Abstract
Apolipoprotein AIV (Apo AIV) and cholecystokinin (CCK) are secreted in response to fat consumption, and both cause satiation via CCK 1 receptor (CCK-1R)-containing vagal afferent nerves to the nucleus of the solitary tract (NTS), where Apo AIV is also synthesized. Fasted male Long-Evans rats received ip CCK-8 or fourth-ventricular (i4vt) Apo AIV alone or in combination. Food intake and c-Fos proteins (a product of the c-Fos immediate-early gene) were assessed. i4vt Apo AIV and/or ip CCK at effective doses reduced food intake and activated c-Fos proteins in the NTS and hypothalamic arcuate nucleus and paraventricular nucleus. Blockade of the CCK-1R by i4vt lorglumide adjacent to the NTS attenuated the satiating and c-Fos-stimulating effects of CCK and Apo AIV, alone or in combination. Maintenance on a high-fat diet (HFD) for 10 weeks resulted in weight gain and attenuation of both the behavioral and c-Fos responses to a greater extent than occurred in low-fat diet-fed and pair-fed HFD animals. These observations suggest that NTS Apo AIV or/and peripheral CCK requires vagal CCK-1R signaling to elicit satiation and that maintenance on a HFD reduces the satiating capacity of these 2 signals.
Cholecystokinin (CCK) is secreted by intestinal endocrine cells after lipid and protein consumption, and this fat-induced stimulation is dependent on the formation and secretion of chylomicrons (1–3). CCK is involved in many functions, such as triggering gallbladder contraction, stimulating pancreatic enzyme secretion, modulating intestinal motility, and regulating food intake (4–6). CCK elicits a short-term satiation by reducing meal size (5, 7), and this effect is attenuated by vagotomy, deactivation of vagal afferents with capsaicin, or administration of a CCK 1 receptor (CCK-1R) antagonist; thus the satiation signals are relayed via CCK-1R on vagal afferent nerves (3, 8–11). Apolipoprotein AIV (Apo AIV) is secreted by enterocytes lining the small intestine in response to dietary lipids (12). Peripheral administration of Apo AIV reduces food intake (13–15). Conversely, the application of either CCK-1R antagonists or vagal deafferention abolishes the satiation effect of peripheral Apo AIV when given alone (15). Thus, CCK and Apo AIV-induced satiation signals use a common pathway on vagal afferent nerves passing to the hindbrain, and this is consistent with the findings that neither circulating Apo AIV nor CCK is able to cross the blood-brain barrier (16–18). Recently, we found that ip administration of CCK and Apo AIV interact to suppress food intake and that peripheral Apo AIV requires an intact CCK system as well as intact vagal afferent nerves to relay satiating signals to the hindbrain (14, 15). Interestingly, the nucleus of the solitary tract (NTS) in the hindbrain where vagal afferent axons terminate expresses both CCK-1R and Apo AIV (18, 19). Apo AIV knockout (KO) mice have a greater satiating response to CCK possibly due to up-regulation of CCK-1R expression in the nodose ganglia and/or NTS, suggesting that NTS CCK-1R may be important for the satiation effect induced by Apo AIV (20). However, the role of Apo AIV in the hindbrain and whether it interacts with CCK in the control of food intake remain unknown. We hypothesized that Apo AIV in or near the NTS locally inhibits food intake and also interacts with the peripheral CCK signal and that the interaction requires CCK-1R in the NTS.
Chronic consumption of a high-fat diet (HFD) increases plasma CCK in humans and rodents but does not alter plasma Apo AIV (2, 21–23). It also reportedly reduces sensitivity to the satiating effect of peripheral CCK (24–26), although this is controversial (27, 28). In contrast, HFD decreases hypothalamic levels of Apo AIV and CCK (29, 30) and also reportedly reduces sensitivity to the satiating effect of CCK (24–26). However, neither the effects of fourth-ventricular (i4vt) Apo AIV alone or in combination with CCK on food intake have been assessed in HFD-induced obese animals. We hypothesized that maintenance on a HFD would attenuate sensitivity to the satiating effect elicited by combinations of CCK and Apo AIV. The objectives of the current experiments were to determine: 1) whether Apo AIV functions locally in the hindbrain as a satiating signal; 2) whether the satiation signal from peripheral CCK interacts with NTS Apo AIV to induce satiation; 3) whether NTS CCK-1R are involved; and 4) whether maintenance on a HFD attenuates sensitivity to the satiating effect of combined peripheral CCK and central Apo AIV.
Materials and Methods
Materials
Lorglumide and sulfated CCK-8 were obtained from Sigma-Aldrich, and rat recombinant Apo AIV was produced by a bacterial expression system. The detailed procedure is described in a previous report (31).
