg), or NMDA (15 or 30 mg/kg). Regardless of dose, NMDA and kisspeptin resulted in significantly more LH release, GnRH/c-Fos colabeling, and c-Fos immunoreative cells in young than in middle-aged females. Estradiol priming significantly increased Kiss1r, Nr1, and Nr2b receptor transcript and protein abundance in young but not middle-aged female hypothalamus. JB-1 attenuated kisspeptin and NMDA-induced LH release, numbers of GnRH/c-Fos and c-Fos cells, and Kiss1r, Nr1, and Nr2b transcript and protein abundance in young females to levels observed in middle-aged females. IGF-I significantly enhanced NMDA and kisspeptin-induced LH release in middle-aged females without increasing numbers of GnRH/c-Fos or c-Fos immunoreactive cells. IGF-I infusion in middle-aged females also increased Kiss1r, Nr1, and Nr2b protein and transcript to levels that were equivalent to young estradiol-primed females. These findings indicate that age-related changes in estradiol-regulated responsiveness to excitatory input from glutamate and kisspeptin reflect reduced IGF-Ir signaling.
Female reproductive physiology is coordinated by the sequential actions of estradiol (E2) and progesterone (P) in the brain. We and others have demonstrated that a number of E2-regulated processes in the brain require concomitant signaling by IGF-I receptors (IGF-Ir). Estrogen receptors (ERs) and IGF-Ir are colocalized in the hypothalamus (1, 2), and GnRH neurons express both IGF-I and IGF-Ir (3, 4). Brain IGF-Ir antagonism impairs E2-positive feedback, reduces E2- and P-dependent LH surges, and disrupts estrous cycling independent of body weight or food intake changes (5, 6). These observations support the hypothesis that ongoing signaling by brain IGF-Ir is necessary for E2 regulation of female reproductive physiology.
Female reproductive aging is characterized by delayed and attenuated LH surges and reduced responsiveness to E2 (7, 8). Age-related LH surge dysfunction results from reduced hypothalamic excitatory neurotransmission (9–12) and GnRH neuronal activation during conditions of E2-positive feedback (5, 13–16) and decreased brain and peripheral IGF-I levels (17–20). Moreover, in coordination with E2, IGF-I modulates many neurotransmitter pathways that change with reproductive senescence (21, 22). We demonstrated that intracerebroventricular (icv) infusion of IGF-I partially restores LH surge amplitude in middle-aged rats (17), thereby suggesting that decreased brain IGF-Ir signaling contributes to age-related LH surge dysfunction. However, the mechanism(s) by which IGF-I affects E2-dependent, neuroendocrine function is unclear. We hypothesize that IGF-I modulates sensitivity to N-methyl-D-aspartic acid (NMDA) and kisspeptin neurotransmission, inputs that stimulate GnRH-LH release, under E2-positive feedback conditions (23–26). We tested this hypothesis by determining the effect of age and IGF-Ir signaling on hypothalamic-pituitary axis responsiveness to NMDA and kisspeptin under E2-positive feedback conditions, assessed by LH release and percentages of GnRH neurons coexpressing the immediate early gene c-Fos, and on E2 regulation of the expression of NMDA and kisspeptin receptors in the hypothalamus. To modulate IGF-I signaling, young females were chronically infused with an IGF-Ir antagonist, and middle-aged females were chronically infused with an IGF-I dose known to enhance the LH surge (17).
Materials and Methods
Animals
Young (3–4 mo) and middle-aged (retired breeders, 9–11 mo) female Sprague Dawley rats (Taconic Farms) were housed in groups of three and maintained on a 14-hour, light, 10-hour dark cycle with free access to chow and water. Only rats with at least two regular 4- to 5-day estrous cycles were included in the studies. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Einstein College of Medicine.
Stereotaxic surgery and osmotic minipump placement
Females anesthetized with im ketamine(80 mg/kg) and xylazine(4 mg/kg) mixtures were ovariectomized (OVX) and placed in a Kopf stereotaxic apparatus with the nose bar set at +5.0 mm. A 22-gauge guide cannula (Plastics One) was placed into the third ventricle (anterior/posterior ± 0.2 mm; medial/lateral 0.0 mm; dorsal/ventral −7.8 mm with respect to bregma) and anchored with dental acrylic (6). JB-1, IGF-I, or vehicle was delivered continuously with an Alzet minipump (model 2002 delivering 0.5 μl/h) connected to a 26-gauge internal cannula that extended 1 mm below the guide (5). Guide cannula placement was verified with dye infusion. Only rats with confirmed cannula placement were included in the data analysis. Artificial cerebrospinal fluid (aCSF; 140 mM NaCl; 3 mM KCl; 1.2 mM Na2HPO4; 1 mM MgCl2; 0.27 mM NaH2PO4; 1.2 mM CaCl2; and 7.2 mM dextrose, pH 7.4) was used as vehicle and to dissolve drugs. JB-1 (100 μg/mL; Bachem), a peptide that selectively blocks IGF-I binding to IGF-Irs and attenuates IGF-Ir autophosphorylation (27), was infused into some young rats. Human IGF-I (2 μg/mL; Gropep) was infused icv into some middle-aged rats. The doses of JB-1 and IGF-I were selected based on regimens previously used in our laboratory to attenuate LH surges in young and to amplify LH surges in middle-aged female rats, respectively (6, 17). IGF-I, JB-1, or aCSF infusion began immediately after minipump placement and continued until rats were killed.
Jugular vein catheterization, hormone priming, iv drug infusion, blood sampling, and LH assay
Females were anesthetized and a jugular vein catheter was placed into the right atrium for serial blood sampling 7 days after OVX and stereotaxic surgery. Catheters were kept patent by daily heparinized saline (50 U/mL) (9). At 9:00 am on the day of the catheter placement, E2-positive feedback conditions were created by giving rats the first of two daily sc injections of 2 μg of E2 benzoate (E2B; Steraloids Inc). On the day of the anticipated LH surge, females received iv injections of NMDA (15 or 30 mg/kg; Sigma) or mouse kisspeptin-10 (110–119)-amide (Kp-10; 3 or 30 nmol/kg; Phoenix Pharmaceutical) approximately 25 hours after the second E2B. The rodent analog of human C-terminal kisspeptin decapeptide (112–121)-NH2 was chosen because it has equivalent effects on LH release as the full-length peptide, and it rescued the LH surge in middle-aged females (25).
Blood sampling started 48 hours after the first E2B injection (baseline) and continued every 10–30 minutes for a total of six samples. Approximately 300 μL of blood was collected into Eppendorf tubes containing 100 μL of ice-cold heparinized saline (15 IU), refrigerated overnight, and centrifuged at 10 000 × g for 10 minutes. Plasma was removed with a glass pipette and stored at −70 C for LH assays. An equal volume of warmed saline was infused to replace collected blood and to avoid hypovolemia. Plasma LH was determined by the University of Virginia Center Ligand Core Laboratory using a RIA in duplicate with rat double-antibody assays. The lower limit of the LH assay and the intra- and interassay coefficients of variation were 0.04 ng/mL and 3.9% and 5.7%, respectively.
Immunohistochemistry for GnRH and c-Fos staining
The immunohistochemistry technique was adapted from protocols described by Hoffman and colleagues (14, 28) and performed as previously described (5). Briefly, OVX females were primed with E2B and then injected iv with vehicle (controls), NMDA, or Kp10 25 hours as described above. Rats were perfused between 1:00 pm and 2:30 pm with 4% paraformaldehyde in phosphate buffer (pH 6.8). Brains were postfixed in 4% paraformaldehyde overnight at 4°C and cryopreserved in 30% sucrose. Six sets of coronal sections (30 μm) starting at the level of the organum vasclosum of the lamina terminalis (bregma +0.48 mm) and continuing through the medial preoptic area (mPOA; bregma −0.72 mm) were collected from each female, with each set containing every sixth section. Sections were stored in cryoprotectant at −20°C until immunolabeling.
