Abstract
Attenuating myostatin enhances striated muscle growth, reduces adiposity, and improves cardiac contractility. To determine whether myostatin influences tissue potency in a manner that could control such pleiotropic actions, we generated label-retaining mice with wild-type and mstn−/− (Jekyll) backgrounds in which slow-cycling stem, transit-amplifying, and progenitor cells are preferentially labeled by histone 2B/green fluorescent protein. Jekyll mice were born with fewer label-retaining cells (LRCs) in muscle and heart, consistent with increased stem/progenitor cell contributions to embryonic growth of both tissues. Cardiac LRC recruitment from noncardiac sources occurred in both groups, but lasted longer in Jekyll hearts, whereas heightened β-adrenergic sensitivity of mstn−/− hearts was explained by elevated SERCA2a, phospholamban, and β2-adrenergic receptor levels. Jekyll mice were also born with more adipose LRCs despite significantly smaller tissue weights. Reduced adiposity in mstn−/− animals is therefore due to reduced lipid deposition as adipoprogenitor pools appear to be enhanced. By contrast, increased bone densities of mstn−/− mice are likely compensatory to hypermuscularity because LRC counts were similar in Jekyll and wild-type tibia. Myostatin therefore significantly influences the potency of different tissues, not just muscle, as well as cardiac Ca2+-handling proteins. Thus, the pleiotropic phenotype of mstn−/− animals may not be due to enhanced muscle development per se, but also to altered stem/progenitor cell pools that ultimately influence tissue potency.
Myostatin is primarily known to negatively regulate skeletal muscle growth and development, although it is also expressed, albeit at lower levels, in many different adult tissues. These include the heart, fat, and even the brain (1). Embryonic expression was initially isolated to somites, the source of myogenic precursor cells, and has since been demonstrated to occur in hearts of developing sheep, fish, chicken, and mice (2–5). It is therefore surprising that the nonmuscle actions of myostatin are not better known because the null (mstn−/−) phenotype includes “double muscling” as well as reduced fat mass and eccentric cardiac hypertrophy coupled with a heightened response to β-adrenergic stimuli (1, 6–9).
Enhanced muscularity in mstn−/− animals is primarily due to prenatal hyperplasia and postnatal hypertrophy (10–12). Moreover, myostatin may also regulate adult skeletal muscle stem cells (also known as satellite cells) and thereby contribute to the normal muscle regenerative process, although supporting evidence is highly controversial. Satellite cells are located between the basal lamina and the sarcolemma and, when activated, differentiate and fuse with existing fibers (13). Two studies suggest that myostatin maintains these cells in quiescence because more CD34-positive cells can be found in mstn−/− mice than in wild-type mice, and these cells were more active with higher proliferation rates (14, 15). By contrast, conflicting reports also using mstn−/− mice suggest that satellite cell numbers and proliferation rates were either slightly reduced or not different from those in wild-type mice (16, 17). Additional studies are therefore needed to determine myostatin's effects on satellite cells and on other myogenic progenitors because multiple skeletal muscle cell types are ultimately myogenic (18).
Moreover, myogenic precursor cells have the added potential of differentiating into cartilage, bone, or fat (19). Indeed, myostatin attenuation produces changes in muscle insertion regions on bone and ultimately increases bone mineral density and strength (20–23). It is unknown, however, whether these effects on bone are compensatory responses to the increased load brought upon by comparable increases in muscle mass or to direct actions on bone itself. Myostatin and growth/differentiation factor (GDF)-11 coregulate skeletal patterning and although GDF-11 is the primary regulator (24, 25), both cytokines signal via the same activin receptors (eg, ActRIIb, Acvr2b). This suggests that the loss of myostatin may in reality contribute to the mstn−/− bone phenotype and to changes in stem/progenitor cell niches that influence bone development.
Many studies have also demonstrated reduced adiposity in mstn−/− animals of different ages or with direct myostatin attenuation (6, 26–28). Under these conditions, carbohydrates are repartitioned away from adipocytes to support the enhanced musculature. Reduced adiposity in mstn−/− animals may therefore be due to reduced fat deposition and not necessarily to reduced adipogenesis. In fact, in vitro studies with various mesenchymal adipocyte progenitor cells are again highly controversial because myostatin has been demonstrated to both inhibit and stimulate their differentiation into mature adipocytes (29–33). Resolving this controversy has clinical implications because attenuating myostatin both prevents and reverses obesity in different animal models and can even improve insulin sensitivity (26, 28, 34, 35). Thus, novel myostatin-antagonizing therapeutics, which are currently being developed (36–38), could potentially be used to treat type 2 diabetes in addition to muscle-related disorders.
Several recent studies have also implicated myostatin in the regulation of cardiac muscle growth and even function. Indeed, myostatin is expressed in cardiac muscle of different vertebrates (2, 3, 5), and expression of both protein and mRNA increases in various pathophysiological conditions of the heart, including myocardial infarction and dilated cardiomyopathy (2, 39), and with Akt-induced cardiac hypertrophy (9, 40). The myokine blocks basal and IGF-stimulated protein synthesis in primary cardiomyocytes as well as proliferation in a cardiomyoblast cell line (7, 9). These results indicate that myostatin directly regulates cardiomyocytes and that its expression increases with cardiac hypertrophy. It also appears to regulate cardiac muscle function because not only are mstn−/− hearts more responsive to β-adrenergic stimulation in vivo, the contractility and Ca2+-transients of individual cardiomyocytes isolated from mstn−/− hearts are similarly enhanced (7). Furthermore, attenuating myostatin with a soluble Acvr2b receptor enhances both skeletal and cardiac muscle growth in animal models of Duchenne muscular dystrophy and cancer cachexia (36, 41). These results together suggest that myostatin similarly regulates both muscle types, and its attenuation could again be used to treat cardiac as well as muscle disease states.
Although myostatin clearly influences prenatal development, the notable mstn−/− phenotype develops postnatally. Furthermore, adult tissue potency is dependent upon the development of tissue-specific stem and progenitor cells. We therefore determined whether myostatin influences tissue potency in a manner that could contribute to the tissue-specific mstn−/− phenotypes. Our model used a well-established fate mapping system (described below) that labels slow-cycling stem, transit-amplifying (TA), and progenitor cells in vivo, but not mitotic or differentiated cells (42–44). Our studies suggest that myostatin dynamically regulates the putative stem/progenitor cell pools in skeletal muscle, heart, and fat and may even influence their recruitment from external tissue sources. They also indicate that differences in Ca2+-handling proteins and β-adrenergic receptors, between wild-type and mstn−/− mice, similarly explain the functional differences in cardiac and cardiomyocyte contractility previously reported. The pleiotropic phenotypes expressed in mstn−/− mice may therefore result from altered stem/progenitor cell pools and, in addition, to altered expression of genes critical to cardiac function.
Materials and Methods
Animals
All mice used in these studies were housed and bred in environmentally controlled rooms of an AALAC accredited facility under 12-hour light and dark cycles. They were fed ad libitum and were used in strict accordance with protocols preapproved by the Institutional Animal Care and Use Committee of Washington State University.
Label-retaining systems are commonly used to identify stem cells, their immediate progeny (TA cells), and other tissue progenitors because all of these cells divide less frequently and retain label for extended periods (43, 45). These systems, which include labeling with bromodeoxyuridine, cannot distinguish stem, TA, or progenitor cells from one another, although they do distinguish such cells from differentiated cells because quiescent stem/progenitor cells retain label whereas mitotic and differentiated cells do not. Measuring the number of label retaining cells (LRCs) is, therefore, an accurate estimate of the heterologous stem/progenitor cell pool in a particular tissue. We generated label-retaining mice that express a fusion protein composed of histone 2B and green fluorescent protein (H2B-GFP) under the control of a doxycycline-inducible promoter (42, 46). These mice were constructed with both wild-type and myostatin null (mstn−/−) backgrounds and are referred to as wild-type (WT) and Jekyll mice, respectively. Treating pregnant mice with doxycycline results in the labeling of all embryonic cells with H2B-GFP. Label is diluted during a following chase period without doxycycline induction as label is diluted with cell division and as the H2B-GFP chimera is replaced by endogenous H2B in differentiated cells. This results in the retention of label in only quiescent stem/progenitor cells, which help maintain tissue potency. This system was first described and validated by Tumbar et al (42) and has since been used extensively (almost 900 references to date) to quantify and isolate stem/progenitor cell populations in many different tissues.
