Abstract
Introduction
To determine the time to progression (TTP), response rate, and toxicity for North American patients with relapsed small cell lung cancer (SCLC) treated with bendamustine in the second or third line setting.
Methods
Patients with relapsed, histologically confirmed SCLC were eligible for enrollment on study. The study population included patients with both chemotherapy sensitive and resistant disease treated with up to two prior lines of chemotherapy. Patients were treated with bendamustine 120 mg/m2 on days 1 and 2 of a 21-day cycle for up to 6 cycles. Primary endpoint was TTP; secondary endpoints included toxicity, response rate (RR), and overall survival (OS).
Results
Fifty-nine patients were accrued, 50 patients met eligibility for enrollment. The median age of patients was 62, and 56% were male. Twenty-nine patients (58%) had chemotherapy sensitive disease. Median TTP was 4.0 months (95% C.I. 3.3–5.4), median OS was 4.8 months (95% C.I. 3.8–6.3), and the RR was 26% (95% C.I. 13.3%–39.5%). Patients with chemosensitive disease had a median TTP of 4.2 months (95% C.I. 3.3–6.0) compared to 3.4 months (95% C.I. 2.7–∞) for chemotherapy resistant disease. The response rate was 33% (95% C.I. 14.2%–51.8%) in patients with chemosensitive disease and 17% (95% C.I. 0%–34.4%) in those with chemoresistant disease. The most common grade 3/4 adverse events were fatigue (20%), dyspnea (12%), and anemia (12%).
Conclusions
Bendamustine has modest activity in relapsed SCLC similar to other agents evaluated in this patient population.
Introduction
An estimated 246,210 new cases of lung cancer will be diagnosed in the United States in 2013.1 Small cell lung cancer (SCLC) will account for 12 to 15% of these cases. Approximately 60–70% of patients are diagnosed with extensive stage disease at presentation. Despite response rates of up to 80% to first line therapy, the majority of patients eventually relapse with a median survival of 18 to 24 months for patients with limited stage disease and 6 to 12 months for patients with extensive stage disease.2 The prognosis is especially poor for patients who relapse or progress after first-line therapy within the first 3 months. Topotecan is currently the only US Food and Drug Administration (FDA) approved agent for use in patients with relapsed SCLC.3,4 A multitude of trials have investigated other chemotherapy agents in patients with relapsed small cell lung cancer but none have proven efficacy over topotecan’s limited benefit.
It is readily understood that there is a considerable need for improved therapies for patients with SCLC. Bendamustine is a bifunctional alkylating agent that is FDA approved for use as first-line therapy for chronic lymphocytic lymphoma (CLL)5 and for rituximab-refractory indolent Non-Hodgkin’s Lymphoma (NHL)6,7. A European phase II study of single-agent bendamustine in patients with relapsed chemosensitive SCLC, reported a response rate (RR) of 29%, a median overall survival (OS) of 7 months, and the toxicity profile appeared to be superior when compared with toxicities compiled from studies of topotecan8.
Bendamustine has not been evaluated in a North American patient population with relapsed SCLC. To further evaluate the efficacy and toxicity of this promising agent, we conducted a single-arm, phase II study of bendamustine as a single-agent in relapsed SCLC, in patients with both chemosensitive and chemoresistant disease. The primary endpoint was time to progression (TTP). Secondary endpoints included response rate (RR), overall survival (OS), and toxicity.
Materials and Methods
Patient Population
Eligible patients were recruited from Vanderbilt-Ingram Cancer Center Affiliated Network (VICCAN) sites. Patients were ≥ 18 years old, had Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–2, and had received up to two prior lines of chemotherapy, one of which must have been a platinum-containing regimen. Patients with symptomatic or untreated brain metastases were excluded. All patients signed informed consent. Chemoresistant disease was defined as no response to platinumcontaining regimen and/or progression of disease within 90 days of treatment with a platinum-containing chemotherapy regimen; chemosensitive disease was defined as stable disease or response to platinum-containing chemotherapy that lasted at least 90 days. The Vanderbilt Medical Center Institutional Review Board (IRB) approved this study.