Tissue sections were rinsed in potassium PBS (KPBS; 0.05 M, pH 7.4), incubated in 3% H2O2 for 10 minutes, and incubated in KPBS plus 0.04% Triton X-100 (KPBS-Tx) and 1% BSA for 1 hour at room temperature. Sections were next incubated in goat anti-c-Fos antibody (1:1000; Santa Cruz Biotechnology Inc) in KPBS-Tx and 1% BSA for 48 hours at 4°C and incubated in biotinylated antigoat IgG (1:600; Vector Laboratories) in KPBS-Tx for 1 hour at room temperature, rinsed, and incubated for 1 hour in avidin biotin complex (Elite avidin biotin complex kit; Vector Laboratories). Sections were rinsed in KPBS and 0.175 M sodium acetate and stained in nickel sulfate (25 mg/mL) and diaminobenzidine-HCl (0.2 mg/mL) in 0.175 M sodium acetate containing 30% H2O2 for 10 minutes followed by KPBS/sodium acetate rinse. c-Fos immunoreactivity (ir) was visualized as blue-black in the nuclei of neurons. For colocalization of c-Fos in GnRH neurons, the sequence of reactions was repeated, substituting rabbit-anti GnRH antiserum as the primary antibody (1:5000, LR-5; gift from Dr R. Benoit, McGill University, Montréal, Canada) for 24 hours at 4°C. After rinsing, sections were incubated in biotinylated antirabbit IgG (Vector Laboratories) diluted (1:600) in KPBS-Tx for 1 hour at room temperature, rinsed, and reacted with the avidin biotin complex as described above. A mixture of H2O2 and diaminobenzidine-HCl in Tris (Sigma Aldrich Inc; 0.05 M, pH 7.2) was used as the chromogen to yield a brown cytoplasmic staining. Sections were mounted onto Superfrost Plus slides (Fisher Scientific), dried overnight, dehydrated with ascending alcohol concentrations, cleared with xylenes, and coverslipped. A series of tissue sections was treated identically except primary antibody was omitted from the incubation to confirm antibody specificity.
To quantify GnRH and c-Fos-ir neurons, five sections of organum vasclosum of the lamina terminalis and mPOA were viewed under a microscope (Zeiss Axioversion; Carl Zeiss) at ×40 magnification. c-Fos-ir cells are identified by distinct blue-black nuclear staining and GnRH-ir cells by brown cytoplasmic staining. GnRH neurons expressing c-Fos were counted if cells had both brown cytoplasmic and blue-black nuclear staining. Cell counting was performed by two counters blinded to treatment (inter-rater variation < 10.5%) and average cell counts reported. Total numbers of GnRH and c-Fos-ir cells and the percentage of GnRH neurons expressing c-Fos were calculated and analyzed as previously described (5).
Primers, reverse transcription reaction, and real-time quantitative PCR
Independent groups of OVX, young, and middle-aged rats were primed with vehicle or E2B for 2 days as described above. Rats were killed 26–27 hours after the second E2B or oil injection (29), and as previously described, the hypothalamus dissected and transected just posterior to the optic chiasm (10). Because IGF-I modulates synaptic remodeling in the arcuate nucleus (1, 2, 30), only the posterior hypothalamus, which includes the arcuate and ventromedial hypothalamus, were collected, snap frozen on dry ice, and stored at −80 C.
DNA-free total RNA was isolated with RNAeasy minikit (QIAGEN) including a deoxyribonuclease step. RT was performed using Thermo Scientific Verso cDNA kit (Fisher Scientific) and Superscript II reverse transcriptase (Invitrogen Life Technologies). Primer3Plus software (Free Software Foundation, Inc) was used to design esr1, igf, kiss1r, nr1, and nr2b primers (Table 1). Real-time PCR was performed using a Lightcycler Roche 480 Absolute Blue quantitative PCR SYBR Green mix reagents (Fisher Scientific). The β-actin gene was used for transcript normalization. The real-time PCR samples were prepared in triplicate, and the mean values were used to calculate relative transcript levels using the ΔΔcycle threshold method (29).
Table 1.
Forward and Reverse Primers Used for Quantitative Real-Time PCR
| Forward Primer (5′–3′) | Reverse Primer (5′–3′) | |
|---|---|---|
| β-Actina | aggtcatcactatcggcaatg | gcactgtgttggcatagaggt |
| esr1 | tccacgatcaagttcaccttc | ccaccatgccttctacacatt |
| igf-1r | attttgaagggcaatctgctt | ggttcttcaggaaggacaagg |
| kiss1r | gtttttcgctgccctaatgtt | gtacgcagcacagaaggaaag |
| nr2b | acaaagaacgcagtgacgact | tccacattggtcaggttcttc |
| nr1 | cacggctcttggaagatacag | gtgaagtggtcgttgggagta |
Housekeeping gene.
Western blotting
Independent groups of OVX, young, and middle-aged rats primed with oil or E2B as described above were killed 26–27 hours after the second E2B or oil injection. Posterior hypothalamic tissue fragments were dissected as described above, homogenized, and extracted in Tissue-PE LB lysis buffer (G Biosciences) and protease inhibitor cocktail (Roche). Protein concentrations were determined by a Bio-Rad protein assay dye reagent concentrate (Bio-Rad Laboratories). Fifty-microgram protein samples were loaded and separated on 10% SDS-PAGE gels (Bio-Rad Laboratories), blotted with primary antibodies of interest, and incubated with species-specific secondary antibodies, antirabbit IRDye 800CW, and antimouse or antigoat ALEXA680. Membranes were scanned and quantified with the ODYSSEY infrared imaging system (LI-COR). Antibodies used were estrogen receptor alpha (sc-7207, 1:500; Santa Cruz Biotechnology, Inc), IGF-Ir (sc-7952, 1:500; Santa Cruz Biotechnology, Inc), Kiss1r (sc-134499, 1:500, Santa Cruz Biotechnology, Inc), Nr1 (NB100–74473, 1:600; Novus Biological), and Nr2b (06–600, 1:500; Millipore). β-Actin was used for protein normalization and quantification.
Data analysis
GraphPad Prism 6 software was used for statistical analysis. Data are expressed as mean ± SEM. Area under the curve (AUC) was determined with SigmaPlot 10 using the trapezoidal rule. A two-way ANOVA was used for each drug dose with repeated measures on time to determine the effect of age/treatment on Kp10 and NMDA-induced LH release. A two-way ANOVA with dose and age/treatment as factors was used to determine the effect of Kp10 and NMDA dose on total LH release. One-way ANOVA was also used to analyze group differences in GnRH and c-Fos cell numbers, percentage of GnRH neurons expressing c-Fos, and mRNA and protein abundance. Post hoc Turkey's test was used for multiple comparisons of different groups. P < .05 was considered statistically significant. If data were not normally distributed, an appropriate nonparametric test was used.
Results
JB-1 attenuates and IGF-I potentiates Kp10-induced LH release in young and middle-aged females, respectively
To determine the effect of age and IGF-Ir signaling on Kp10-induced peak and total LH (AUC) release under E2-positive feedback conditions, OVX females primed with E2B were injected iv with vehicle (control) or 3 or 30 nmol/kg of Kp10. Samples were collected 60 and 30 minutes before the injection at 10:00 am (baseline) and then every 10–30 minutes after the Kp10 injection for a total of six samples. Some young and middle-aged females were chronically infused icv with the IGF-Ir antagonist JB-1 or IGF-I, respectively (see Materials and Methods).