Wild-type control mice were generated by crossing Tg(tetO-HIST1H2BJ/GFP)47Efu/J (also known as H2B-GFP) mice (The Jackson Laboratory), which possess the tetracycline-responsive promoter linked to a H2B-GFP fusion construct, with B6.Cg-Gt(ROSA)26Sortm1(rtTA*M2)Jae/J (also known as M2) mice (The Jackson Laboratory), which express the reverse tetracycline-controlled transactivator. Jekyll mice were generated by first separately crossing H2B/GFP and M2 mice with mstn−/− mice, producing H2B-GFP/mstn−/− and M2/mstn−/− mice. These latter mice were then crossed to produce Jekyll mice. Tail snips were taken to genotype all offspring using PCR (Table 1). DNA was extracted from the tails using 90 μL of 50 mM NaOH. The solution was heated to 95°C for 40 minutes before adding 10 μL of 1 M Tris-HCl to neutralize. From this, 5 μL were added to a 20-μL PCR and amplified for 33 cycles of 94°C for 30 seconds, an annealing temperature (Table 1) for 30 seconds, and 72°C for 45 seconds.
Table 1.
PCR Primers for Genotyping
| Primer | Sequence (5′–3′) | AT (°C) |
|---|---|---|
| Mstn WT F a | AGAAGTCAAGGTGACAGACACAC | 58 |
| Mstn WT Ra | GGTGCACAAGATGAGTATGCGG | 58 |
| Mstn Null Fa | GGATCGGCCATTGAACAAGATG | 58 |
| Mstn Null Ra | GAGCAAGGTGAGATGACAGGAG | 58 |
| M2 F b | AAAGCTGCTCTGAGTTGTTAT | 58 |
| M2 Mutant Rc | GCGAAGAGTTTGTCCTCAACC | 58 |
| M2 WT Rd | GGAGCGGGAGAAATGGATATG | 58 |
| GFP F | ACGTAAACGGCCACAAGTTC | 56 |
| GFP R | TGTTCTGCTGGTAGTGGTCG | 56 |
| Internal ctrl H2B-GFPe | CTAGGCCACAGAATTGAAAGATCT | 56 |
| Internal ctrl H2B-GFPf | GTAGGTGGAAATTCCTAGCATCATCC | 56 |
AT, annealing temperature; ctrl, control; F or R, forward or reverse.
Reference 24;
oIMR8545;
oIMR8052;
oIMR8546;
oIMR7338;
oIMR7339 (The Jackson Laboratory primer numbers).
Doxycycline (Research Products International Corp) was administered to pregnant females to induce H2B-GFP expression in offspring at a concentration of 400 μg/mL 5% sucrose drinking water that was kept in light-sensitive bottles. Preliminary experiments compared different doxycycline concentrations for in utero labeling and included 200 μg/mL, 400 μg/mL, and 1 mg/mL. No differences in GFP expression, regardless of tissue, were detected (data not shown) and thus, 400 μg/mL was used for this study. Doxycycline was started after plug detection (E0.5) and continued to day E10.5, E14.5, or E19. Drinking solutions were changed every 4 days during the dosing or pulse period.
Imaging of heart, gastrocnemius, and tibia
Tissues were fixed in 4% paraformaldyhde (PFA)/PBS for 2 hours, rinsed in PBS, incubated overnight in 15% sucrose/PBS, and embedded in 7.5% gelatin/15% sucrose/PBS. Tibias were decalcified before embedding in 14% EDTA for 2 days or 1 week (adult tibias) and then placed in 15% sucrose/PBS overnight. Autofluorescence was detected in the primary ossification site only; nevertheless, we quantified regions outside of this area (see Results). Embedded tissues were frozen using 2-methylbutane (Acros) that was cooled to −60°C using liquid nitrogen. Slides containing 5-μm sections were soaked in PBS at 37°C to remove excess gelatin, incubated in 0.3% Sudan Black B (Sigma Aldrich)/70% ethanol for 5 minutes, and stained with Prolong gold + 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Tissues were imaged at ×100 using a Leica DM2000 and DFC 360Fx microscope. Three images were acquired for each section: one for DAPI-stained nuclei and 2 for GFP-labeled LRCs. The histogram function in the Leica Application Suite software v3.6 was then used to select for total GFP-positive cells and brightest GFP-positive cells. The latter were differentiated by threshold gating as described (Supplemental Figure 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). For gastrocnemius, 12 sections were analyzed per sample with 2–4 images per section, which included both longitudinal and cross-sections. For heart, 12 sections were analyzed per sample, and the entire section was imaged and analyzed. One end of each tibia was imaged and included the epiphysis, metaphysic, and part of the diaphysis, although the articular surface and the remaining portion of the epiphysis and diaphysis were analyzed separately. Because the secondary ossification center appears by day 14, growth plate measurements were used for epiphyseal counts on days 14 and 62.
Imaging of adipose tissue
Inguinal white and intrascapular brown fat pads were collected and fixed in 4% PFA/PBS for 2 hours. Tissues were rinsed in PBS, lysed with 1 mM EGTA, 1 mM EDTA, 0.5% Triton-X and 150 mM NaCl in PBS for 1 hour, incubated in 10 μg/mL RNAse A (Fermentas) for 1 hour at 37°C, and stained with propidium iodide at 1 μg/mL (Sigma-Aldrich) for 40 minutes. After rinsing in PBS, tissues were embedded in 1% low-melt agarose and submerged in PBS before imaging at ×250 on a confocal microscope (Leica TCS Sp511, DM 6000 CS). Two separate areas of each tissue were analyzed by imaging 32 frames per area with constant emission-excitation spectra for GFP and propidium iodide. Tissues were then embedded in paraffin, and 6-μm sections were stained with hematoxylin and eosin (Thermo Fisher Scientific) before again imaging at ×200 using a Nikon Eclipse 90i and DS-Ri1 microscope. Cell counts were calculated from hematoxylin and eosin images of inguinal white adipose tissue (WAT) and brown adipose tissue (BAT) from adult C57Bl/6 and mstn−/− mice, and the total number was divided by adipose weight.
Cell count analysis
Color images were first converted to color-free 8-bit images using Image J 1.46. An upper GFP threshold limit of 255 was used for all tissues. By contrast, the lower limit varied and was set to 7 for gastrocnemius, heart, and adipose tissues, 8 for the epiphysis, and 10 for the articular surface and diaphysis of the tibia. A free-hand selection tool was then used to select the 3 areas to count in tibia images (see Results). Because threshold limits varied with nuclear stained images, the watershed tool was used to separate nearby cells. In addition, the background was subtracted from adipose images using a radius of 15 pixels. All GFP-positive cells, regardless of tissue, were then normalized to nuclei counts.
Histology
Slides were immersed in PBS to remove gelatin or to remove coverslips (gastrocnemius) and incubated in Bouin's solution overnight. They were then rinsed in dH2O and stained with Masson's trichrome stains (American MasterTech) according to the manufacturer's protocol. Slides were then incubated in 1% acetic acid for 5 minutes followed by dehydration in 95% ethanol, 100% ethanol, and xylene solution. Sections were imaged at ×100 for gastrocnemius and ×40 or ×100 for tibias using Nikon Eclipse 90i and DS-Ri1.
Immunofluorescence
Tissues were fixed in 4% PFA/PBS for 2 hours, rinsed in PBS, and lysed in 1 mM EGTA, 1 mM EDTA, 0.5% Triton-X, 150 mM NaCl in PBS overnight. The hearts were cut in half to view the ventricles. Tissues were blocked for 1 hour in PBS containing 0.1% BSA, 1% normal serum, and 0.3% Triton-X and incubated overnight in Actin 555 phalloidin (Cytoskeleton) at 100 nm. Samples were then embedded in 1% low-melt agarose, submerged in PBS, and imaged at ×50 and ×250 on a confocal microscope. 3-D images were constructed using Metamorph software. For phosphohistone H3 (PHH3) staining, sections were cut at 5 μm, and gelatin was removed by incubating slides in PBS at 37°C for 40 minutes. Slides were blocked for 1 hour in TN solution (100 mM Tris, 150 mM NaCl, 1% BSA, 1% normal goat serum, 0.3% Triton-X) and incubated overnight with antirabbit PHH3 (Millipore Corp) at 1:750 dilution. After washing with TN solution, slides were incubated with a goat antirabbit Alexafluor 555 (Life Technologies) secondary at 1:5000 for 1 hour. Prolong Gold + DAPI (Life Technologies) was then added, and slides were cured overnight before imaging at ×200.