Animals
Male Long-Evans rats (200–220 g; Harlan) were housed in an American Association for the Accreditation of Laboratory Animal Care (AAALAC)-accredited facility under conditions of controlled illumination (12-h light, 12-h dark cycle, lights from 6 am to 6 pm) for 14 days before surgery. Rats in most experiments had free access to pelleted chow (7002 Teklad 6% fat mouse/rat diet; Harlan Teklad) and water. In one experiment, rats were maintained on one of 2 semipurified diets, a low-fat diet (LFD) (4% by weight butter fat, D03082705; Research Diets) or a HFD (20% butter fat, D03082706; Research Diets). A third group (pair-fed HFD) received the HFD from 4 to 5 pm every day, but with the same daily caloric intake as the LFD group. Rats were adapted to the assigned diets for 10 weeks before testing. All animals were transferred to clean cages and deprived of food for 17 hours (5 pm to 10 am) before each experiment. Food was returned immediately after the injections of vehicle, CCK, and/or Apo AIV, and body weight, food intake, and water consumption were assessed by weighing food and water bottles (±0.01 g; Adenturer SL, Ohaus Corp). All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use.
In experiment 1, we first completed individual dose-response studies for ip CCK and i4vt Apo AIV. Rats (n = 6–7 rats/group) received ip CCK-8 (0.06, 0.25, or 0.5 μg/kg) or saline. Other rats (n = 5–6/group) received rat recombinant Apo AIV at 2, 4, or 8 μg or cerebrospinal fluid (CSF) (5 μL) into the fourth ventricle. For the combination study in experiment 1.1, rats (n = 8–10/group) received combinations of ip CCK-8 or saline and i4vt Apo AIV or CSF. In experiment 1.2, the CCK-1R antagonist, lorglumide, was used to block CCK-1R in the hindbrain (14). On the test day, rats (n = 8/group) were administered with 2 injections, which included the first injection (CSF or lorglumide) and the second injection (CSF or Apo AIV). In experiment 1.3, rats received 3 injections: the first injection was CSF or lorglumide at 3 μg, the second injection was CSF or Apo AIV, and the third injection was saline or CCK. The first injection (CSF or lorglumide at 3 μg) occurred 15 minutes before the second injection (CSF or Apo AIV at 4 μg) i4vt and followed by the third injection (saline or CCK-8 at 0.25 μg/kg) ip. In experiment 1.4, fasted rats (4–5 rats/group) were killed, and their brains were perfused after 2 administrations of CCK (0.25 μg/kg), Apo AIV (4 μg), or vehicles to determine the number of c-Fos (a product of the c-Fos immediate-early gene)-positive cells in the brain. In the CCK-1R study, rats (5 rats/group) received 3 injections of CCK (0.25 μg/kg), Apo AIV (4 μg), lorglumide (3 μg), or vehicles.
In experiment 2, rats (40/group) were adapted to 10 weeks of LFD or HFD. On the eighth week, all animals were surgically implanted with i4vt cannulas. Fasted rats received combinations of ip CCK-8 (0.25 μg/kg), i4vt Apo AIV (4 μg), or vehicle (saline + CSF). c-Fos-positive proteins were assessed in separate rats.
Surgical implantation of fourth-cerebroventricular infusion cannulas
Rats (n = 10/group) were anesthetized with ketamine (40 mg/100 g) and acepromazine (20 mg/100 g) and placed into a stereotaxic device (Kopf Instruments). A 24-gauge stainless steel guide cannula (Plastic One, Inc) was aimed 2.0 mm above the fourth-cerebral ventricle at the following coordinates: 2.5 mm anterior to the occipital suture, 4.5 mm ventral to dura, and on the midline (32). After 5 days of recovery from surgery, cannulated rats received 210-μg 5-thio-D-glucose (Sigma-Aldrich) in 2-μL artificial CSF (Harvard Apparatus); animals whose plasma glucose at least doubled after 60 minutes were included in the present study (32).
Immunohistochemistry
Ninety minutes after treatment, the rats' brains were perfused through the left ventricle of the heart with 0.9% saline, followed by perfusion of 4% paraformaldehyde to fix the brain (33, 34). The hindbrains and hypothalamus were postfixed in 4% paraformaldehyde and in 30% sucrose-phosphate buffer. Regions of the hindbrain and hypothalamus were identified according to the information provided by the mouse/rat brain stereotaxic atlas of Paxinos and Franklin (35). Based on our published protocols, frozen brain was sliced with a microtome, and brain sections (30 μm) were stained with diluted c-Fos Ab-5 (1:30 000; Calbiochem) and biotinylated goat antirabbit secondary antibody (dilution 1:200) in normal goat serum plus 0.3% Triton X-100 buffer (34). The sections were incubated with horseradish peroxidase avidin-biotin complex (dilution 1:200) and developed by diaminobenzidine with nickel sulfate. As negative controls, sections were incubated with nonimmune rabbit serum instead of the primary antibody to test for nonspecific staining.
Data analysis and statistical analysis
Quantitative assessment of the c-Fos data was achieved by counting the number of c-Fos-positive cells per slide in the brains (34). All slides were numbered without the treatment information to avoid bias in counting, and each hindbrain was bilaterally counted in 5–8 different sections for rats. c-Fos-positive neurons were identified according to the photomicrographs depicted in NTS from bregma −13.24 to −14.6 mm, paraventricular nucleus (PVN) from −1.44 to −1.56 mm, and arcuate nucleus (ARC) from −2.04 to −2.28 mm according to the rat brain in stereotaxic coordinates (35). Cells with distinct brown nuclear c-Fos-like immunoreactivity staining were manually counted under light microscopy with low magnification (×20) with the aid of a 1-mm2 ruler (15, 34). Data are presented as means ± SEM of the average number of cells per slide and of food intake. Parametric statistical analyses, one-way ANOVA and two-way ANOVA were performed using GraphPad Prism (version 5.0), followed by post hoc Newman-Keuls or Bonferroni test, respectively. All differences were considered significant if the P < .05.