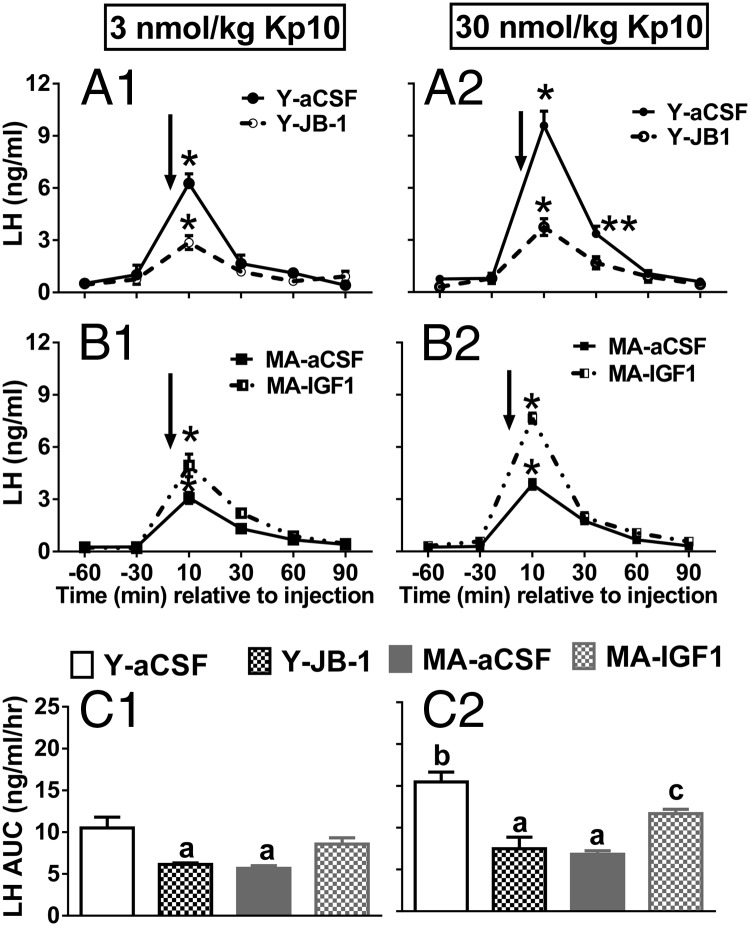
To determine the effect of age and IGF-Ir signaling on Kp10-induced LH release, a two-way ANOVA was done for each Kp10 dose with repeated measures on time. Age did not affect baseline LH levels (Figure 1, A1–B2), indicating that E2-negative feedback is not compromised in older females. There was a main effect of time with peak LH release occurring 10 minutes after Kp10 infusion in all animals (Figure 1, A1–B2; P < .001 vs all other time points). LH levels were still significantly increased at 30 minutes only in young females treated with 30 nmol/kg of Kp10 (Figure 1, A2; P < .01). At both Kp10 doses, peak LH release in young controls was greater than in middle-aged females infused icv with either vehicle or IGF-I (Figure 1, A1–1B2; P < .001). JB-1 significantly reduced the Kp10-stimulated peak and total LH release in young females at both peptide doses (P < .01) to levels observed in middle-aged controls (Figure 1, A1–1A2). Increasing brain IGF-I levels by chronic IGF-I infusion significantly increased Kp10-induced peak LH release in middle-aged females (Figure 1, B1–B2; P < .01).
Figure 1.
Kp10-induced LH release in young (Y) and middle-aged (MA) females chronically infused with JB-1 or IGF-I. A1, Temporal LH release in young females injected with 10 nmol/kg Kp10. A2, Temporal LH release in young females injected with 30 nmol/kg Kp10. B1, Temporal LH release in middle-aged females injected with10 nmol/kg Kp10. B2,Temporal LH release in middle-aged females injected with 30 nmol/kg Kp10. C1, AUC for panels A1 and B1. C2, AUC for panels A2 and B2. Arrow reflects time zero and the point in which Kp10 was injected. Values are shown as means ± SEM (n = 4). a, P < .05 vs Y-aCSF; b, P < .01 vs 3 nmol/kg Y-aCSF; c, P < .05 vs MA-aCSF, Y-JB-1. *, P < .001 vs baseline LH values for all groups. Peak LH release occurred in all groups at 10 minutes after the Kp10 injection; **, P < .01 vs young and middle-aged-aCSF controls and middle-aged, IGF-I at 30 minutes.
To determine the effect of Kp10 dose on total LH release, a two-way ANOVA was performed with dose and age/treatment as factors. There were dose-dependent effects of Kp10 on total LH release in young but not middle-aged females; 30 nmol/kg induced more LH release than 3 nmol/kg in young females (Figure 1, C1–C2; P < .001). IGF-I potentiated the effect of 30 but not 3 nmol/kg of Kp10 on total LH release in middle-aged females (Figure 1, C2). These data show IGF-Ir antagonism reduces LH release in young females to levels observed in middle-aged controls, these data suggest that middle-aged females are less responsive to Kp10 than young females, and that increasing brain IGF-I levels can enhance the response to Kp10 in middle-aged females.
JB-1 attenuates and IGF-I augments NMDA-induced LH release in young and middle-aged females, respectively
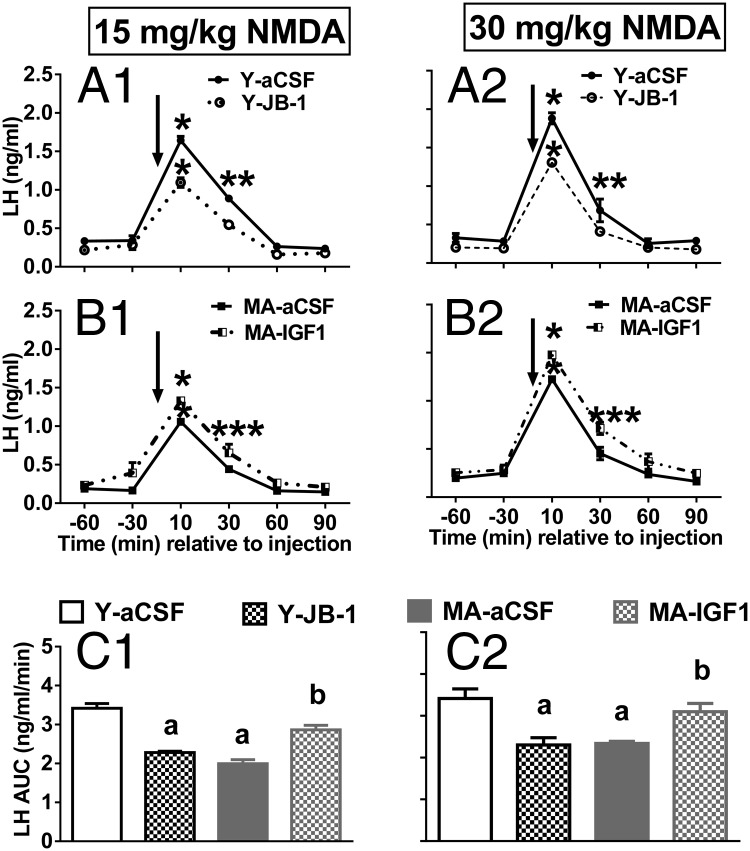
To determine the effect of age/treatment on NMDA-induced LH release, we conducted a two-way ANOVA for each NMDA dose with repeated measures on time. As shown in Figure 1, age did not affect baseline LH levels (Figure 2, A1–B2). There was a main effect of time with peak LH release occurring 10 minutes after NMDA infusion in all animals (Figure 2, A1–B2; P < .001 vs all other time points). Regardless of NMDA dose, young females had higher peak LH release than middle-aged females (Figure 2, A1–A2; P < .001). JB-1 significantly attenuated peak NMDA-induced LH release in young females (P < .01) to levels observed in middle-aged controls (Figure 2, A1–B2) at both NMDA doses. IGF-I infusion potentiated the effects of both doses of NMDA in middle-aged females (Figure 2, B1–B2; P < .05); however, peak LH levels were still significantly less than in young females injected with 15 or 30 mg/kg of NMDA.
Figure 2.
NMDA-induced LH release in young (Y) and middle-aged (MA) females chronically infused with JB-1 or IGF-I. A1, Temporal LH release in young females injected with 15 mg/kg NMDA. A2,Temporal LH release in young females injected with 30 mg/kg NMDA. B1, Temporal LH release in middle-aged females injected with 15 mg/kg NMDA. B2, Temporal LH release in middle-aged females injected with 30 mg/kg NMDA. C1, AUC for panels A1 and B1. C2, AUC for panels A2 and B2. Arrow reflects time zero and the point at which NMDA was injected. Values are shown as means ± SEM (n = 4). a, P < .0001 vs Y-aCSF; b, P < .05 vs MA-aCSF, Y-JB-1. *, P < .001 vs all baseline LH values for each treatment group (peak LH release occurred in all groups at 10 min after NMDA injection); **, P < .01 vs MA-aCSF and Y-JB-1 at 30 minutes; ***, P < .05 vs MA-aCSF control and Y-JB-1 at 30 minutes.