Western blotting
Hearts of C57Bl/6 and mstn−/− mice were removed and rinsed in PBS, and total protein was extracted by pulverizing the tissue in liquid nitrogen and resuspending in radioimmune precipitation assay buffer supplemented with Na3VO4, NaF, and Protease cocktail (Sigma Aldrich). Total protein was quantified using a Bradford assay (Bio-Rad Laboratories), and Western blotting was performed with 35 μg protein/lane on 12% SDS-PAGE gels. Protein was transferred to either 0.45-μm polyvinylidene difluoride or 0.2-μm nitrocellulose membranes (phospholamban, [PLN], and phosphorylated-phospholamban [P-PLN] only) that were subsequently blocked with either 5% milk in Tris-buffered saline/0.1% Tween-20 (TBST) or 3% BSA in TBST (sarcoplasmic reticulum Ca2+ ATPase [SERCA2a] only) before probing with primary antisera overnight at 4°C. Membranes were then washed 3 × 15 minutes with TBST, incubated with secondary antisera for 1 hour, washed again, and imaged with a 2000-mm Kodak Imager. The following antibodies and titers were used: rabbit polyclonal β1AR (1:500, Pierce Chemical Co), rabbit polyclonal β-adrenergic receptor 2 (β2AR) (1:500, Gentex), mouse monoclonal SERCA2a (1:2000, Pierce), mouse monoclonal phospholamban (1:2500, Millipore), rabbit polyclonal phospho-phospholamban Ser16 (1:50, Millipore), and rabbit polyclonal glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5000, Cell Signaling Technology). Goat antimouse (1:10,000, Chemicon) or mouse antirabbit (1:10 000, The Jackson Laboratory) horseradish peroxidase-conjugated secondaries were used to detect primary antibodies. Band intensities and sizes were quantified using a 2000-mm Kodak Imager and normalized to GAPDH loading controls. For SERCA2a, PLN, and P-PLN, protein extracts were not heated prior to loading and thus, the entire oligomer for these proteins was quantified.
Statistical analysis
Differences between means were determined by a two-way ANOVA coupled to Tukey or Fisher Least Significant Difference test for multiple mean comparisons or by Student's t test when appropriate, eg, when no interactions were identified between one of two independent variables. In each comparison, P ≤ .05 was used to determine significance unless otherwise noted.
Results
Model characterization
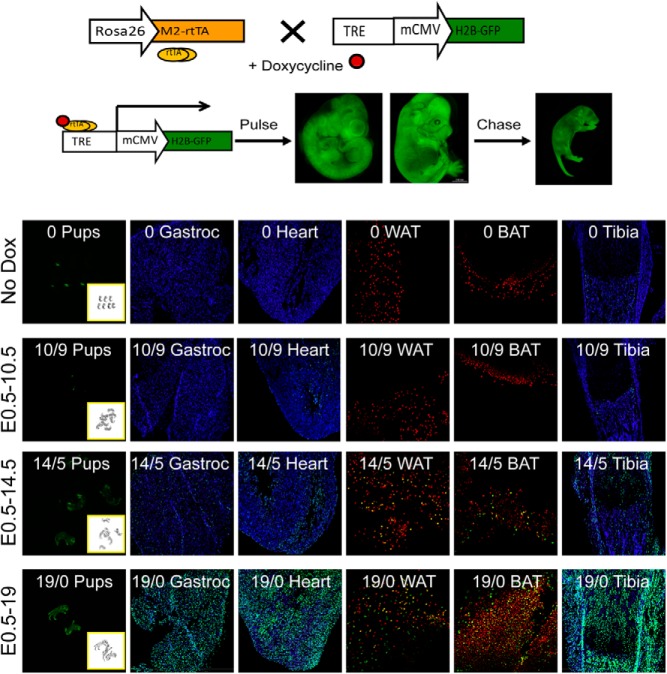
Because this is the first study to use the H2B-GFP tetracycline system for labeling LRCs in heart and skeletal muscle, we first determined the level of autoflourescence and in utero dosing period for these and other tissues. Although some auto fluorescence was detected in animals that did not receive doxycycline (Figure 1, No Dox), it was exceedingly low as, for example, in milk-filled stomachs of day 0 neonates. In individual tissues, autofluorescence was detected in 0.17% of cells in the heart, 0% in gastrocnemius and adipose tissue, and 0.02%, 0.6%, and 5% in the articular surface, epiphysis, and diaphysis of tibia, respectively, although the latter was limited almost exclusively to bone marrow (see Results). This indicates a very low level of leaky H2B/GFP expression in noninduced animals.
Figure 1.
Model characterization. WT H2B-GFP mice were crossed with WT M2 mice and pregnant mothers were given normal drinking water (No Dox) or pulsed with water supplemented with doxycycline (Dox) from E0.5 to E10.5, E14.5, or E19 (birth). Pregnant mice pulsed from E0.5 to E10.5 or E14.5 were subsequently chased with normal water. Neonates expressing GFP were identified by GFP- and brightfield imaging (first column). Gastrocnemius (Gastroc), heart, and tibia were sectioned, nuclei were stained with DAPI (blue), and sections were imaged at ×100 (green, LRCs). WAT and BAT were stained with propidium iodide (red nuclei) and imaged at ×250. Inset numbers indicate the days pulsed with Dox/days chased without Dox.
Day 0 pups were screened for GFP expression using a Kodak Imager 2000 mm and confirmed by genotyping. This technique worked on animals pulsed with doxycycline from E0.5 to E14.5 or E19 because labeled (M2/H2B positive) and nonlabeled (M2/H2B negative) mice could be easily distinguished by imaging (Figure 1). However, animals pulsed to E10.5 were not sufficiently labeled to screen by imaging alone and were therefore genotyped. The heart is the first organ to develop and by E10.5, heartbeat and circulation are established (47). Pregnant mothers were therefore administered a doxycycline pulse from E0.5 to E10.5 in order to label cardiac LRCs, which was followed by a 9-day chase period without doxycycline (Figure 1). Myogenic, chondrogenic, osteogenic, and adipogenic precursors originate from compartments within embryonic somites, the formation of which is mostly complete by E14. Nonsomite origins of these cells develop within the same time period and long bones are already present when secondary myogenesis begins. Thus, pulse periods from E0.5 to E14.5 and birth (E19), followed by 5 or 0 days chase, respectively, were also performed. Pulsing to E14.5 selectively labeled a minority of cells in gastrocnemius, fat, and tibia, suggesting that differentiated cells were for the most part unlabeled, as has been described previously (42, 44). This is supported by the fact that relative estimates of LRCs are very similar to published estimates of stem/progenitor cell numbers in these tissues (see Discussion). By contrast, pulsing to birth without a chase period labeled all cells. We therefore pulsed until E10.5 when labeling cardiac LRCs and until E14.5 for the other tissues.
Skeletal muscle LRCs
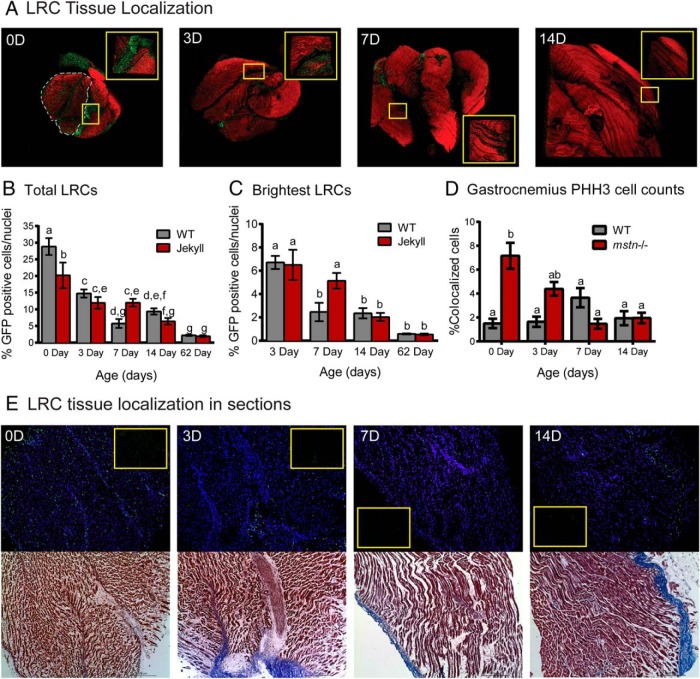
Confocal imaging determined that most LRCs were located in the connective tissue regions of the gastrocnemius and also in the major head (Figure 2A). As expected, the number of LRCs decreased over time, which is consistent with satellite cell expansion and incorporation into mature fibers. Nevertheless, approximately 2% of total nuclei remained labeled even after 2 months (Figure 2, B and C). This LRC tissue localization pattern was further confirmed by Masson's trichrome staining of individual muscle sections (Figure 2E; connective tissue stains blue).
Figure 2.