Results
Experiment 1. The combination of peripheral CCK and i4vt Apo AIV suppresses food intake via a circuit requiring local CCK-1R
In experiment 1.1, higher doses of CCK-8 caused greater reductions of food intake, with the lowest dose (0.06 μg/kg) consequently being selected to be used in further experiments (Figure 1A). These data are consistent with previous reports that CCK-8 at 0.25 μg/kg and above is effective at suppressing food intake in Long-Evans rats, as well as in mice, whereas CCK-8 at 0.06 μg/kg is an ineffective dose (14, 15). Apo AIV at 4 and 8 μg inhibited food intake at 60 and 120 minutes (Figure 1B), whereas there was no reduction of intake after 2 μg. The combination of ip CCK-8 (0.06 μg/kg) and i4vt Apo AIV (4 μg) suppressed food intake, and it reached significance by 60 minutes (P < .05) (Figure 1C). When the dose of CCK-8 was increased to 0.25 μg/kg, the combination had a prolonged satiating effect for 4 hours (P < .05) (Figure 1C). Combinations of ip CCK-8 and i4vt Apo AIV did not significantly alter 24 hours of food intake relative to control injections (Figure 1D).
Figure 1.
Effect of CCK and Apo AIV on ability to control food intake. Food intake was determined in fasted rats after receiving one of the following treatments. A, Intraperitoneal injection of either CCK-8 or saline (SAL). B, i4vt administration of Apo AIV or CSF. C, Two injections of CCK ip, Apo AIV i4vt, or vehicles (CSF or SAL). D, Two combined doses of CCK ip, Apo AIV i4vt, or vehicles at 24 hours. E, Two i4vt injections of Apo AIV (4 μg), lorglumide (Lorg) (3 μg), or vehicles. F, Three combinations of CCK (0.25 μg/kg), Apo AIV (4 μg), and Lorg (3 μg). Data are expressed as mean ± SEM, n = 10, values with asterisks represent significant differences relative to the vehicle-treated group (P < .05). BW, body weight.
To determine whether the combined doses of CCK and Apo AIV are more efficacious at inhibiting food intake than the individual doses, we calculated the percentage change relative to a saline or/and CSF injection for CCK at 0.25 μg/kg or/and Apo AIV at 4 μg at 3 time points (60, 120, and 240 min). Compared with the control group, the percentage of intake reduction in CCK alone at 0.25 μg/kg was 47%, 36%, and 13% at 60, 120, and 240 minutes, respectively (Figure 1A), whereas the group treated with Apo AIV at 4 μg was 56%, 43%, and 27% (Figure 1B). The means of intake reduction in the combination of CCK-8 (0.06 μg/kg) and Apo AIV (4 μg) were 56%, 42%, and 36%, respectively, whereas in the group of CCK-8 (0.25 μg/kg) and Apo AIV (4 μg) were 73%, 58%, and 43% (Figure 1C). A two-away ANOVA followed by post hoc tests revealed that there were significant differences in the percentage of intake reduction between treatments and times. In contrast, no significance was observed in the percentage of intake reduction between Apo AIV alone and the combination of CCK-8 (0.25 μg/kg) and Apo AIV (4 μg).
In experiment 1.2, i4vt lorglumide at 3 μg did not reliably alter food intake compared with vehicle (Figure 1E). Thus, i4vt lorglumide, like ip lorglumide at these doses, does not alter food intake (15, 36). Lorglumide was, however, able to attenuate the satiating action of i4vt Apo AIV at 120 minutes (Figure 1E). In experiment 1.3, the combined administration of ip CCK-8, i4vt Apo AIV, and i4vt CSF again inhibited food intake at 120 and 240 minutes (P < .05) (Figure 1F). In contrast, administering lorglumide (3 μg) either ip or i4vt attenuated the satiating action of ip CCK and i4vt Apo AIV (Figure 1F). The attenuation by ip lorglumide is consistent with our previous reports (14, 15); the attenuation by i4vt lorglumide suggests that Apo AIV acting in or near the NTS uses local CCK-1R to induce a satiating effect.