To determine whether the NMDA dose affected total LH release, we performed a two-way ANOVA with dose and age/treatment as factors. There was no effect of NMDA dose on total LH release in young or middle-aged females (Figure 2, C1–C2). This analysis showed that IGF-Ir antagonism reduces LH release in young females to levels observed in middle-aged controls and that IGF-I infusion potentiates the effect of NMDA on LH release in middle-aged females. Thus, the reduced responsiveness of middle-aged females to NMDA (10, 12, 31) may reflect reduced hypothalamic IGF-Ir signaling.
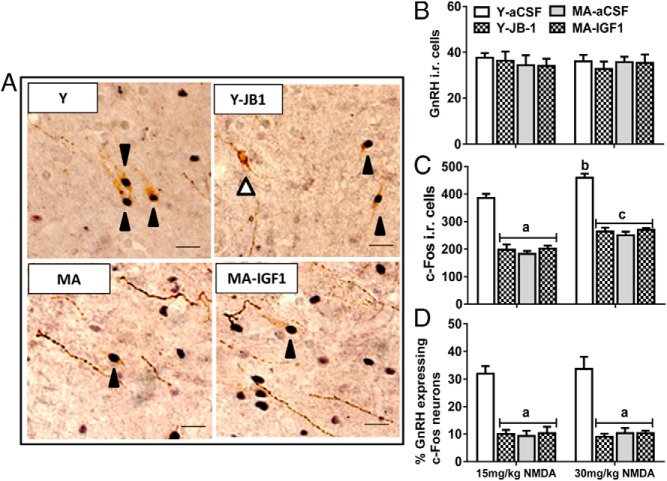
JB-1 decreases but IGF-I does not affect GnRH neuron activation by Kp10 and NMDA in young and middle-aged females, respectively
To determine whether JB-1 modulates Kp10 and/or NMDA-induced LH release in young females by preventing activation of GnRH neurons, we used dual-label immunohistochemistry to quantify the percentage of GnRH neurons expressing c-Fos-ir after iv injection of Kp10 or NMDA. At both Kp10 and NMDA doses, approximately 30–35% of GnRH neurons coexpress c-Fos in E2-primed young females (Figures 3D and 4D). Blockade of IGF-Irs with JB-1 significantly reduced the percentage of GnRH/c-Fos-ir cells at both doses of Kp10 and NMDA (Figures 3 and 4). Kp10 at both doses induced equivalent total numbers of c-Fos-ir cells in the POA of young adults, and JB-1 significantly reduced the numbers of c-Fos-ir cells (Figure 3; P < .01). In contrast, NMDA caused a dose-dependent increase in total c-Fos-ir cell numbers (Figure 4C; P < .05).
Figure 3.
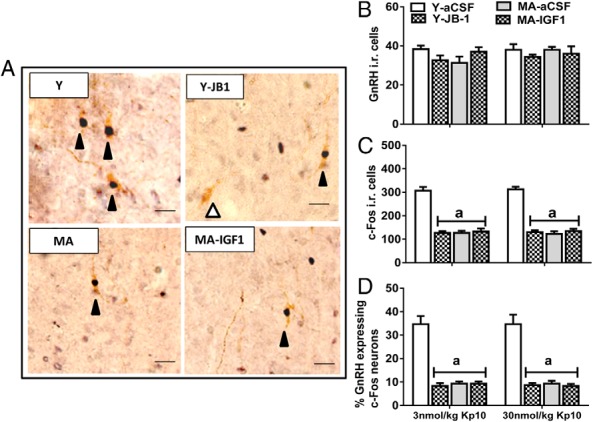
Kp10-induced GnRH neuron activation. A, Representative photos for c-Fos-ir (black arrowhead) and negative (white arrowhead) in GnRH neurons (scale bar, 10 μm, magnification, ×40). B, Number of GnRH immunopositive cells. C, Number of c-Fos-ir cells. D, Percentage of GnRH neurons coexpressing c-Fos. Values are shown as means ± SEM (n = 3). a, P < .01 vs Y-aCSF.
Figure 4.
NMDA-induced GnRH neuron activation. A, Representative photos for c-Fos-ir (black arrowhead) and negative (white arrowhead) in GnRH neurons (scale bar, 10 μm, magnification, 40×). B, Number of GnRH-ir cells. C, Number of c-Fos-ir cells. D, Percentage of GnRH neurons coexpressing c-Fos. Values are shown as means ± SEM (n = 3). a, P < .0001 vs all Y-aCSF; b, P < .001 vs 15 mg/kg Y-aCSF; c, P < .001 vs 15 mg/kg Y-JB1+E2, MA-aCSF+E2, and MA-IGF-I+E2.
To determine whether IGF-Ir signaling modulated Kp10 and NMDA-induced LH release in middle-aged females by preventing activation of GnRH neurons, we quantified the percentage of GnRH neurons coexpressing c-Fos (5). As expected, icv infusion of IGF-I did not affect the number of GnRH-ir cells (Figures 3B and 4B) (5). Regardless of the Kp10 or NMDA dose and in agreement with our previous work (5), middle-aged females had fewer GnRH neurons that expressed c-Fos (10% for middle aged vs 30–35% for young; Figures 3D and 4D, P < .001), and IGF-I did not affect the percentage of GnRH/c-Fos-ir cells in Kp10- or NMDA-treated females (Figures 3D and 4D). At 30 mg/kg of NMDA, there was more c-Fos expression in non-GnRH cells than in middle-aged females treated with 15 mg/kg (Figure 4C; P < .05).
IGF-Ir signaling regulates E2-induced changes in hypothalamic mRNA expression of kiss1r, nr1, and nr2b NMDA receptor subunits in young and middle-aged females
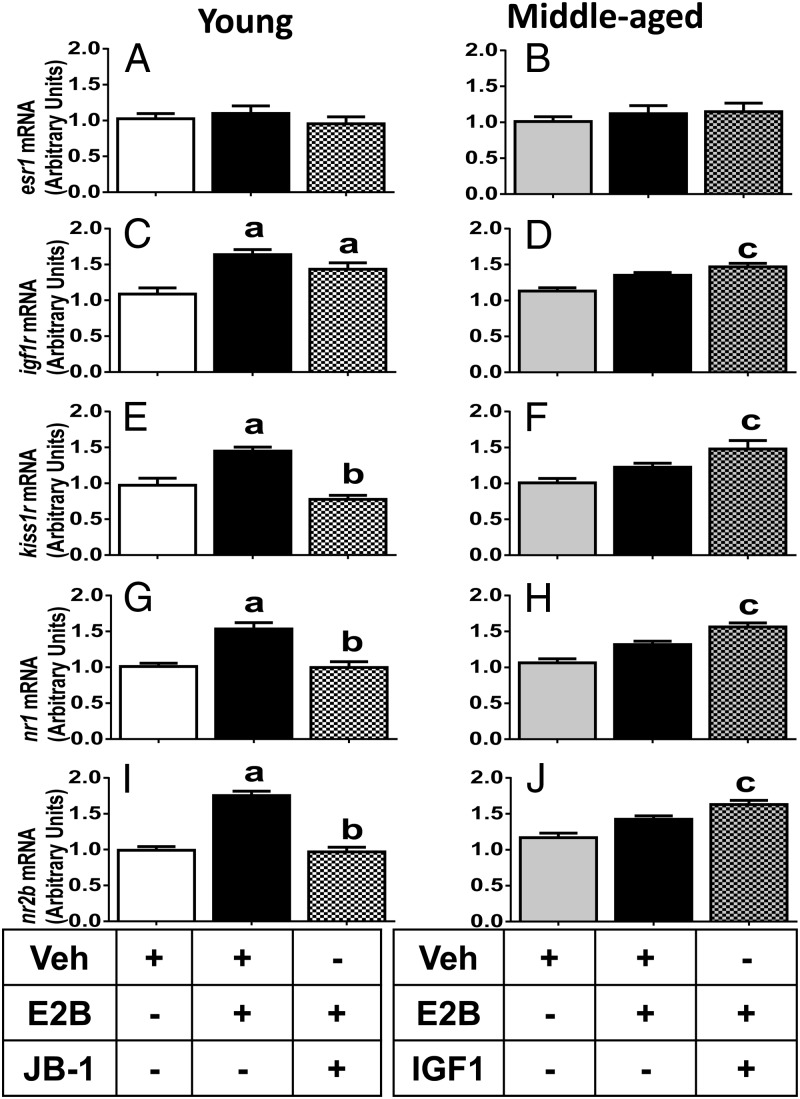
To determine whether IGF-Ir signaling affects E2-dependent LH release by modulating the expression of its cognate receptor, IGF-Ir, or through effects on the expression of esr1, the mediator of E2 action on hypothalamic kisspeptin and NMDA-mediated neurotransmission, we performed quantitative RT-PCR on hypothalamic tissues collected from oil or E2-primed, OVX females infused icv with aCSF (controls; young and middle aged), JB-1 (young), or IGF-I (middle aged) (Figure 5, A–D). Neither JB-1 nor IGF-I affected hypothalamic esr1 mRNA expression in control or E2-treated females of any age (Figure 5, A and B). E2 priming significantly increased the igf-Ir mRNA expression in young but not middle-aged females (P < .05; Figure 5, C and D). JB-1 did not inhibit the E2-induced elevation of igf-Ir mRNA expression in young adult females. However, infusion of IGF-I in middle-aged females rescued the response to E2, which significantly increased igf-Ir mRNA to levels observed in young females (Figure 5D).