Skeletal muscle LRCs. A, Three dimensional confocal images of whole gastrocnemius (×50) constructed using Metamorph software. Tissues were sampled from 0-, 3-, 7-, and 14-day-old pups (0 day old, 0D, etc; red, phalloidin-stained actin; green, LRCs) pulsed from E0.5 to E14.5. Inset images (×250) correspond to areas within yellow boxes. The greater head is outlined in the 0-day (0D) image and all tissues are oriented with the cranial end on top. B and C, The number of total and brightest LRCs was normalized to total nuclei and quantified in tissues sampled on the indicated days (n = 5–11/group). D, The number of GFP-positive cells also positive for PHH3 was quantified in tissues sampled on the indicated days (n = 3/group). In each histogram, different letters indicate statistical significance (P ≤ .05) between any particular group whereas the same letters indicate no differences. E, Sections of gastrocnemius were stained with DAPI (blue nuclei) and counterstained with Masson's trichrome stain (bottom, red muscle and blue connective tissue) before imaging at ×100. The top panels are of DAPI/GFP-merged images; inset are GFP only.
Jekyll mice were born with significantly fewer LRCs compared with WT mice (∼8% different) (Figure 2B), even though tissue weights were similar (data not shown). This is consistent with increased hyperplastic muscle growth and satellite cell activation in mstn−/− mice (10, 14). The pattern of change in the number of LRCs during the first 2 weeks differed between WT and Jekyll mice. Indeed, the latter niche was more stable in week 1 and only declined by 8% compared with more than 20% in WT muscle. In order to detect potential differences in proliferation or LRC recruitment from nonmuscle sources, we gated our measurements to include only the brightest cells: those that have not proliferated and remain quiescent. The patterns remained the same, however, and by day 62 there was no difference in either total or brightest LRCs between genotypes. There was also no difference in staining of the proliferation marker PHH3 (Figure 2D), confirming these results, except for at day 0 where there were almost 7-fold more proliferating cells in Jekyll neonates. This is consistent with fewer LRCs at day 0 as the label is diluted with proliferation.
Cardiac LRCs
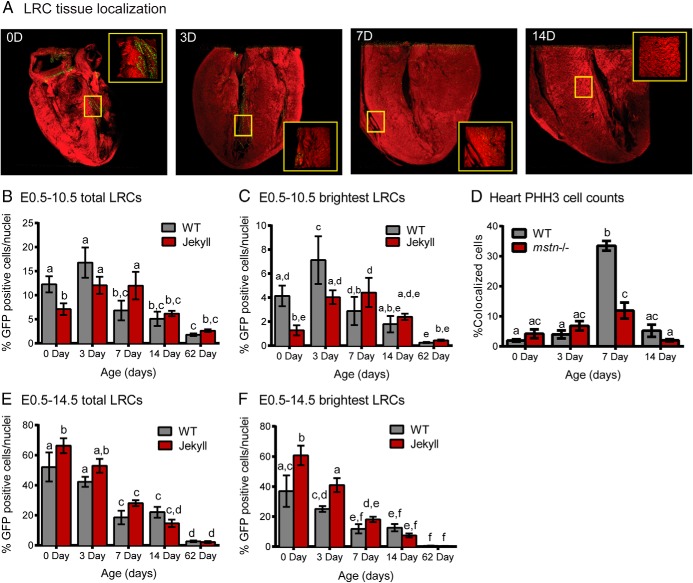
In the heart, LRCs were located near the ventricles, septum, and epicardium at all time points analyzed (Figure 3A), and by 2 months there were fewer total LRCs and most were concentrated near the epicardium. As with skeletal muscle, Jekyll mice had fewer total cardiac LRCs at birth compared with wild type (Figure 3B). Interestingly, total LRC counts increased from birth to day 3 in both genotypes, but the difference was only significant in Jekyll mice (Figure 3B). Total LRCs then steadily declined in WT mice (>40% in 4 days), but remained constant during the first week in Jekyll mice before declining. A similar pattern emerged when quantifying the brightest LRCs (Figure 3C), although the increase on day 3 was now significantly different in both groups. Indeed, brightest LRC counts increased 1.5- and 4-fold in WT and Jekyll mice, respectively. Staining for PHH3 increased on day 7 only (Figure 3D) and particularly in WT mice. This suggests that the changes in total cardiac LRCs is not due to proliferation per se because the increase also occurred with the brightest LRCs, which have not proliferated, and did not coincide with increased PHH3 staining. It is possible, therefore, that this increase is due to LRC recruitment from tissues outside the heart.
Figure 3.
Cardiac LRCs. A, 3-dimensional confocal images of whole hearts (×50) constructed using Metamorph software. Hearts were sampled from 0-, 3-, 7-, and 14-day-old pups (0 day old, 0D etc; red, phalloidin-stained actin; green, LRCs) pulsed with doxycycline from E0.5–E10.5. Inset images (×250) correspond to areas within yellow boxes. B and C, The number of total and brightest LRCs was normalized to total nuclei and quantified in tissues sampled on the indicated days. D, The number of GFP-positive cells also positive for PHH3 was quantified in tissues sampled on the indicated days (n = 3/group). E and F, Total and brightest LRCs in mice pulsed with doxycycline from E0.5–E14.5. In all graphs, different letters indicate statistical significance (P ≤ .05, n = 5–7/group) between any particular group whereas the same letters indicate no differences.
Skeletal muscle of mstn−/− mice contain more nuclei and DNA per myofiber as a result of increased satellite cell activation. A similar increase in cardiac myogenesis in utero could conceivably explain the lower amount of LRCs in Jekyll mice at birth. Thus, we increased the pulse period to E14.5 in order to label and quantify the number of differentiated nuclei in cardiac muscle. This produced 4- and 9-fold more LRC nuclei in WT and Jekyll mice, respectively, when compared with the E0.5–E10.5 pulse period (Figure 3, B and E). It additionally resulted in the detection of significantly more Jekyll LRC nuclei at birth and day 3 (Figure 3E). This is in contrast to data obtained using the shorter pulse protocol, which labels mostly quiescent stem/progenitor cells, and is consistent with a greater number of differentiated nuclei within the myocardium. The lower number of stem/progenitor cells in Jekyll mice (Figure 3, B and C) may therefore be a result of increased cardiac myogenesis. Moreover, gating for the brightest LRCs in mice pulsed until E14.5 reduced counts by 15% in 0 day WT hearts compared with only 6% in Jekyll hearts (Figure 3, E and F). The larger reduction in WT counts is again consistent with a larger population of stem/progenitor cells within the WT pool of LRC nuclei whereas the relative stability of the mstn−/− population suggest that the nuclei belong to a more committed cell type (eg, differentiated cardiomyocytes). The number of LRCs detected on day 62 was similar whether mice were dosed until E10.5 or E14.5 and suggests that the labeled pool, by day 62, is the same regardless of labeling protocol.
Expression of cardiac β-adrenergic receptors and Ca2+-handling proteins
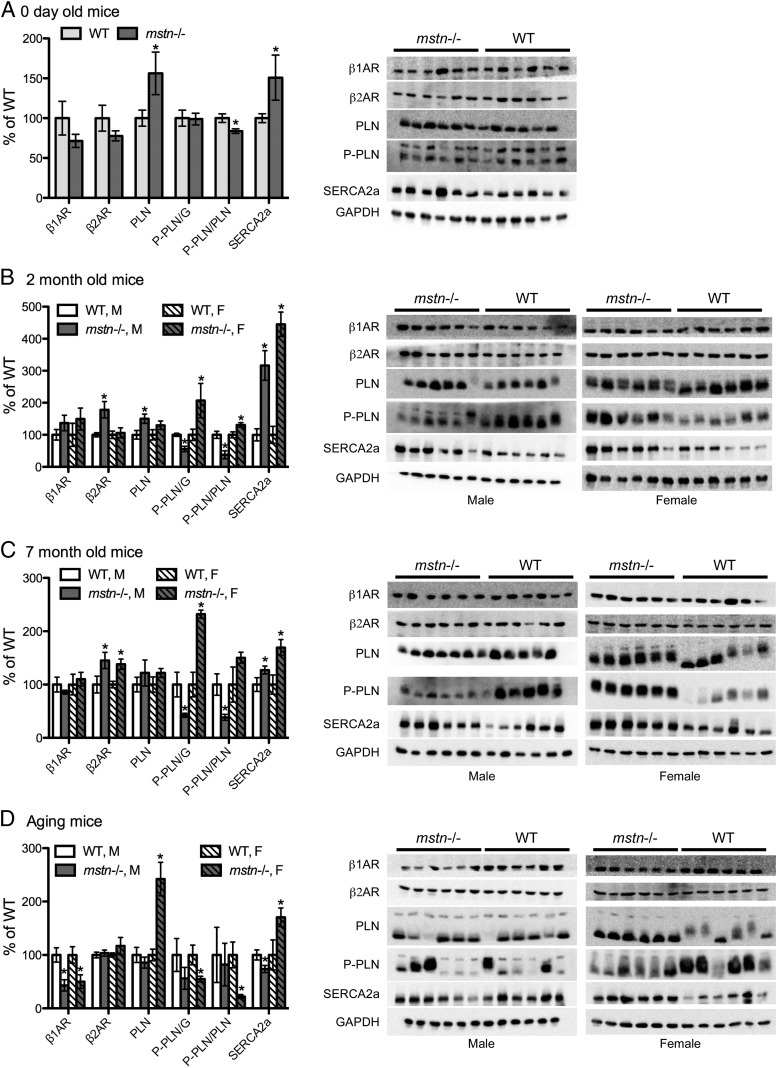
Protein expression of β1AR, β2AR, PLN, P-PLN, and SERCA2a was quantified in hearts from WT and mstn−/− mice of different ages in order to elucidate the mechanisms responsible for enhanced cardiac function, contractility, and Ca2+-transients in mstn−/− mice (7). Levels of PLN and SERCA2a were both elevated in mstn−/− neonates (Figure 4A) whereas SERCA2a levels were also elevated in 2- and 7-month-old (m.o.) mstn−/− mice (Figure 4, B and C). This was particularly evident in 2-m.o. mice because levels in mstn−/− hearts were 3 to 4-fold higher than those in WT hearts. Expression of β2AR and PLN was also elevated in 2-m.o. male mstn−/− mice, and β2AR levels were elevated in both sexes at 7 months. This is highly novel and indicates that the enhanced performance of mstn−/− cardiac muscle is due specifically to the absence of myostatin during development and not to compensatory changes brought upon by enhanced muscle growth that, in fact, only becomes apparent after 3 months of age (6, 7). These data also suggest that increased β2AR and, in particular, SERCA2A levels could mechanistically explain the heightened responsiveness of mstn−/− hearts and cardiomyocytes to β-adrenergic agonists.