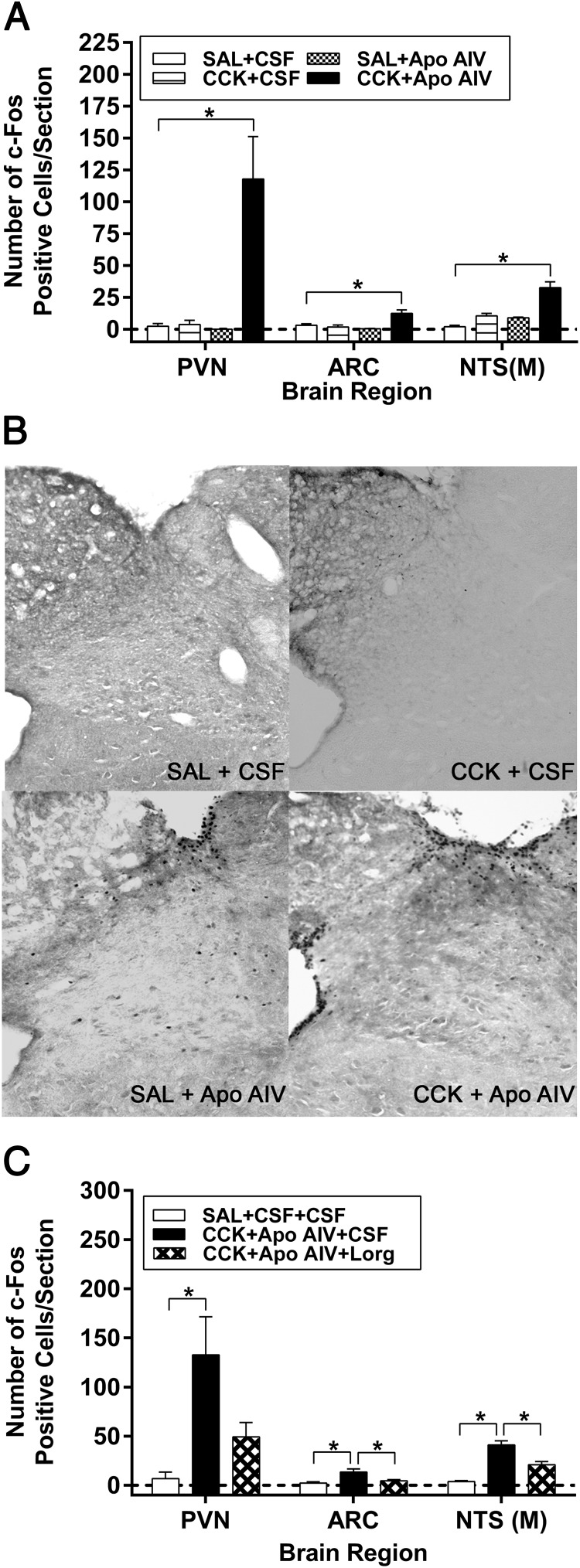
c-Fos immunoreactive protein is commonly used as a marker of neuronal activity in brains (37). Relative to vehicle, injections of CCK-8 or Apo AIV given individually had no effect on the number of c-Fos-positive cells in the NTS, PVN, and ARC (Figure 2, A and B). In contrast, the combination of CCK-8 and Apo AIV significantly increased the number of c-Fos-positive cells in the NTS, ARC, and PVN (P < .001). Administering lorglumide i4vt near the NTS significantly attenuated the increased number of c-Fos-positive cells in the ARC and NTS (P < .05) (Figure 2C) and halved the count in the PVN, although the latter did not reach significance. The findings imply that neuronal activation in the hindbrain and hypothalamus induced by the combination of ip CCK and i4vt Apo AIV is mediated via CCK-1R near the NTS.
Figure 2.
Effect of CCK and Apo AIV on c-Fos-positive proteins via CCK-1R. A, The number of c-Fos-positive cells in the hindbrain and hypothalamus in rats treated with CCK (0.25 μg/kg), Apo AIV (4 μg), alone or combination, or vehicles. B, The images of DAB-stained c-Fos-positive proteins in the NTS with various treatments. C, The number of c-Fos-positive cells in the hindbrain and hypothalamus in rats treated with the combination of CCK (0.25 μg/kg), Apo AIV (4 μg), lorglumide (3 μg), or vehicles. Data are expressed as mean ± SEM for 4–5 animals per group, and values with asterisks represent significant differences relative to the control rats (P < .05). SAL, saline; Lorg, lorglumide.
Experiment 2. Combinations of ip CCK and i4vt Apo AIV suppress food intake in HFD-induced obese rats
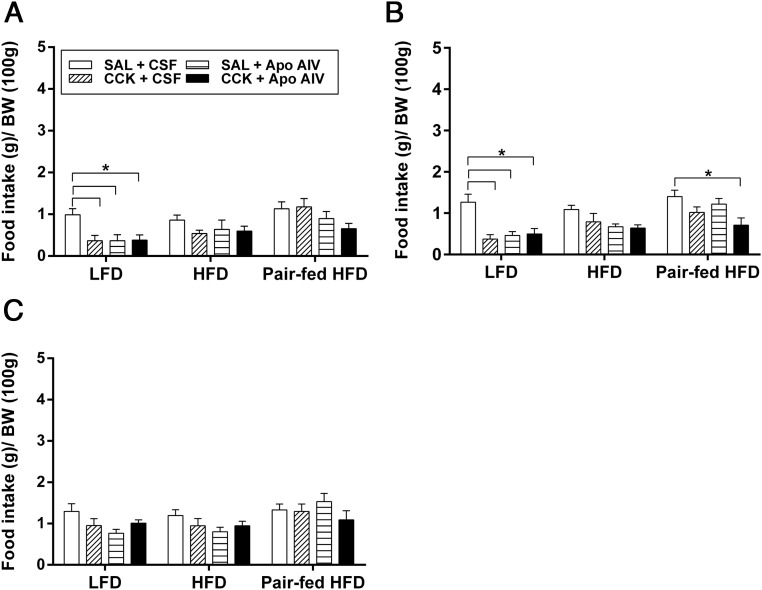
Rats with comparable body weights were assigned to different groups (Table 1). After 10 weeks of either LFD or HFD, HFD-fed rats consumed 10% more calories, and their body weight was 6% greater than their control groups fed the LFD (P < .05) (Table 1). Pair-fed HFD rats had daily caloric intake and body weight comparable with LFD-fed rats (P = .8) (Table 1) as we have seen previously (29, 38). At 30 and 60 minutes, CCK and Apo AIV, either alone or in combination, significantly decreased food intake in LFD-fed rats relative to their controls (P < .05) (Figure 3A). In contrast, CCK and Apo AIV, either alone or in combination, did not significantly reduce food intake in HFD-fed obese rats, suggesting that either excess calories from a HFD, or obesity per se, attenuate peripheral CCK or i4vt Apo AIV sensitivity. In pair-fed HFD rats, only the combination reduced food intake at 60 minutes, but neither CCK nor Apo AIV alone suppressed food intake at 30 and 60 minutes. After 120 minutes, no treatments reliably altered food intake (Figure 3C).