Figure 5.
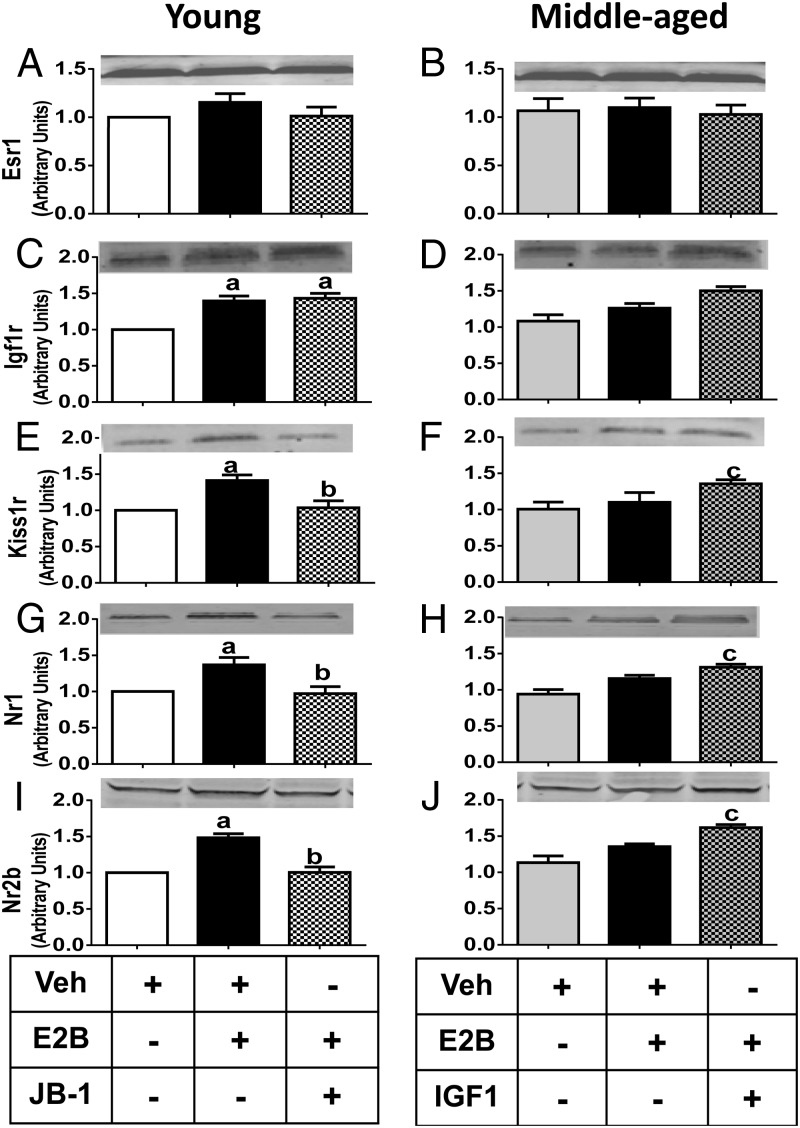
Effects of chronic IGF-I or JB-1 infusion in E2- or oil-injected young (Y) and middle-aged (MA) females on mRNA expression in the posterior hypothalamus. esr1 in Y (A) and MA (B) females; IGF-Ir in Y (C) and MA (D) females; kiss1r in Y (E) and MA (F) females; nr1 in Y (G) and MA (H) females; and nr2b in Y (I) and MA (J) females. β-Actin was used as the housekeeping gene for transcript normalization. Values are shown as means ± SEM (n = 4). a, P < .05 vs Y-aCSF-oil; b, P < .05 vs Y-aCSF-E2; c, P < .05 vs MA-aCSF-oil and MA-aCSF-E2. Veh, vehicle.
IGF-I potentiated and JB-1 attenuated NMDA and Kp10-induced LH release (Figures 1 and 2). It is possible that IGF-I affects NMDA and Kp10 responsiveness by modulating E2 regulation of gene expression patterns of kiss1r, the cognate receptor for Kp10, and NMDA receptor subunits nr1 and/or nr2b. We therefore performed quantitative RT-PCR to assess this possibility. E2 increased and JB-1 attenuated E2-dependent increases in kiss1r mRNA expression in young rats (Figure 5E; P < .05). In contrast, E2 did not significantly affect kiss1r mRNA expression in middle-aged females (Figure 5F). However, infusion of IGF-I in middle-aged females rescued the ability of E2 to significantly increase kiss1r mRNA expression (Figure 5F; P < .05). E2 also significantly increased nr1 and nr2b mRNA expression in young females, and JB-1 significantly attenuated this effect (P < .05; Figure 5, G and I). In contrast, E2 did not affect nr1 or nr2b mRNA expression in middle-aged females. However, infusion of IGF-I in middle-aged females rescued the ability of E2 to significantly increase nr1 and nr2b mRNA expression to levels observed in young females (Figure 5, H and J; P < .05).
IGF-Ir signaling regulates E2-induced changes in hypothalamic protein expression of Kiss1r and NMDA receptor subunits in young and middle-aged females
To determine whether IGF-Ir-related transcript changes translate into altered protein expression, we performed Western blots on hypothalamic protein extracts from young and middle-aged animals treated as described above (Figure 6, A–D). Like mRNA, neither JB-1 nor IGF-I affected hypothalamic Esr1 protein abundance in control or E2-treated females (Figure 6, A and B). IGF-Ir protein abundance was similar in young and middle-aged females. Similar to mRNA findings, E2 priming increased Igf-Ir protein in young females (P < .05; Figure 6C). However, JB-1 did not attenuate E2-induced increases in Igf-Ir protein abundance in young females (Figure 6C). Neither E2 priming nor IGF-I significantly affected Igf-Ir protein abundance in middle-aged females (Figure 6D).
Figure 6.
Effects of chronic IGF-I or JB-1 in E2- or oil-injected young (Y) and middle-aged (MA) females on protein expression in the posterior hypothalamus. Esr1 in Y (A) and MA (B) females; Igf-Ir in Y (C) and MA (D) females; Kiss1r in Y (E) and MA (F) females; Nr1 in Y (G) and MA (H) females; and Nr2b in Y (I) and MA (J) females. β-Actin was used as the housekeeping gene for protein normalization. Values are shown as means ± SEM (n = 4). a, P < .01 vs Y-aCSF-oil; b, P < .05 vs Y-aCSF-E2; c, P < .05 vs MA-aCSF-oil. Veh, vehicle.