Figure 4.
Expression of cardiac β-ARs and Ca2+-handling proteins. Hearts were removed from male (M) and female (F) WT and mstn−/− pups on the indicated days and equal amounts of protein were analyzed by Western blotting for β1AR and β2ARs, PLN, P-PLN, and SERCA2a. Band intensities for each of these proteins were normalized to those of GAPDH whereas P-PLN was normalized to GAPDH or PLN (P-PLN/G or P-PLN/PLN). Aging mice (D) were 18–24 m.o., and significant differences in all graphs are indicated by asterisks (P ≤ .05, n = 6/group). Comparisons were made between WT and msnt−/− mice for a particular sex but not between sexes.
Several notable differences were also detected in aging mice (18–24 months of age). Like young mstn−/− mice, this age group is also more responsive to isoproterenol stress tests than similarly aged WT mice (6, 7). Differences include 50% lower levels of β1AR protein expression in mstn−/− mice of both genders (Figure 4D). This was somewhat surprising considering that β1AR levels were similar in the younger age groups and because β2AR levels were higher, not lower, in these mice (Figure 4, B and C). The aged mstn−/− females also maintained nearly 2- and 2.5-fold higher levels of SERCA2a and PLN, respectively, than aged WT mice, although SERCA2a levels were slightly lower in aged mstn−/− male mice.
We normalized P-PLN to GAPDH or PLN to determine the total and relative ratio of P-PLN, respectively, in cardiac muscle extracts. Both normalizations produced similar results in all age groups except neonates and appeared to change independently of total PLN. In neonates, relative P-PLN (normalized to PLN) was slightly reduced, but total P-PLN was not. In 2- and 7-m.o. mice, however, P-PLN was higher in female and lower in male mstn−/− mice, regardless of normalization, compared with their WT counterparts (Figure 4, B and C). Aged mice, on the other hand, had lower P-PLN levels that were significant in female mstn−/− mice only.
Adipose tissue LRCs
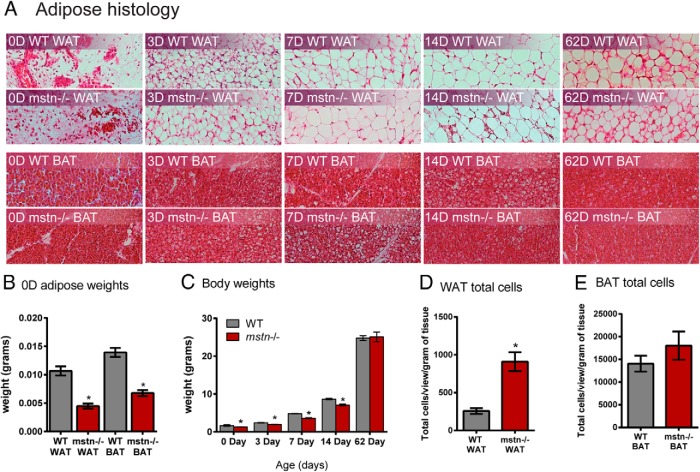
A histologic assessment of inguinal fat pads indicated no differences between WT and mstn−/− tissues until day 62 when adipocyte size appeared smaller in mstn−/− mice (Figure 5A). The latter is consistent with previous reports demonstrating reduced adiposity and a greater distribution of smaller adipocytes in inguinal fat pads from adult male and female mstn−/− mice (6, 26). Brown adipose histology was again similar in WT and mstn−/− neonates, although by day 14, cell size appeared smaller in mstn−/− mice. At birth, adipose tissue weights were smaller by 2-fold in mstn−/− mice (Figure 5B). The body weights of mstn−/− mice were also smaller than WT up until day 62 (Figure 5C), consistent with the rapid phase of muscle growth that occurs in juvenile mstn−/− mice (26). This suggests that the caloric demand of enhanced musculature influences adipose tissue weights and cell size. To determine whether mstn−/− mice had more adipocytes, despite smaller tissue weights, we normalized adipocyte cell counts in adult mice to tissue weights and determined that the number of cells in mstn−/− inguinal fat pads were 3 times higher than those in WT pads (Figure 5D). Cell counts were also higher in BAT, but not significantly different (Figure 5E).
Figure 5.
Adipose tissue histology and weights in WT and mstn−/− neonates. A, Representative hematoxylin and eosin-stained images from inguinal WAT and BAT (×200) removed from mice at day (D) 0, 3, 7, 14, and 62. B and C, Inguinal WAT and BAT weights from neonates (n = 4/group) and body weights from mice of the indicated ages (n = 20–69/group). D and E, Inguinal WAT and BAT cell counts (quantified from 5–6 images/mouse tissue) normalized to tissue weights (n = 10/group). Significant differences between WT and mstn−/− mice are indicated by asterisks (P ≤ .05).
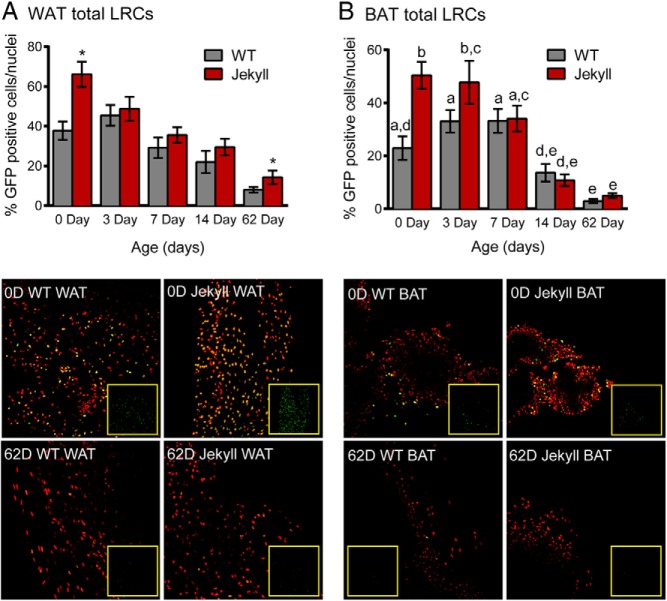
In contrast to muscle and heart LRC counts, Jekyll mice were born with approximately twice the number of WAT and BAT LRCs (Figure 6, A and B). These counts then steadily declined in WAT of both genotypes and, by day 62, Jekyll mice still maintained twice the number of LRCs. In Jekyll BAT, LRC counts remained significantly higher than WT counts until day 7 (Figure 6B) and eventually declined almost 10-fold by day 62 in both groups. Unlike WAT, however, there was no significant difference between groups at this time. Comparing LRC counts in WAT and BAT indicated that the number of LRCs in inguinal WAT of WT mice was almost double that of BAT even at 2 months of age. This suggests that the progenitor pool in BAT is smaller than the WAT pool and may be due to the different primary functions of WAT vs BAT. Nevertheless, this difference was far less pronounced in Jekyll mice due to disproportionately elevated LRC counts in mstn−/− BAT.