Table 1.
Body Weight and Caloric Intake in Long-Evans Rats
| LFD | HFD | Pair-fed HFD | |
|---|---|---|---|
| Initial BW(g) | 209.6 ± 1.7 | 208.3 ± 1.8 | 209.6 ± 1.9 |
| Final BW (g) | 446.0 ± 4.9 | 471.0 ± 5.9a | 444.7 ± 4.8 |
| Daily caloric intake (kcal) | 87.4 ± 2.1 | 95.8 ± 2.4a | 87.3 ± 2.0 |
Body weight (BW) and caloric intake in Long-Evans rats (n = 40/group) were collected before or after 10 weeks of LFD and HFD. Pair-fed HFD group consumed HFD with the same daily caloric intake to LFD-fed group. Values represent mean ± SEM for 7–8 animals per group.
Significant difference (P < .05) compared with LFD-fed rats.
Figure 3.
Effect of CCK and Apo AIV on food intake of rats fed on LFD and HFD. After 10 weeks of LFD, HFD, and pair-fed HFD, fasted rats (n = 10/group) received 2 injections of CCK ip (0.25 μg/kg) and Apo AIV (4 μg), alone or combination, or vehicles. Food intake was monitored at 30 minutes (A), 60 minutes (B), and 120 minutes (C). Data are expressed as means ± SEM, and values with asterisks indicate significant differences from the vehicle-treated group (P < .05). BW, body weight; SAL, saline.
CCK or Apo AIV alone increased the number of c-Fos cells in the NTS, ARC, and PVN of LFD-fed rats relative to vehicle-treated LFD rats, but the difference was not significant (Figure 4, A–C). The combination of CCK and Apo AIV significantly increased the number of c-Fos-positive cells in the NTS, PVN, and ARC of LFD-fed rats, but only in the NTS and ARC of pair-fed HFD rats (P < .05) (Figure 4, A–C). In contrast, no significant difference in c-Fos proteins was observed in the NTS, PVN, and ARC of HFD-fed rats after the treatments of CCK and Apo AIV, alone or the combination. Two-way ANOVA revealed that there were significant differences in diets, treatments, and interactions. Therefore, although the trends were similar to what was observed in chow-fed rats, these observations suggest that HFD attenuates neuronal activation in the hindbrain and hypothalamus induced by CCK and Apo AIV.
Figure 4.
Effect of CCK and Apo AIV on c-Fos-positive cells of rats fed on LFD and HFD. Fasted rats (n = 4–5/group) received CCK ip (0.25 μg/kg), Apo AIV i4vt (4 μg), or vehicles and the number of c-Fos-positive cells in the NTS (A), PVN (B), and ARC (C) was determined in the LFD, HFD, and pair-fed HFD rats. Data are expressed as means ± SEM, and values with asterisks indicate significant differences from the vehicle-treated group (P < .05). SAL, saline.
Discussion
The hindbrain solitary complex is an important site that receives satiating signals elicited by peripheral signals (39–42). Fasted Apo AIV KO mice have up-regulated CCK-1R expression in the nodose ganglia as well as in the NTS and increased sensitivity to CCK-induced satiation (20, 43). Thus, CCK-1R in the hindbrain is important for transmitting satiating signals due to the interaction of CCK and Apo AIV. Apo AIV is synthesized in the NTS, and its physiological effect or the signals that release Apo AIV in the NTS remain unknown (18). Administration of Apo AIV near to the NTS in the present study mimics the effect of endogenous Apo AIV in the NTS on the food intake. The present studies demonstrate that local Apo AIV near or within the NTS (i4vt), as well as the combination of ip CCK and i4vt Apo AIV, has a satiating effect in lean rats. The combination of CCK and Apo AIV causes a greater percentage reduction of food intake than individual doses of the 2, but the difference between the combination and individual doses is not significant. Thus, they interact to prolong the satiating effect rather than to increase the satiation signals. Blockade of peripheral or near-NTS CCK-1R attenuates CCK and/or Apo AIV-induced satiating signals. Thus, the vagal CCK signaling pathway in the periphery as well as a circuit in or near the NTS requires CCK-1R activation, and both are required for Apo AIV alone and the interaction of ip CCK and i4vt Apo AIV to reduce food intake in lean animals.