In agreement with its effects on kiss1r mRNA, E2 significantly increased Kiss1r protein abundance, and JB-1 blocked this effect in young females (P < .05; Figure 6E). Additionally, E2 significantly increased Nr1 and Nr2b protein expression in young adult females (Figure 6, G and I; P < .05), and JB-1 attenuated this effect (Figure 6, G and I; P < .05). In agreement with the mRNA data, E2 alone failed to increase Nr1 and Nr2b protein abundance in middle-aged rats. However, IGF-I increased Nr1 and Nr2b protein expression in E2-treated, middle-aged females to levels that were equivalent to young adults (Figure 6, H and J; P < .05).
Discussion
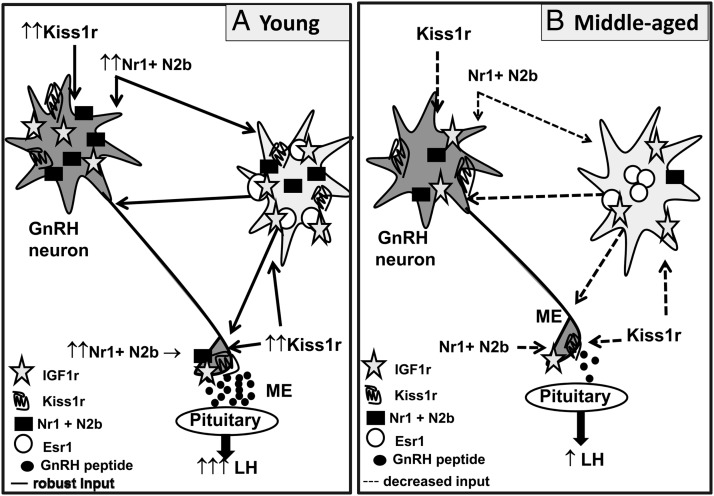
The present study provides novel evidence that age-related changes in brain IGF-Ir signaling adversely affect the ability of E2 to maximize kisspeptin as well as NMDA receptor-mediated neurotransmission in the hypothalamus of middle-aged females. We provide evidence that under E2-positive feedback conditions, icv infusion of the IGF-Ir antagonist JB-1 in young adult females reduces GnRH neuron activation, as determined by the percent GnRH/c-Fos coexpressing cells, and reduces responsiveness to excitatory input from kisspeptin and NMDA, evidenced by reduced GnRH/LH release. These changes in LH release are correlated with reduced E2-dependent increases in mRNA and protein expression of Kiss1r and NMDA receptor subunits, Nr1 and Nr2b. The phenotype observed in JB-1 treated young females is very similar to that of reproductively senescing, middle-aged females. Furthermore, supplemental brain IGF-I rescues hypothalamic responsiveness to NMDA and kisspeptin under E2-positive feedback conditions in middle-aged females. However, increased IGF-Ir signaling in middle-aged females does not increase GnRH neuron activation. Thus, these data suggest that the potentiation of the LH surge by IGF-I supplementation in middle-aged females may reflect IGF-Ir-mediated increases in the ability of E2 to enhance Kiss1r and NMDA Nr1 and Nr2b receptor subunit mRNA expression and protein abundance, which subsequently results in enhanced responsiveness to excitatory kisspeptinergic and glutamatergic inputs received by GnRH nerve terminals (Figure 7).
Figure 7.
E2 and IGF-I receptor signaling regulate LH release through effects on kisspeptin and NMDA neurotransmitter pathways. A, Representative model of how IGF-Ir and ESR1 cosignaling enhance LH release and the expression of Kiss1R and Nr1 and N2b (NMDA receptor subunits) in young adult females. IGF-Ir and ESR1 cosignaling in young females facilitates robust LH release in response to kisspeptin (Figure 1, A1 and A2) and NMDA (Figure 2, A1 and A2). The effect of IGF-Ir and ESR1 cosignaling on LH release is accompanied by increased expression of hypothalamic Kiss1r, Nr1, and N2b expression (Figure 6). Icv infusion of JB-1, a selective IGF-Ir antagonist, reduces kisspeptin (Figure 1, A1–A2) and NMDA (Figure 2A1–2)-induced LH release, and attenuates E2-dependent increases in Kiss1r, Nr1, and N2b expression (Figure 6). B, Representative model of the effect of female reproductive senescence on LH release, IGF-Ir and ESR1 association, and the expression of Kiss1r and Nr1 and N2b, NMDA receptor subunits. Female reproductive senescence is characterized by reduced hypothalamic IGF-I and IGF-Ir signaling. Reduced or dysfunctional ESR1 and IGF-Ir cosignaling in hypothalamus decreases responsiveness to kisspeptin (Figure 1, B1–B2) and NMDA (Figure 2, B1–B2) and reduces E2-induced expression of Kiss1r, Nr1 and N2b (Figure 6). Intracerebroventricular infusion of IGF-I in middle-aged females potentiates kisspeptin- (Figure 1, B1 and B2) and NMDA (Figures 2B1 and B2)-induced LH release and rescues the effects of E2 on Kiss1r, Nr1, and N2b protein expression. These data suggest that age-related decreases in brain IGF-I compromise the effect of E2 and IGF-I on the interaction and cosignaling of IGF-Ir and ESR1. Reduced IGF-Ir and ESR1 cosignaling disrupts LH release in part by compromising kisspeptinergic and glutamatergic neurotransmission (N2b = Nr2b).
Reproductive age and IGF-Ir signaling modulate Kp10-induced LH release and alter Kiss1r transcript and protein expression in female rats
This study provides new evidence that IGF-Irs work in concert with E2 to modulate hypothalamic sensitivity to kisspeptin in both young adult and middle-aged females. Reproductive age significantly reduces hypothalamic responsiveness to kisspeptin under E2-positive feedback conditions, and icv infusion of IGF-I rescues the responsiveness of middle-aged females to excitatory input mediated by kisspeptin. Specifically, E2-primed middle-aged females respond less robustly to iv kisspeptin, releasing about half as much LH compared with dose-matched young adult females. To our knowledge, this is the first demonstration that middle-aged females exhibit impaired responsiveness to Kp10. We previously demonstrated that exogenous Kp10 rescued LH surge amplitude in middle-aged females to levels equivalent to that observed in young adult females, thereby suggesting that middle-aged females respond appropriately to Kp10 (25). However, in that study middle-aged females were primed with both E2 and P, and they received continuous icv infusion of Kp10 on the day of the LH surge. Thus, our previous experimental design may have masked reduced responsiveness of middle-aged females to kisspeptin.
IGF-I regulates hypothalamic kiss1 gene expression in prepubertal females (21, 32). Of note, we and others reported that reproductive aging is characterized by reduced hypothalamic kisspeptin (25, 29, 33, 34) and brain IGF-I (18) protein expression and reduced the ability of E2 to increase IGF-I synthesis (17, 18). We also demonstrated that the LH surge in middle-aged females is enhanced by icv infusion of IGF-I (6), thereby raising the possibility that IGF-I rescues age-related changes in kisspeptin. However, unlike its action in peripubertal females (21), the addition of IGF-I did not significantly affect posterior hypothalamic kiss1 mRNA expression in E2-primed middle-aged (5.3 ± 2.1 vs 8.9 ± 4.8 arbitrary units; n = 4) or young (3.5 ± 0.8 vs 3.5 ± 1.1 arbitrary units; n = 4) adult females. Similarly, administration of IGF-I did not further affect anterior hypothalamic kiss1 mRNA expression in E2-primed middle-aged (0.5 ± 0.08 vs 0.6 ± 0.26 arbitrary units; n = 4) or young (0.76 ± 0.2 vs 0.79 ± 0.27 arbitrary units; n = 4) adult females.