Figure 6.
Adipose tissue LRCs. A, Inguinal WAT was removed from mice on the indicated days and LRCs were quantified (histogram) and normalized to total nuclei stained with propidium iodide (red). Inset images are of GFP only and correspond to the surrounding merged nuclei/GFP image. Significant differences between WT and mstn−/− mice are indicated by asterisks (P ≤ .05, n = 5–11/group). B, BAT LRCs were similarly quantified and imaged. Different letters indicate statistical significance between any group, whereas the same letters indicate no differences.
Tibia LRCs
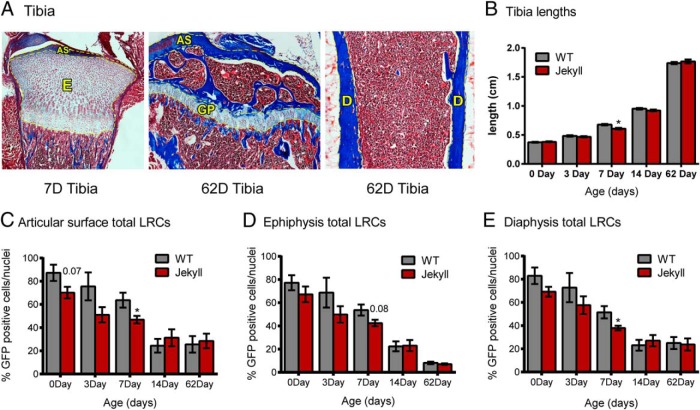
Bone strength is correlated to muscle mass due to the mechanical stresses imposed on bone (48, 49), which may explain the increased strength of mstn−/− bone (22, 50). Nevertheless, myostatin coregulates bone development with GDF-11, and its influence on osteo- and chondroprogenitor pools is unknown. We therefore quantified tibia LRCs in the articular surface, ephiphysis, growth plate, and diaphysis of WT and Jekyll mice (Figure 7A). Although the body weights of mstn−/− neonates are smaller than WT weights (Figure 5C), tibia lengths were similar at all time points except for day 7 when they were smaller (Figure 7B). The number of LRCs steadily decreased after birth in both groups, at apparently the same rate and in all 3 bone regions (Figure 7, C–E). There were also no differences in total LRC numbers between groups except at day 7 when the Jekyll numbers were slightly smaller. Gating to measure only the brightest LRCs again produced identical results in both groups (data not shown). Thus, myostatin does not appear to regulate the number of LRCs or pattern of proliferation or recruitment in tibia. This is consistent with preliminary assessments of other tissues (eg, neonate pituitaries, data not shown) that are also not different between groups.
Figure 7.
Tibial LRCs. A, Representative images of Masson's trichrome-stained tibias (×40). Yellow dashed lines indicate areas where LRCs were quantified (AS, articular surface; E, epiphysis; GP, growth plate; D, diaphysis). B, Lengths of tibia removed from mice on the indicated days (n = 20–69/group). C–E, Total LRC counts for articular surface, epiphysis, and diaphysis. When quantifying the epiphysis, the large region labeled E in panel A was used for 0-, 3-, and 7-day tibias whereas the growth plate was used for 14- and 62-day tibias. Significant differences between WT and mstn−/− mice are indicated by asterisks (P ≤ .05, n = 5–7/group).
Discussion
To our knowledge, this is the first study to quantify putative stem/progenitor cells using the doxycycline-inducible H2B/GFP system and embryonic pulsing. It is also the first study to quantify muscle, heart, fat, and tibia LRCs using this model and more importantly, to assess myostatin's influences on the putative stem/progenitor cell pools in the latter three tissues. The results together suggest that myostatin's actions are far more pleiotropic than generally presumed and include the regulation of these pools as well as the expression of Ca2+-handling proteins in the heart. It is important to note that the differences reported herein are not due to differential dosing of doxycycline between WT and Jekyll mice because LRC counts in different tissues were higher, lower, or similar between groups.
This H2B/GFP doxycycline-inducible system described is superior to the more common method of bromodeoxyuridine labeling, which only labels S phase cells and requires tissues to be fixed and denatured. By contrast, the H2B/GFP system labels all cells independently of cell cycle, and the H2B/GFP label itself is stable for long chase periods, enabling LRCs to be isolated and further characterized in vitro or in vivo. In fact, this system has been used to identify stem cell niches in many diverse tissues (42, 44, 51, 52) as well as to analyze cycling rates and distinct pools of hematopoietic stem cells (53, 54). A potential weakness of the system, however, is the possibility of “leaky” H2B/GFP transactivation in some tissues. This proved to be extremely rare except for ossified regions of tibia diaphysis in which approximately 5% of cells were labeled in the absence of induction (Figure 1), which commonly occurs in bone marrow (55). Nevertheless, this was not problematic because ossification regions were easily excluded when quantifying tibia LRCs.
Developmental expression of myostatin is most evident in somites, and its ablation increases the number of primary myotubes as well as Pax7-positive satellite cells by E14.5 (10). The fewer number of LRCs in Jekyll gastrocnemius at birth is consistent with the elevated levels of satellite cell proliferation that occur in mstn−/− skeletal muscle during primary and secondary myogenesis (10) as this would result in the dilution of label among satellite cells, even among reserve populations that renew through limited proliferation (18). In mature skeletal muscle, satellite cells constitute 35% of skeletal muscle nuclei at birth and 5% by sexual maturity (18). Our study indicates that by 2 months, LRC numbers were similar in Jekyll and WT mice. This was confirmed even when we quantified LRCs in mice pulsed from E0.5 to birth (data not shown). In both situations, LRCs represented 2%–5% of all nuclei, which is consistent with proposed satellite cell numbers in adult mice.
Satellite cells express M-cadherin, c-Met, and CD34, although Pax7 is the most reliable biomarker because it is expressed exclusively in these cells whether quiescent or proliferating. Despite having reliable markers, it is still unclear how myostatin influences these cells in vivo. McCroskery et al (14) first reported higher numbers in mstn−/− mice, compared with WT, which contradicts subsequent studies reporting no difference or slightly lower cell numbers (16, 17). However, McCroskery et al quantified CD34-positive cells whereas the latter studies used Pax7 as a marker. Other myogenic cells also populate the satellite cell niche including muscle-derived stem cells and pericytes as well as the CD34-positive side population, PW1+ interstitial, and fibroadipoprogenitor (also known as preadipocyte) cells (18). Thus, McCroskery et al likely quantified differences in several cell types within the heterologous pool rather than satellite cells per se. Our study quantified the entire stem/progenitor pool, in contrast to all 3 studies, and suggests that it is indeed regulated by myostatin. Furthermore, the LRC pool in Jekyll skeletal muscle appeared more stable than the WT pool despite a greater number of proliferating cells at day 0. This could either be due to a greater number of fully formed myotubes in mstn−/− mice, which is evident by E18.5 (10) and even in adult mice (11), or to differences in the heterologous progenitor cell populations. Future studies are therefore needed to determine whether the noted differences are due to changes in one or multiple populations of distinct cell types and whether the composition of the resulting adult pool is different in WT and mstn−/− mice.
The discovery of stem cells in vertebrate hearts has transformed the field's understanding of cardiac developmental biology and of regenerative medicine (56). Although cardiac muscle may differ from skeletal muscle in lacking a large heterologous pool of myogenic progenitor cells, it nevertheless maintains the ability to regenerate, especially in fish (57), and cardiac stem/progenitor cells are critical to this process as well as to the normal maintenance of muscle mass (58). Attenuating myostatin receptor signaling prevents cancer-induced cardiac cachexia, and hearts of mstn−/− mice are hypertrophic (6, 7, 36). Conversely, overexpressing myostatin can cause cardiac atrophy (59), and the myokine directly inhibits many growth processes in primary cardiomyocytes and in H9C2 cardiomyoblasts (8). The differences described herein should therefore come as no surprise because myostatin clearly regulates cardiac muscle growth.