It is well known that ip CCK induces satiation in lean rats maintained on chow diets and LFD (5, 14) and that chronic consumption of HFD decreases sensitivity to this action of ip CCK (24–26, 44). The attenuated sensitivity could be due to reduced levels of CCK-1R transcript and membrane excitability in the nodose ganglia (2, 23, 44–46). In the present studies, exogenous administration of comparable doses of CCK and Apo AIV, alone and in combination, does not effectively suppress food intake in HFD-induced obese rats. We reported that maintenance on a HFD attenuated the induction of hypothalamic Apo AIV in response to acute administration of lipid (29, 38). Because the same animal model and diets were used in the present and previous studies (29, 47), HFD-fed rats in the current study presumably had lower levels of Apo AIV in the brain. However, the level of NTS Apo AIV induced by LFD and HFD feeding remains unknown in the present study, and further experiments will be pursued. The present studies indicate that HFD-fed rats have a 10% increase in daily caloric intake, which results in a 6% increase in body weight, suggesting that HFD-induced obesity likely has a minimal effect on altering intake reduction. In addition, HFD itself attenuates the sensitivity to peripheral CCK or i4vt Apo AIV in pair-fed HFD lean animals. Peripheral Apo AIV has been reported to require an intact CCK system and vagal afferents to relay satiation signals (14, 15). Thus, it seems plausible that reduced sensitivity in HFD-fed rats to the response of CCK and Apo AIV could be due to decreased levels of central Apo AIV and CCK or/and decreased sensitivity in vagal CCK system.
The NTS receives numerous satiating signals from the afferent vagus or else via the area postrema (48, 49), and the integrated signals directly project anteriorly to areas, including the hypothalamus, that regulate food intake and energy homeostasis (41, 50–52). The PVN receives myriad signals from the NTS and ARC and plays a key role in the modulation of food intake (53–56). Previous studies have documented that ip CCK dose dependently activates neurons in the PVN, which contains CCK-1R, CCK-2R, and CCK-positive nerve terminal, but not in the ARC (40, 57–65). In the present study, either CCK or Apo AIV at minimally effective doses had little effect on c-Fos activation in the brain of rats maintained on chow, and the doses of CCK were much lower than those doses used in previous studies (40, 65). Further experiments in neuronal activation in other feeding-relevant brain regions, such as nucleus accumbens, will be pursed to investigate Apo AIV-induced satiation (66). In contrast, the combination of ip CCK and Apo AIV administered near the NTS activates neurons in the hindbrain and hypothalamus in rats maintained on chow or LFD and the pair-fed HFD. This suggests that with greater neuronal activation induced by CCK and Apo AIV, the PVN might be the key hypothalamic nucleus where CCK and Apo AIV interact to increase satiation and that a HFD attenuates neuronal activation in the PVN. In the study of CCK-1R blockade using lorglumide, the neuronal activation in the hindbrain and hypothalamus induced by i4vt Apo AIV and ip CCK required NTS CCK-1R. Long-term exposure to HFD attenuates CCK-induced neuronal activation in the hindbrain (67) and also abolishes neuronal activation in the hindbrain induced by Apo AIV near the NTS or/and peripheral CCK. Further experiments of the effect of Apo AIV on isolated neurons in the nodose ganglia and hypothalamus of LFD or HFD-fed rats will be studied to investigate whether Apo AIV activity attenuated by HFD is due to abolished vagal CCK sensitivity. Thus, the vagal CCK system has a key role in relaying satiation signals induced by CCK and Apo AIV after high-fat consumption.
Conclusion
Apo AIV administered near the NTS, alone or in combination with ip CCK-8, locally elicits satiation, and the action is mediated via CCK-1R-dependent pathways in the periphery as well as within the NTS. Maintenance on a HFD attenuates these satiating effects.
Acknowledgments
We thank Dr Min Liu and Dr Matthew R Hayes for their excellent training assistance in surgical implantation of fourth-cerebroventricular cannulae.
This work was supported by the National Institutes of Health Grants DK83550, DK97436, DK17844, DK59630, DK92138, and GM98458.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Apo AIV
- apolipoprotein AIV
- ARC
- arcuate nucleus
- CCK
- cholecystokinin
- CCK-1R
- CCK 1 receptor
- c-Fos proteins
- a product of the c-Fos immediate-early gene
- CSF
- cerebrospinal fluid
- HFD
- high-fat diet
- KO
- knockout
- i4vt
- fourth-ventricular
- LFD
- low-fat diet
- NTS
- nucleus of the solitary tract.