Our data do not support the hypothesis that IGF-Ir signaling regulates kiss1 mRNA expression in either group. Thus, it is unlikely that icv infusion of IGF-I affects LH release by changing the abundance of kisspeptin neurotransmitter. Instead, our data suggest that IGF-Irs modulate E2-dependent changes in Kiss1r protein abundance. In support of this conclusion, icv infusion of IGF-I potentiated and JB-1 attenuated the abundance of Kiss1r protein in E2-primed, middle-aged, and young adult females, respectively. We also observed that under E2-positive feedback conditions, IGF-I increased the sensitivity of middle-aged females to Kp10. Conversely, JB-1 significantly reduced Kp10-induced LH release in young females to levels equivalent with middle-aged control females.
These new data extend our previous studies and strongly suggest that age-related decreases in brain IGF-I (5, 17, 25) may blunt E2-dependent increases in Kiss1r protein, which in turn produce submaximal responsiveness to kisspeptin. Our present findings cannot eliminate the possibility that female reproductive senescence affects the binding kinetics or second-messenger signaling pathways used by the Kiss1r. Future experiments will include a detailed neuroanatomical evaluation of the NMDA and kisspeptin neurotransmitter systems with immunohistochemistry.
It is important to note that although JB-1 attenuated LH release in young adult females to levels seen in middle-aged females, IGF-I infusion in middle-aged females did not restore the effect of the highest dose of Kp10 on LH release to levels observed in young females. Thus, the function of hypothalamic IGF-Irs in middle-aged females may also be compromised. However, neither JB-1 nor IGF-I affected hypothalamic igf-Ir mRNA or Igf-Ir protein levels in young adult or middle-ages females. Thus, it is unlikely that reduced availability of Igf-Ir accounts entirely for age-related differences in kisspeptin neurotransmission (25, 29, 33) or Kiss1r protein and mRNA expression. Nonetheless, we cannot rule out the possibility that middle-aged females are less sensitive to IGF-I. Assessment of age-related differences in IGF-I-induced Akt phosphorylation may answer this question (32). Additionally, there is a large body of literature that demonstrates the synergistic effects of estradiol and IGF-I as well as the cross talk between IGF-Ir and ERs, specifically Esr1(for review see Reference 35). Future experiments should determine whether advancing reproductive age disrupts cross talk between ERs and IGF-Irs.
Reproductive age and IGF-Ir signaling modulate NMDA-induced LH release and alter NR1 and NR2b transcript and protein expression in female rats
Similar to our findings with kisspeptin, E2-primed middle-aged females were less responsive to NMDA than young adults, releasing 60% less LH than young females injected with the same dose of NMDA. Reduced responsiveness to NMDA in middle-aged females correlated with a reduced ability of E2 to increase nr1 and nr2b mRNA expression and protein abundance. Our findings support others studies that suggest reproductive aging may be associated with altered NMDA receptor subunit stoichiometry (36–39) as well as reduced responsiveness to NMDA signaling (12, 31). Recent studies in the hippocampus suggest that age-dependent differences in glutamatergic neurotransmission may reflect alterations in the effectiveness and the type of signaling pathway triggered by the IGF-I in young compared with senescing rats (40). Our findings that IGF-I infusion increases E2-induced LH release and NMDA receptor subunit expression in middle-aged females support the hypothesis that age-related differences in NMDA responsiveness may reflect a generalized reduction in cross talk between hypothalamic IGF-Ir and Esr1 signaling.
Our findings in young females also support the hypothesis that IGF-Ir signaling is a key player in E2-positive feedback that involves NMDA neurotransmission. Regardless of the dose of NMDA, JB-1 reduced NMDA-induced LH release by 30% in young adults and completely blocked the ability of E2 to increase the relative abundance of nr1 and nr2b mRNA and Nr1 and Nr2b protein. These studies extend previous work in our and other laboratories by demonstrating IGF-Ir signaling affects the sensitivity to NMDA in young and middle-aged adult females, most likely by modulating the abundance of NR1 and NR2b subunit abundance under E2-positive feedback conditions. These data are consistent with studies in the hippocampus that suggest IGF-I increases the expression of Nr2b (22) and enhances glutamatergic neurotransmission (22, 40).
Reproductive age and IGF-Ir signaling modulate GnRH neuronal activation in young and middle-aged females injected with NMDA or kisspeptin
Between 30% and 35% of GnRH neurons were activated, as defined by the presence of c-Fos-ir, in E2-primed young females injected with Kp10 or NMDA. When IGF-Ir was blocked with JB-1 in young females, c-Fos expression in GnRH neurons was dramatically reduced, and this correlated with reduced LH release. However, the magnitude of LH release is not linearly correlated with the percentage of GnRH neurons expressing c-Fos. For example, although 30 nmol/kg of Kp10 induced 52% more LH release in young females than 3 nmol/kg, the percentage of GnRH neurons expressing c-Fos was identical. Kp10- and NMDA-treated young females had comparable numbers of GnRH neurons expressing c-Fos, and icv NMDA induced more c-Fos-ir in non-GnRH neurons than Kp10. However, the effect of NMDA was not dose dependent, and it did not translate into more LH release than Kp10. Instead, Kp10 induced 3- to 4-fold more LH release than NMDA. These data suggest that during E2-positive feedback conditions, NMDA-induced activation of GnRH and non-GnRH neurons is not sufficient for maximal GnRH-LH release in young or middle-aged rats. Additionally, because NMDA receptors are expressed in diverse hypothalamic cells, it is possible that NMDA infusion also affects neurotransmitter pathways that inhibit GnRH neurons (41) and blunt GnRH-LH release. Lastly, the ability of kisspeptin to mediate a dose-dependent effect on LH release but not c-Fos-ir most likely reflects the modulatory effects of kisspeptin on excitatory and inhibitory neurotransmitter systems such as glutamate and γ-aminobutyric acid (GABA), respectively (42, 43). This hypothesis is consistent with our previous work demonstrating that intrahypothalamic application of kisspeptin increased extracellular glutamate and decreased extracellular GABA levels in the mPOA, respectively (25). We also showed that increased hypothalamic glutamateric neurotransmission in middle-aged females is accompanied by increased GABA release (10).
Conversely, chronic icv infusion of IGF-I did not increase the percentage of GnRH/c-Fos-ir cells in middle-aged females but did increase LH release in response to NMDA and Kp10. We previously reported that the IGF-I enhancement of the LH surge in middle-aged females does not correlate with increased c-Fos expression in GnRH neurons (5). Taken together, these new data support our previous work as well as reports from Miller and Gore (18) and suggest IGF-I most likely modulates GnRH release at the level of the median eminence, especially in middle-aged females (17, 18). These data also provide strong evidence that IGF-Ir signaling may facilitate GnRH-LH release by permitting E2 to increase excitatory neurotransmission in the hypothalamus of middle-aged females (10, 25).
Conclusions
The present study provides compelling evidence that brain IGF-Ir signaling plays an essential role in mediating E2 modulation of kisspeptin and NMDA neurotransmission in the hypothalamus of young and middle-aged females, with important consequences for GnRH-LH release. The data also provide novel evidence that attenuated LH release patterns that characterize reproductive aging in females are associated with reduced responsiveness to kisspeptin as well as NMDA. This most likely reflects reduced brain IGF-Ir signaling, which impairs the ability of E2 to increase Kiss1r and NMDA receptor subunit mRNA and protein abundance in the hypothalamus of middle-aged females. This in turn may adversely affect GnRH-LH release. Studies using JB-1, a selective IGF-Ir antagonist, in young females complement findings in middle-aged females. These data imply that reproductive age significantly affects the ability of IGF-Ir signaling to activate GnRH neurons and terminals under E2-positive feedback conditions, thereby raising the possibility that reproductive senescence may also be characterized, in part, by hypothalamic desensitization to IGF-I.