The localization of cardiac LRCs is similar to stem cell niches previously described, near the epicardium (60) and ventricles (61), although we did not detect a particular concentration near the apex (61). The number of cardiac stem/progenitor cells is still in debate and estimates range up to 2% depending on whether cardiac muscle or endothelial progenitors are distinguished (62). This is consistent with the approximate 2% of cardiac LRCs identified in 2-m.o. mice regardless of pulse period. Future studies are needed to characterize the exact identity of each cardiac LRC pool (based on location), which should not detract from the demonstrable fact that myostatin clearly regulates the overall population and in a manner similar to skeletal muscle. Indeed, our results suggest that cardiac myogenesis is enhanced in Jekyll neonates (ie, fewer LRCs and more differentiated nuclei at birth) and that the Jekyll pool is more stable during the first week. This is identical to the skeletal muscle pattern and again suggests that the heterologous pool may differ between WT and mstn−/− mice. Evidence of stem/progenitor cell recruitment was also detected in both groups, but was enhanced in Jekyll mice. This is highly novel because circulating mesenchymal (bone marrow-derived) stem cells also contribute to the growth, maintenance, and repair of cardiac muscle and although such cells also contribute to other tissues (63), they likely play a lesser role in tissues with pronounced stem/progenitor niches (eg, skeletal muscle). Myostatin may therefore regulate the circulating pool itself or alternatively, the deposition/recruitment of these cells.
Hearts and cardiomyocytes from mstn−/− mice are also more responsive to β-adrenergic stimuli whereas Ca2+ transients and cellular loads are similarly enhanced (6, 7). Our results suggest that this is due to the up-regulation in βAR2 and SERCA2a, at least in young adult mice. The latter was also elevated in aging female, but not male, mice (6) and possibly explains why enhanced β-adrenergic responsiveness is only lost in aging male mstn−/− mice. Many cardiac contractility disorders are associated with reduced levels or activity of SERCA2a that result in Ca2+ dysregulation (64–66). Restoring SERCA2a can restore cardiac function in such cases (67–69), and SERCA2a is in fact a target for gene therapy (70). Studies have also demonstrated elevated SERCA2a expression with exercise-induced cardiac improvement (71), a condition that resembles the mstn−/− cardiac phenotype. This together suggests that attenuating myostatin may improve cardiac function as well as mass, whether or not the myokine directly or indirectly controls SERCA2a expression.
PLN negatively regulates SERCA2a and its deactivation is stimulated by βAR/protein kinase A signaling that phosphorylates PLN (72). Ablation of PLN enhances Ca2+ reuptake and relaxation in cardiac muscle, but also impairs βAR sensitivity, whereas PLN overexpression compromises mechanical performance, all of which can be rescued with βAR activation. Although total PLN levels were often elevated in mstn−/− hearts, this is likely not a direct compensatory response to up-regulated SERCA2a because their expression is independently regulated (73). The sexual dimorphism in P-PLN levels among 2- and 7-m.o. mstn−/− mice (elevated in females, reduced in males) can also not be explained by changes in βAR levels alone. Thus, PLN levels and activity may also be under the control of myostatin or a myostatin-regulated factor, as with SERCA2a. Estradiol inhibits, rather than stimulates, PLN activation and does not appear to regulate PLN expression (74). Gonadal steroids may therefore influence myostatin sensitivity, but are likely not directly responsible for the noted sexual dimorphism.
The effects of myostatin on adipogenesis are still under debate. Myostatin inhibits the differentiation of bone marrow-derived mesenchymal stem cells as well as primary preadipocytes (31), but stimulates C3H10T(1/2) commitment toward an adipogenic rather than myogenic fate (32, 75). Adding further confusion is the demonstration that myostatin blocks bone morphogenetic protein 7-stimulated adipogenesis in C3H10T(1/2) and 3T3-L1 cells (76). Differentiation in each of these studies was induced prior to myostatin treatment and the myokines effects on adipogenesis in vivo is currently unknown despite the well-documented reductions in total adiposity with myostatin attenuation (1). The greater number of mature adipocytes in mstn−/− WAT and LRCs in Jekyll WAT and BAT supports the hypothesis that myostatin inhibits preadipocyte differentiation in vivo. This suggests that reduced adiposity in mstn−/− animals is, therefore, due to reduced fat deposition and not necessarily to reduced adipogenesis.
The myostatin/activin receptor (Acvr2b) is expressed in many nonmuscle tissues including cartilage and bone (77, 78). In addition, the proliferation rate of chondrocytes isolated from the epiphyseal growth plates of mstn−/− mice is greater than that of cells from WT mice (79). Notwithstanding, our study failed to detect significant differences in the number of bone LRCs in WT and mstn−/− mice, regardless of region. This suggests that the previously noted differences in bone mineral density or morphology (80) are compensatory responses to hypermusclarity or possibly to enhanced somatomediation (81), but not to differences in bone stem/progenitor pools. This is contrasted by our studies with muscle, heart, and fat that clearly indicate that myostatin regulates the putative stem/progenitor pools in these tissues.
Future experiments are of course needed to determine cellular identities, stemness, and relative compositions of each LRC pool. The Jekyll mouse will no doubt prove invaluable to such studies and will aid in the isolation, in vitro characterization, and fate mapping of these cells. This in turn will help determine myostatin's influence on the growth and development of these tissues and may even contribute to novel therapies that rely upon or target stem/progenitor cells.
Acknowledgments
This work was supported by the National Science Foundation Grant 1147275 (to B.D.R.) and the National Institutes of Health (5T32GM083864).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- β2AR
- β-adrenergic receptor 2
- BAT
- brown adipose tissue
- DAPI
- 4′,6-diamidino-2-phenylindole
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GDF
- growth/differentiation factor
- GFP
- green fluorescent protein
- H2B
- histone 2B
- LRC
- label retaining cell
- m.o.
- month old
- PFA
- paraformaldyhde
- PHH3
- phosphohistone H3
- PLN
- phospholamban
- P-PLN
- phosphorylated PLN
- SERCA
- sarcoplasmic reticulum Ca2+ ATPase
- TBST
- Tris-buffered saline/0.1% Tween 20
- WAT
- white adipose tissue
- WT
- wild type.
References
- 1. Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008;29(5):513–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma M, Kambadur R, Matthews KG, et al. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180(1):1–9 [DOI] [PubMed] [Google Scholar]
- 3. Kubota K, Sato F, Aramaki S, Soh T, Yamauchi N, Hattori MA. Ubiquitous expression of myostatin in chicken embryonic tissues: its high expression in testis and ovary. Comp Biochem Physiol A Mol Integr Physiol. 2007;148(3):550–555 [DOI] [PubMed] [Google Scholar]
- 4. Amthor H, Huang R, McKinnell I, et al. The regulation and action of myostatin as a negative regulator of muscle development during avian embryogenesis. Dev Biol. 2002;251(2):241–257 [DOI] [PubMed] [Google Scholar]
- 5. Østbye TK, Galloway TF, Nielsen C, Gabestad I, Bardal T, Andersen Ø. The two myostatin genes of Atlantic salmon (Salmo salar) are expressed in a variety of tissues. Eur J Biochem. 2001;268(20):5249–5257 [DOI] [PubMed] [Google Scholar]
- 6. Jackson MF, Luong D, Vang D, et al. The aging myostatin null phenotype: cardiac hypertrophy, enhanced stress response, and sexual dimorphism. J Endocrinol. 2012;213(3):263–275 [DOI] [PubMed] [Google Scholar]
- 7. Rodgers BD, Interlichia JP, Garikipati DK, et al. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J Physiol. 2009;587(Pt 20):4873–4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Artaza JN, Reisz-Porszasz S, Dow JS, et al. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrinol. 2007;194(1):63–76 [DOI] [PubMed] [Google Scholar]
- 9. Morissette MR, Cook SA, Foo S, et al. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res. 2006;99(1):15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsakas A, Otto A, Elashry MI, Brown SC, Patel K. Altered primary and secondary myogenesis in the myostatin-null mouse. Rejuvenation Res. 2010;13(6):717–727 [DOI] [PubMed] [Google Scholar]
- 11. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387(6628):83–90 [DOI] [PubMed] [Google Scholar]
- 12. Elashry MI, Otto A, Matsakas A, El-Morsy SE, Patel K. Morphology and myofiber composition of skeletal musculature of the forelimb in young and aged wild type and myostatin null mice. Rejuvenation Res. 2009;12(4):269–281 [DOI] [PubMed] [Google Scholar]
- 13. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162(6):1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102(7):2519–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amthor H, Otto A, Vulin A, et al. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci USA. 2009;106(18):7479–7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Q, McPherron AC. Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J Physiol. 2012;590(Pt 9):2151–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9(10):642–647 [DOI] [PubMed] [Google Scholar]