References
- 1. Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green GM, Taguchi S, Friestman J, Chey WY, Liddle RA. Plasma secretin, CCK, and pancreatic secretion in response to dietary fat in the rat. Am J Physiol. 1989;256:G1016–G1021 [DOI] [PubMed] [Google Scholar]
- 3. Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol. 1998;274:R1834–R1838 [DOI] [PubMed] [Google Scholar]
- 4. Zieve L, Mulford B, McHale A. Secretion of pancreatic enzymes. II. Comparative response following test meal or injection of secretin and pancreozymin. Am J Dig Dis. 1966;11:685–694 [DOI] [PubMed] [Google Scholar]
- 5. Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84:488–495 [DOI] [PubMed] [Google Scholar]
- 6. Debas HT, Farooq O, Grossman MI. Inhibition of gastric emptying is a physiological action of cholecystokinin. Gastroenterology. 1975;68:1211–1217 [PubMed] [Google Scholar]
- 7. Mueller K, Hsiao S. Specificity of cholecystokinin satiety effect: reduction of food but not water intake. Pharmacol Biochem Behav. 1977;6:643–646 [DOI] [PubMed] [Google Scholar]
- 8. Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol. 1997;272:R1245–R1251 [DOI] [PubMed] [Google Scholar]
- 9. Raybould HE. Capsaicin-sensitive vagal afferents and CCK in inhibition of gastric motor function induced by intestinal nutrients. Peptides. 1991;12:1279–1283 [DOI] [PubMed] [Google Scholar]
- 10. Sayegh AI, Ritter RC. Vagus nerve participates in CCK-induced Fos expression in hindbrain but not myenteric plexus. Brain Res. 2000;878:155–162 [DOI] [PubMed] [Google Scholar]
- 11. Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1005–R1012 [DOI] [PubMed] [Google Scholar]
- 12. Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res. 1990;31:1613–1625 [PubMed] [Google Scholar]
- 13. Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–G1006 [DOI] [PubMed] [Google Scholar]
- 14. Lo CM, Zhang DM, Pearson K, et al. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1490–R1494 [DOI] [PubMed] [Google Scholar]
- 15. Lo CC, Langhans W, Georgievsky M, et al. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology. 2012;153:5857–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oldendorf WH. Blood-brain barrier permeability to peptides: pitfalls in measurement. Peptides. 1981;2(suppl 2):109–111 [DOI] [PubMed] [Google Scholar]
- 17. Zhu XG, Greeley GH, Jr, Lewis BG, Lilja P, Thompson JC. Blood-CSF barrier to CCK and effect of centrally administered bombesin on release of brain CCK. J Neurosci Res. 1986;15:393–403 [DOI] [PubMed] [Google Scholar]
- 18. Shen L, Pearson KJ, Xiong Y, et al. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav. 2008;95:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179 [DOI] [PubMed] [Google Scholar]
- 20. Yoshimichi G, Lo CC, Tamashiro KL, et al. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1336–G1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weinberg RB, Dantzker C, Patton CS. Sensitivity of serum apolipoprotein A-IV levels to changes in dietary fat content. Gastroenterology. 1990;98:17–24 [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez AI, Manso MA, Garcia-Montero AC, Orfao A, de Dios I. Long-term blockade of cholecystokinin (CCK): effects of L 364,718 (a CCK receptor antagonist) on pancreatic enzyme storage and secretion. Pancreas. 1997;15:314–322 [PubMed] [Google Scholar]
- 23. Little TJ, Feltrin KL, Horowitz M, et al. A high-fat diet raises fasting plasma CCK but does not affect upper gut motility, PYY, and ghrelin, or energy intake during CCK-8 infusion in lean men. Am J Physiol Regul Integr Comp Physiol. 2008;294:R45–R51 [DOI] [PubMed] [Google Scholar]
- 24. Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19:1407–1415 [DOI] [PubMed] [Google Scholar]
- 25. Covasa M, Marcuson JK, Ritter RC. Diminished satiation in rats exposed to elevated levels of endogenous or exogenous cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2001;280:R331–R337 [DOI] [PubMed] [Google Scholar]
- 26. Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. J Nutr. 2005;135:1953–1959 [DOI] [PubMed] [Google Scholar]
- 27. Torregrossa AM, Smith GP. Two effects of high-fat diets on the satiating potency of cholecystokinin-8. Physiol Behav. 2003;78:19–25 [DOI] [PubMed] [Google Scholar]
- 28. Chandler PC, Wauford PK, Oswald KD, Maldonado CR, Hagan MM. Change in CCK-8 response after diet-induced obesity and MC3/4-receptor blockade. Peptides. 2004;25:299–306 [DOI] [PubMed] [Google Scholar]
- 29. Liu M, Shen L, Liu Y, et al. Obesity induced by a high-fat diet downregulates apolipoprotein A-IV gene expression in rat hypothalamus. Am J Physiol Endocrinol Metab. 2004;287:E366–E370 [DOI] [PubMed] [Google Scholar]
- 30. Morris MJ, Chen H, Watts R, Shulkes A, Cameron-Smith D. Brain neuropeptide Y and CCK and peripheral adipokine receptors: temporal response in obesity induced by palatable diet. Int J Obes (Lond). 2008;32:249–258 [DOI] [PubMed] [Google Scholar]
- 31. Liu M, Maiorano N, Shen L, et al. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav. 2003;78:149–155 [DOI] [PubMed] [Google Scholar]
- 32. Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verbalis JG, Stricker EM, Robinson AG, Hoffman GE. Cholecystokinin activates C-fos expression in hypothalamic oxytocin and corticotropin-releasing hormone neurons. J Neuroendocrinol. 1991;3:205–213 [DOI] [PubMed] [Google Scholar]