Treatment Strategy
Patients were treated with bendamustine 120 mg/m2 intravenously (IV) on days 1 and 2 of a 21-day schedule. Erythropoesis stimulating agents (ESAs) and granulocyte colony stimulating factors (G-CSF) were allowed at the discretion of the investigator, consistent with American Society of Clinical Oncology (ASCO) guidelines. Disease assessment with CT scans was repeated every two cycles. Toxicities were tabulated using NCI-CTC version 2.0. Response was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) 1.0. Patients with evidence of a response to treatment or stable disease (SD) in the absence of unacceptable toxicity were treated up to a maximum of 6 cycles.
Statistical Methods
Sample size estimation and power calculation
The primary objective of this phase II trial was to evaluate the median TTP. Secondary objectives included RR, OS and toxicity. The sample size estimation was completed a priori using the one sample test based on exponential distribution. A sample size of 42 provided at least 80% power to detect a 50% improvement of the median TTP from previous studies of topotecan with two-sided type I error = 5%. The null hypothesis was that treatment with bendamustine would not result in a TTP of 4.5 months. The power analysis was based on the assumptions of an accrual time of 18 months and an additional 12 months of follow-up.
Statistical Analysis Plan
TTP and OS were estimated using the Kaplan-Meier method with the 95% confidence interval. Survival for the chemosensitive population and the chemoresistant population were compared with the log-rank test. Adverse medical events, including National Cancer Institute (NCI) toxicity grade 3 and grade 4 laboratory abnormalities were tabulated.
Results
From September 2009 to February 2012, a total of 59 patients were consented and 50 patients underwent treatment on study. Of the 9 patients that did not begin treatment, 4 were excluded due to laboratory abnormalities, 3 required urgent radiation therapy, and 2 had a decline in performance