- 20. Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec A DiscovMol Cell Evol Biol. 2003;272A(1):388–391 [DOI] [PubMed] [Google Scholar]
- 21. Hamrick MW, McPherron AC, Lovejoy CO. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif Tissue Int. 2002;71(1):63–68 [DOI] [PubMed] [Google Scholar]
- 22. Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21(3):477–483 [DOI] [PubMed] [Google Scholar]
- 23. Hamrick MW, McPherron AC, Lovejoy CO, Hudson J. Femoral morphology and cross-sectional geometry of adult myostatin-deficient mice. Bone. 2000;27(3):343–349 [DOI] [PubMed] [Google Scholar]
- 24. McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev Biol. 2009;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22(3):260–264 [DOI] [PubMed] [Google Scholar]
- 26. McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109(5):595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun. 2002;291(3):701–706 [DOI] [PubMed] [Google Scholar]
- 28. Akpan I, Goncalves MD, Dhir R, et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes (Lond). 2009;33(11):1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geng J, Peng F, Xiong F, et al. Inhibition of myostatin promotes myogenic differentiation of rat bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2009;11(7):849–863 [DOI] [PubMed] [Google Scholar]
- 30. Artaza JN, Singh R, Ferrini MG, Braga M, Tsao J, Gonzalez-Cadavid NF. Myostatin promotes a fibrotic phenotypic switch in multipotent C3H 10T1/2 cells without affecting their differentiation into myofibroblasts. J Endocrinol. 2008;196(2):235–249 [DOI] [PubMed] [Google Scholar]
- 31. Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/β-catenin signaling pathways. J Biol Chem. 2008;283(14):9136–9145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Artaza JN, Bhasin S, Magee TR, et al. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146(8):3547–3557 [DOI] [PubMed] [Google Scholar]
- 33. Lei H, Yu B, Yang X, et al. Inhibition of adipogenic differentiation by myostatin is alleviated by arginine supplementation in porcine-muscle-derived mesenchymal stem cells. Sci China Life Sci. 2011;54(10):908–916 [DOI] [PubMed] [Google Scholar]
- 34. Dilger AC, Spurlock ME, Grant AL, Gerrard DE. Myostatin null mice respond differently to dietary-induced and genetic obesity. Anim Sci J. 2010;81(5):586–593 [DOI] [PubMed] [Google Scholar]
- 35. Hamrick MW, Pennington C, Webb CN, Isales CM. Resistance to body fat gain in 'double-muscled' mice fed a high-fat diet. Int J Obes (Lond). 2006;30(5):868–870 [DOI] [PubMed] [Google Scholar]
- 36. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–543 [DOI] [PubMed] [Google Scholar]
- 37. Khurana TS, Davies KE. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2(5):379–390 [DOI] [PubMed] [Google Scholar]
- 38. Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19(6):543–549 [DOI] [PubMed] [Google Scholar]
- 39. George I, Bish LT, Kamalakkannan G, et al. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail. 2010;12(5):444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277(25):22528–22533 [DOI] [PubMed] [Google Scholar]
- 41. Pistilli EE, Bogdanovich S, Goncalves MD, et al. Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. Am J Pathol. 2011;178(3):1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137(5):811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sada A, Tumbar T. New insights into mechanisms of stem cell daughter fate determination in regenerative tissues. Int Rev Cell Mol Biol. 2013;300:1–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121(3):465–477 [DOI] [PubMed] [Google Scholar]
- 47. Savolainen SM, Foley JF, Elmore SA. Histology atlas of the developing mouse heart with emphasis on E11.5 to E18.5. Toxicol Pathol. 2009;37(4):395–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruff C, Holt B, Trinkaus E. Who's afraid of the big bad Wolff?: “Wolff's law” and bone functional adaptation. Am J Phys Anthropol. 2006;129(4):484–498 [DOI] [PubMed] [Google Scholar]
- 49. Robling AG. Is bone's response to mechanical signals dominated by muscle forces? Med Sci Sports Exerc. 2009;41(11):2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamrick MW, Pennington C, Byron CD. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF-8 (myostatin). J Orthop Res. 2003;21(6):1025–1032 [DOI] [PubMed] [Google Scholar]
- 51. Oliver JA, Klinakis A, Cheema FH, et al. Proliferation and migration of label-retaining cells of the kidney papilla. J Am Soc Nephrol: JASN. 2009;20(11):2315–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roth S, Franken P, van Veelen W, et al. Generation of a tightly regulated doxycycline-inducible model for studying mouse intestinal biology. Genesis. 2009;47(1):7–13 [DOI] [PubMed] [Google Scholar]
- 53. Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129 [DOI] [PubMed] [Google Scholar]
- 54. Foudi A, Hochedlinger K, Van Buren D, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27(1):84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Challen GA, Goodell MA. Promiscuous expression of H2B-GFP transgene in hematopoietic stem cells. PLoS One. 2008;3(6):e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bergmann O, Zdunek S, Frisen J, Bernard S, Druid H, Jovinge S. Cardiomyocyte renewal in humans. Circ Res. 2012;110(1):e17–18; author reply e19–21 [DOI] [PubMed] [Google Scholar]
- 57. Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190 [DOI] [PubMed] [Google Scholar]
- 58. Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reisz-Porszasz S, Bhasin S, Artaza JN, et al. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285(4):E876–E888 [DOI] [PubMed] [Google Scholar]
- 60. Kuhn EN, Wu SM. Origin of cardiac progenitor cells in the developing and postnatal heart. J Cell Physiol. 2010;225(2):321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sussman MA, Anversa P. Myocardial aging and senescence: where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48 [DOI] [PubMed] [Google Scholar]
- 62. Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113(11):1451–1463 [DOI] [PubMed] [Google Scholar]
- 63. Anversa P, Leri A, Kajstura J. Cardiac regeneration. J Am Coll Cardiol. 2006;47(9):1769–1776 [DOI] [PubMed] [Google Scholar]
- 64. Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing human heart. Basic Res Cardiol. 1997;92(Suppl 1):87–93 [DOI] [PubMed] [Google Scholar]
- 65. Hasenfuss G, Reinecke H, Studer R, et al. Calcium cycling proteins and force-frequency relationship in heart failure. Basic Res Cardiol. 1996;91(Suppl 2):17–22 [DOI] [PubMed] [Google Scholar]
- 66. Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85(1):38–46 [DOI] [PubMed] [Google Scholar]
- 67. del Monte F, Williams E, Lebeche D, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104(12):1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Loukianov E, Ji Y, Grupp IL, et al. Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circ Res. 1998;83(9):889–897 [DOI] [PubMed] [Google Scholar]
- 69. Niwano K, Arai M, Koitabashi N, et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16(6):1026–1032 [DOI] [PubMed] [Google Scholar]
- 70. Periasamy M, Kalyanasundaram A. SERCA2a gene therapy for heart failure: ready for primetime? Mol Ther. 2008;16(6):1002–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kemi OJ, Ceci M, Condorelli G, Smith GL, Wisloff U. Myocardial sarcoplasmic reticulum Ca2+ ATPase function is increased by aerobic interval training. Eur J Cardiovasc Prev Rehabil. 2008;15(2):145–148 [DOI] [PubMed] [Google Scholar]
- 72. Cerra MC, Imbrogno S. Phospholamban and cardiac function: a comparative perspective in vertebrates. Acta Physiol (Oxf). 2012;205(1):9–25 [DOI] [PubMed] [Google Scholar]
- 73. Haghighi K, Gregory KN, Kranias EG. Sarcoplasmic reticulum Ca-ATPase-phospholamban interactions and dilated cardiomyopathy. Biochem Biophys Res Commun. 2004;322(4):1214–1222 [DOI] [PubMed] [Google Scholar]
- 74. Filice E, Angelone T, De Francesco EM, Pellegrino D, Maggiolini M, Cerra MC. Crucial role of phospholamban phosphorylation and S-nitrosylation in the negative lusitropism induced by 17β-estradiol in the male rat heart. Cell Physiol Biochem. 2011;28(1):41–52 [DOI] [PubMed] [Google Scholar]
- 75. Feldman BJ, Streeper RS, Farese RV, Jr, Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci USA. 2006;103(42):15675–15680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23(20):7230–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hamrick MW, Shi X, Zhang W, et al. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40(6):1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shuto T, Sarkar G, Bronk JT, Matsui N, Bolander ME. Osteoblasts express types I and II activin receptors during early intramembranous and endochondral bone formation. J Bone Miner Res. 1997;12(3):403–411 [DOI] [PubMed] [Google Scholar]
- 79. Elkasrawy M, Fulzele S, Bowser M, Wenger K, Hamrick M. Myostatin (GDF-8) inhibits chondrogenesis and chondrocyte proliferation in vitro by suppressing Sox-9 expression. Growth Factors. 2011;29(6):253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10(1):56–63 [PMC free article] [PubMed] [Google Scholar]
- 81. Williams NG, Interlichia JP, Jackson MF, Hwang D, Cohen P, Rodgers BD. Endocrine actions of myostatin: systemic regulation of the IGF and IGF binding protein axis. Endocrinology. 2011;152(1):172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]