- 34. Lo CM, Ma L, Zhang DM, et al. Mechanism of the induction of brain c-Fos-positive neurons by lipid absorption. Am J Physiol Regul Integr Comp Physiol. 2007;292:R268–R273 [DOI] [PubMed] [Google Scholar]
- 35. Paxinos G, Franklin KBJ. The Mouse/Rat Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Science; 2004 [Google Scholar]
- 36. Kaltwasser MT, Petrack B, Crawley JN. Potency of CR 1409, a new proglumide analog, on cholecystokinin-mediated behaviors and receptor binding. Neurochem Int. 1987;10:547–553 [DOI] [PubMed] [Google Scholar]
- 37. Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451 [DOI] [PubMed] [Google Scholar]
- 38. Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087 [DOI] [PubMed] [Google Scholar]
- 39. Palkovits M, Kiss JZ, Beinfeld MC, Williams TH. Cholecystokinin in the nucleus of the solitary tract of the rat: evidence for its vagal origin. Brain Res. 1982;252:386–390 [DOI] [PubMed] [Google Scholar]
- 40. Mönnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 1997;770:277–288 [DOI] [PubMed] [Google Scholar]
- 41. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17 [DOI] [PubMed] [Google Scholar]
- 42. Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820 [DOI] [PubMed] [Google Scholar]
- 43. Whited KL, Lu D, Tso P, Kent Lloyd KC, Raybould HE. Apolipoprotein A-IV is involved in detection of lipid in the rat intestine. J Physiol. 2005;569:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nefti W, Chaumontet C, Fromentin G, Tomé D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1681–R1686 [DOI] [PubMed] [Google Scholar]
- 45. Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Ma W, Wang S. Slower gastric emptying in high-fat diet induced obese rats is associated with attenuated plasma ghrelin and elevated plasma leptin and cholecystokinin concentrations. Regul Pept. 2011;171:53–57 [DOI] [PubMed] [Google Scholar]
- 47. Woods SC, D'Alessio DA, Tso P, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–578 [DOI] [PubMed] [Google Scholar]
- 48. Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465–471 [DOI] [PubMed] [Google Scholar]
- 49. Michel S, Becskei C, Erguven E, Lutz TA, Riediger T. Diet-derived nutrients modulate the effects of amylin on c-Fos expression in the area postrema and on food intake. Neuroendocrinology. 2007;86:124–135 [DOI] [PubMed] [Google Scholar]
- 50. Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26 [DOI] [PubMed] [Google Scholar]
- 51. Broberger C, Holmberg K, Shi TJ, Dockray G, Hökfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001;903:128–140 [DOI] [PubMed] [Google Scholar]
- 52. Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701 [DOI] [PubMed] [Google Scholar]
- 53. Leibowitz SF. Paraventricular nucleus: a primary site mediating adrenergic stimulation of feeding and drinking. Pharmacol Biochem Behav. 1978;8:163–175 [DOI] [PubMed] [Google Scholar]
- 54. Tribollet E, Dreifuss JJ. Localization of neurones projecting to the hypothalamic paraventricular nucleus area of the rat: a horseradish peroxidase study. Neuroscience. 1981;6:1315–1328 [DOI] [PubMed] [Google Scholar]
- 55. Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325 [DOI] [PubMed] [Google Scholar]
- 56. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 57. Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res. 1992;569:238–248 [DOI] [PubMed] [Google Scholar]
- 58. Vanderhaeghen JJ, Lotstra F, De Mey J, Gilles C. Immunohistochemical localization of cholecystokinin- and gastrin-like peptides in the brain and hypophysis of the rat. Proc Natl Acad Sci USA. 1980;77:1190–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schick RR, Reilly WM, Roddy DR, Yaksh TL, Go VL. Neuronal cholecystokinin-like immunoreactivity is postprandially released from primate hypothalamus. Brain Res. 1987;418:20–26 [DOI] [PubMed] [Google Scholar]
- 60. Honda T, Wada E, Battey JF, Wank SA. Differential Gene Expression of CCK(A) and CCK(B) Receptors in the Rat Brain. Mol Cell Neurosci. 1993;4:143–154 [DOI] [PubMed] [Google Scholar]
- 61. Hinks GL, Poat JA, Hughes J. Changes in hypothalamic cholecystokininA and cholecystokininB receptor subtypes and associated neuropeptide expression in response to salt-stress in the rat and mouse. Neuroscience. 1995;68:765–781 [DOI] [PubMed] [Google Scholar]
- 62. Mercer LD, Beart PM. Histochemistry in rat brain and spinal cord with an antibody directed at the cholecystokininA receptor. Neurosci Lett. 1997;225:97–100 [DOI] [PubMed] [Google Scholar]
- 63. Mercer LD, Le VQ, Nunan J, Jones NM, Beart PM. Direct visualization of cholecystokinin subtype2 receptors in rat central nervous system using anti-peptide antibodies. Neurosci Lett. 2000;293:167–170 [DOI] [PubMed] [Google Scholar]
- 64. Kobelt P, Tebbe JJ, Tjandra I, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2005;288:R751–R758 [DOI] [PubMed] [Google Scholar]
- 65. Noetzel S, Stengel A, Inhoff T, et al. CCK-8S activates c-Fos in a dose-dependent manner in nesfatin-1 immunoreactive neurons in the paraventricular nucleus of the hypothalamus and in the nucleus of the solitary tract of the brainstem. Regul Pept. 2009;157:84–91 [DOI] [PubMed] [Google Scholar]
- 66. Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–14457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86:83–88 [DOI] [PubMed] [Google Scholar]