Acknowledgments
We thank Ms Zunjun Hu for excellent technique assistance with GnRH/c-Fos immunohistochemistry and neuron counting and Dr R. Benoit for providing the GnRH primary antibody.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54 HD058155 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and by the Department of Obstetrics and Gynecology and Women's Health, Albert Einstein College of Medicine.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- AUC
- area under the curve
- E2
- estradiol
- E2B
- E2 benzoate
- ER
- estrogen receptor
- GABA
- γ-aminobutyric acid
- icv
- intracerebroventricular
- IGF-Ir
- IGF-I receptor
- ir
- immunoreactivity
- mPOA
- medial preoptic area
- KPBS
- potassium PBS
- KPBS-Tx
- KPBS plus Triton X-100
- NMDA
- N-methyl-D-aspartic acid
- OVX
- ovariectomized
- P
- progesterone.
References
- 1. Cardona-Gomez GP, Trejo JL, Fernandez AM, Garcia-Segura LM. Estrogen receptors and insulin-like growth factor-I receptors mediate estrogen-dependent synaptic plasticity. Neuroreport. 2000;11:1735–1738 [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Segura LM, Rodriguez JR, Torres-Aleman I. Localization of the insulin-like growth factor I receptor in the cerebellum and hypothalamus of adult rats: an electron microscopic study. J Neurocytol. 1997;26:479–490 [DOI] [PubMed] [Google Scholar]
- 3. Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: relationship to gonadotropin-releasing hormone neurons. Endocrinology. 2003;144:2034–2045 [DOI] [PubMed] [Google Scholar]
- 4. Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol. 2004;16:160–169 [DOI] [PubMed] [Google Scholar]
- 5. Sun Y, Todd BJ, Thornton K, Etgen AM, Neal-Perry G. Differential effects of hypothalamic IGF-I on gonadotropin releasing hormone neuronal activation during steroid-induced LH surges in young and middle-aged female rats. Endocrinology. 2011;152:4276–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Todd BJ, Fraley GS, Peck AC, Schwartz GJ, Etgen AM. Central insulin-like growth factor 1 receptors play distinct roles in the control of reproduction, food intake, and body weight in female rats. Biol Reprod. 2007;77:492–503 [DOI] [PubMed] [Google Scholar]
- 7. Cooper RL, Conn PM, Walker RF. Characterization of the LH surge in middle-aged female rats. Biol Reprod. 1980;23:611–615 [DOI] [PubMed] [Google Scholar]
- 8. Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146:4331–4339 [DOI] [PubMed] [Google Scholar]
- 10. Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008;79:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brann DW, Zamorano PL, De Sevilla L, Mahesh VB. Expression of glutamate receptor subunits in the hypothalamus of the female rat during the afternoon of the proestrous luteinizing hormone surge and effects of antiprogestin treatment and aging. Neuroendocrinology. 2005;81:120–128 [DOI] [PubMed] [Google Scholar]
- 12. Arias P, Carbone S, Szwarcfarb B, et al. Effects of aging on N-methyl-D-aspartate (NMDA)-induced GnRH and LH release in female rats. Brain Res. 1996;740:234–238 [DOI] [PubMed] [Google Scholar]
- 13. Krajnak K, Rosewell KL, Wise PM. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod. 2001;64:1160–1164 [DOI] [PubMed] [Google Scholar]
- 14. Le W-W, Wise PM, Murphy AZ, Coolen LM, Hoffman GE. Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology. 2001;142:4976–4982 [DOI] [PubMed] [Google Scholar]
- 15. Rubin BS, King JC. The number and distribution of detectable luteinizing hormone (LH)-releasing hormone cell bodies changes in association with the preovulatory LH surge in the brains of young but not middle-aged female rats. Endocrinology. 1994;134:467–474 [DOI] [PubMed] [Google Scholar]
- 16. Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod. 1994;51:1264–1272 [DOI] [PubMed] [Google Scholar]
- 17. Todd BJ, Merhi ZO, Shu J, Etgen AM, Neal-Perry GS. Hypothalamic insulin-like growth factor-I receptors are necessary for hormone-dependent luteinizing hormone surges: implications for female reproductive aging. Endocrinology. 2010;151:1356–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol. 2001;13:728–736 [DOI] [PubMed] [Google Scholar]
- 19. Bartke A. Is growth hormone deficiency a beneficial adaptation to aging? Evidence from experimental animals. Trends Endocrinol Metab: TEM. 2003;14:340–344 [DOI] [PubMed] [Google Scholar]
- 20. Bartke A, Chandrashekar V, Dominici F, et al. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8 [DOI] [PubMed] [Google Scholar]
- 21. Hiney JK, Srivastava VK, Pine MD, Les Dees W. Insulin-like growth factor-I activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Greves M, Le Greves P, Nyberg F. Age-related effects of IGF-1 on the NMDA-, GH- and IGF-1-receptor mRNA transcripts in the rat hippocampus. Brain Res Bull. 2005;65:369–374 [DOI] [PubMed] [Google Scholar]
- 23. Ottem EN, Godwin JG, Petersen SL. Glutamatergic signaling through the N-methyl-D-aspartate receptor directly activates medial subpopulations of luteinizing hormone-releasing hormone (LHRH) neurons, but does not appear to mediate the effects of estradiol on LHRH gene expression. Endocrinology. 2002;143:4837–4845 [DOI] [PubMed] [Google Scholar]
- 24. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roseweir AK, Kauffman AS, Smith JT, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res. 1992;52:6447–6451 [PubMed] [Google Scholar]
- 28. Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci USA. 1990;87:5163–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lederman MA, Lebesgue D, Gonzalez VV, et al. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology. 2010;58:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandez-Galaz MC, Naftolin F, Garcia-Segura LM. Phasic synaptic remodeling of the rat arcuate nucleus during the estrous cycle depends on insulin-like growth factor-I receptor activation. J Neurosci Res. 1999;55:286–292 [DOI] [PubMed] [Google Scholar]
- 31. Bonavera JJ, Swerdloff RS, Sinha Hikim AP, Lue YH, Wang C. Aging results in attenuated gonadotropin releasing hormone-luteinizing hormone axis responsiveness to glutamate receptor agonist N-methyl-D-aspartate. J Neuroendocrinol. 1998;10:93–99 [DOI] [PubMed] [Google Scholar]
- 32. Hiney JK, Srivastava VK, Les Dees W. Insulin-like growth factor-1 stimulation of hypothalamic KiSS-1 gene expression is mediated by Akt: effect of alcohol. Neuroscience. 2010;166:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272 [DOI] [PubMed] [Google Scholar]
- 36. Gore AC. Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Brain Res Rev. 2001;37:235–248 [DOI] [PubMed] [Google Scholar]
- 37. Gore AC, Oung T, Woller MJ. Age-related changes in hypothalamic gonadotropin-releasing hormone and N-methyl-d-aspartate receptor gene expression, and their regulation by oestrogen, in the female rat. J Neuroendocrinol. 2002;14:300–309 [DOI] [PubMed] [Google Scholar]
- 38. Maffucci JA, Noel ML, Gillette R, Wu D, Gore AC. Age- and hormone-regulation of N-methyl-D-aspartate receptor subunit NR2b in the anteroventral periventricular nucleus of the female rat: implications for reproductive senescence. J Neuroendocrinol. 2009;21:506–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maffucci JA, Walker DM, Ikegami A, Woller MJ, Gore AC. NMDA receptor subunit NR2b: effects on LH release and GnRH gene expression in young and middle-aged female rats, with modulation by estradiol. Neuroendocrinology. 2008;87:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Molina DP, Ariwodola OJ, Weiner JL, Brunso-Bechtold JK, Adams MM. Growth hormone and insulin-like growth factor-I alter hippocampal excitatory synaptic transmission in young and old rats. Age (Dordr). 2013;35:1575–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kantamneni S, Gonzalez-Gonzalez IM, Luo J, et al. Differential regulation of GABAB receptor trafficking by different modes of NMDA receptor signalling. J Biol Chem. 2014;289:6681–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garcia-Galiano D, Pineda R, Ruiz-Pino F, et al. Differential modulation of gonadotropin responses to kisspeptin by aminoacidergic, peptidergic, and nitric oxide neurotransmission. Am J Physiol Endocrinol Metab. 2012;303:E1252–E1263 [DOI] [PubMed] [Google Scholar]
- 43. Garcia-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153:316–328 [DOI] [PubMed] [Google Scholar]