status after signing consent and prior to starting on therapy. Patient characteristics are included in Table 1. The median age was 62 years, 56% were male and 84% of patients had ECOG PS 0–1. Twenty-one (42%) patients had received two prior lines of chemotherapy. The median TTP from 1st line chemotherapy was 6 months. Twenty-nine (58%) patients had chemosensitive disease and 21 (42%) had chemoresistant disease.
Table 1.
Patient Characteristics
| Characteristic | Number of Patients (%) |
|---|---|
| Age (years) | |
| Median | 62 |
| Range | 44–77 |
| Sex | |
| Male | 28 (56%) |
| Female | 22 (44%) |
| Race | |
| Caucasian | 48 (96%) |
| African-American | 2 (4%) |
| ECOG PS | |
| 0 | 14 (28%) |
| 1 | 28 (56%) |
| 2 | 8 (16%) |
| Previous # chemotherapies | |
| 1 | 29 (58%) |
| 2 | 21 (42%) |
| Previous radiation therapy | |
| Yes | 38 (76%) |
| No | 12 (24%) |
| Chemosensitive disease | 29 (58%) |
| Chemoresistant disease | 21 (42%) |
Legend: ECOG PS = Eastern Cooperative Oncology Group Performance Status
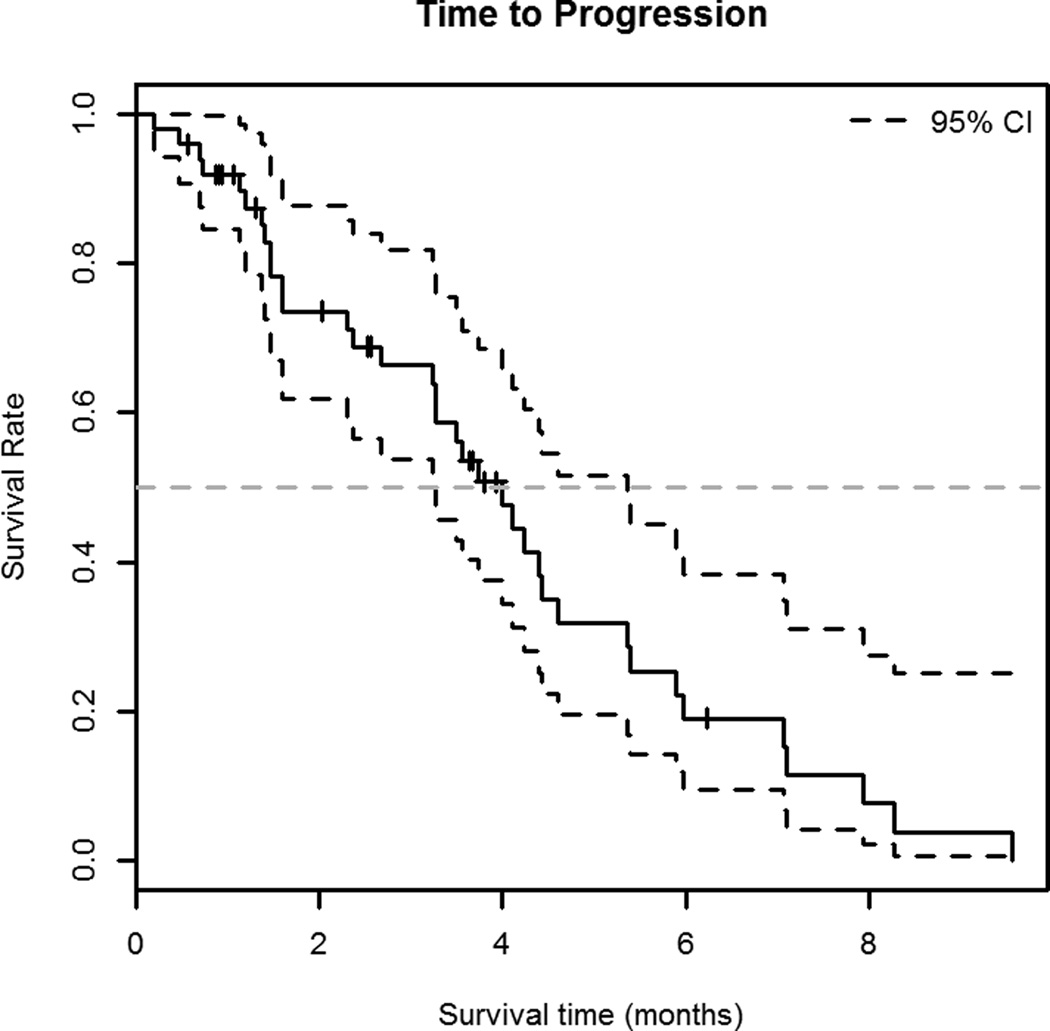
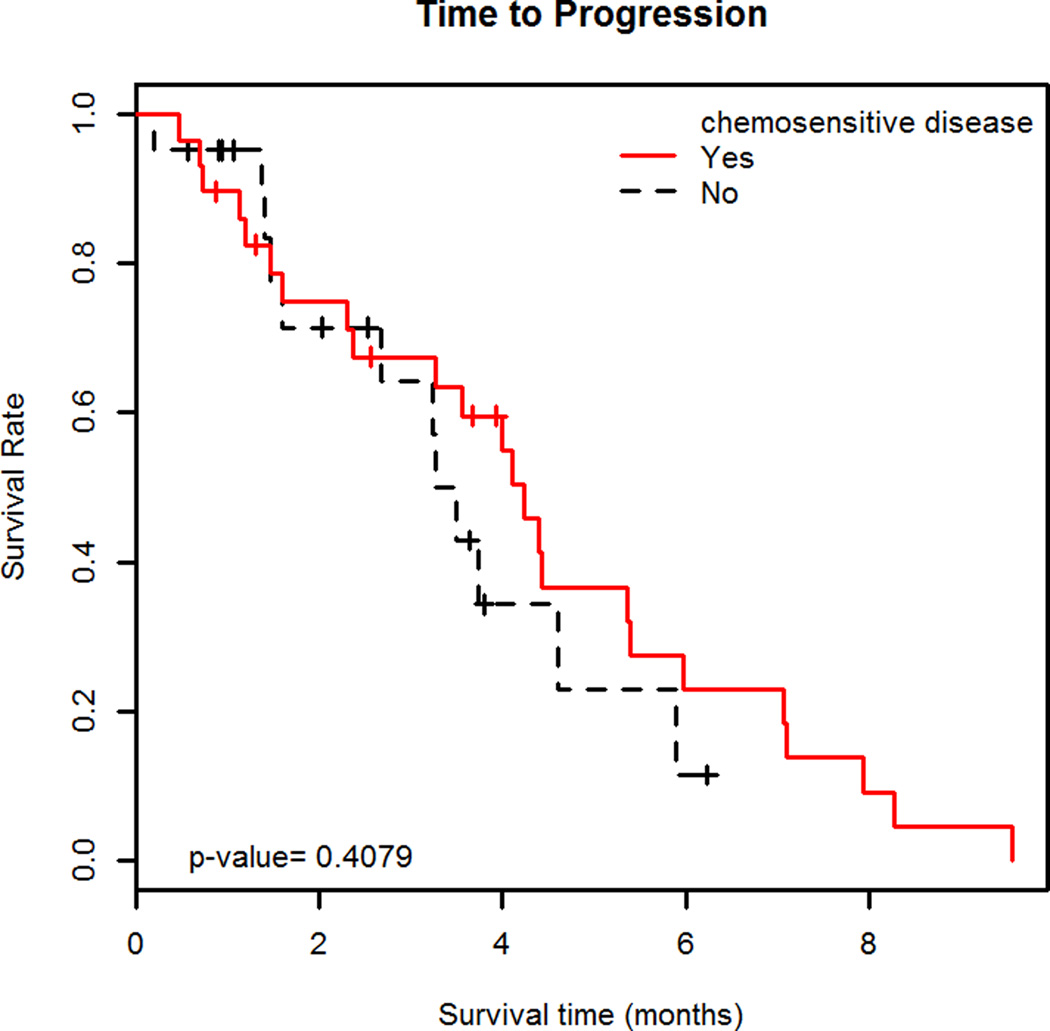
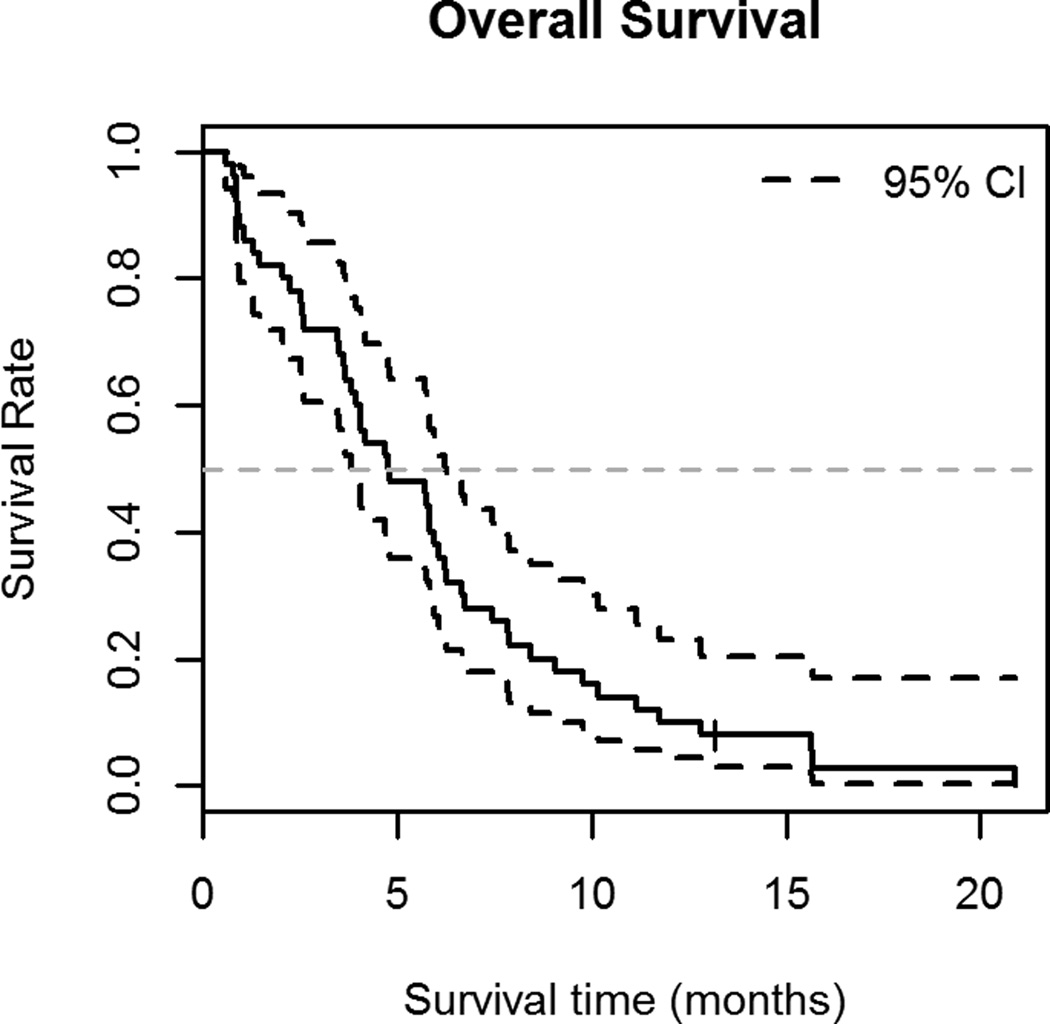
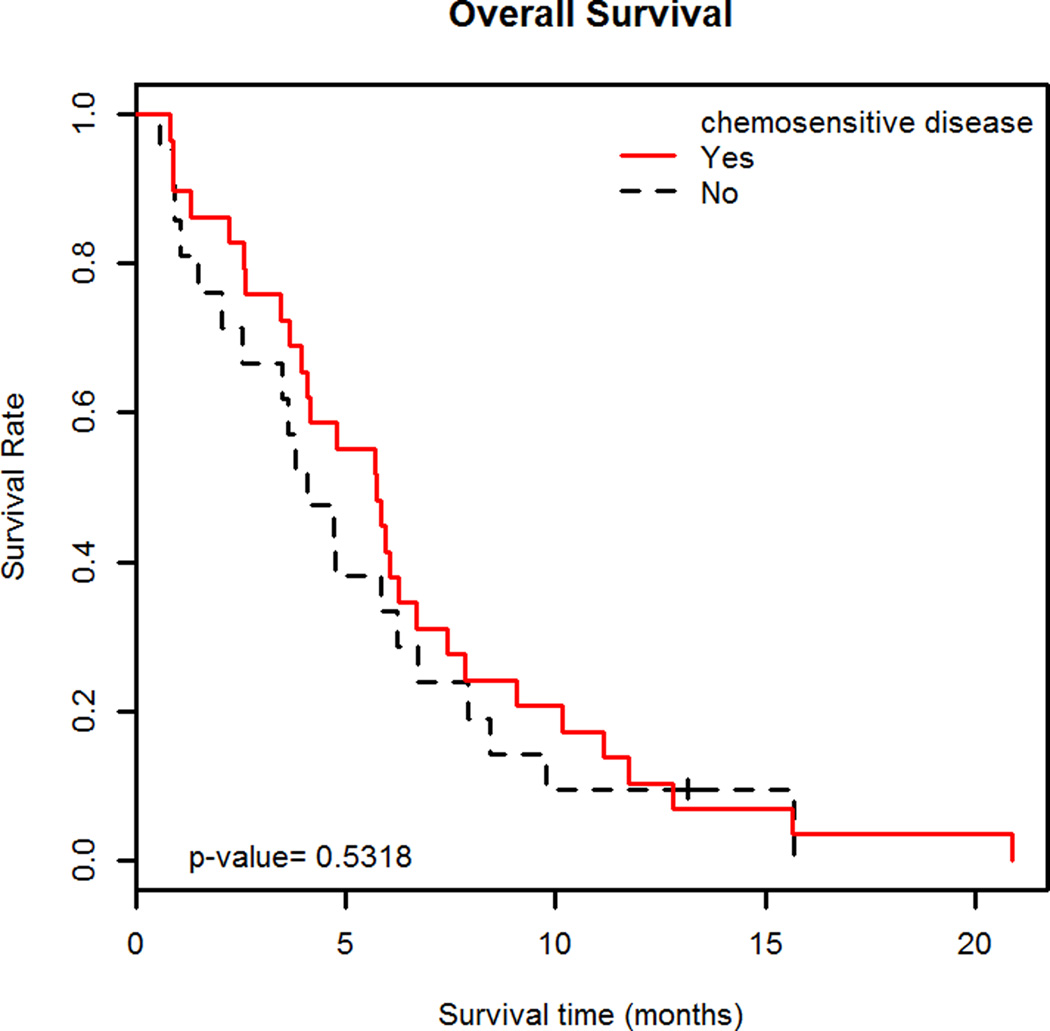
Median TTP was 4.0 months (95% C.I. 3.3–5.4 months) (Figure 1A); patients with chemosensitive disease had a median TTP of 4.2 months (95% C.I. 3.3–6.0 months) and those with chemoresistant disease had a median TTP of 3.4 months (95% C.I. 2.7-∞ months). (Figure 1B) Median OS was 4.8 months (95% C.I. 3.8–6.3 months) (Figure 2A). Patients with chemosensitive disease had a median OS of 5.7 months (95% C.I. 4.1–7.4 months) and patients with chemoresistant disease had a median OS of 4.1 months (95% C.I. 2.5–7.9 months). (Figure 2B) Forty-two patients were evaluable for response, one patient had a complete response (CR), 10 had partial response (PR), and 17 patients had SD as the best response. Therefore, the RR was 26% (95% C.I. 13.3%–39.5%) and the overall clinical benefit (CR +PR + SD) was 67%. Fourteen patients had progressive disease (PD) at the first evaluation. Thirty-three percent (95% C.I. 14.2%–51.8%) of patients with chemosensitive disease had a response to therapy and 17% (95% C.I. 0%–34.4%) of patients with chemoresistant disease had a response to therapy.
Figure 1.
A. Time to progression
Kaplan-Meier curve showing time to progression (TTP) with 95% C.I. of 50 patients treated with bendamustine for relapsed small cell lung cancer (SCLC). The median TTP in this population was 4.0 months (95% C.I. 3.3–5.4 months).
B. Time to progression in chemosensitive and chemoresistant disease
Kaplan-Meier curves showing time to progression (TTP) with 95% C.I. of 29 patients treated with bendamustine for relapsed small cell lung cancer (SCLC) with chemosensitive disease and TTP with 95% C.I. of 21 patients treated with bendamustine for relapsed SCLC with chemoresistant disease. The median TTP in patients with chemosensitive disease was 4.2 months (95% C.I. 3.3–6.0 months) and those with chemoresistant disease had a median TTP of 3.4 months (95% C.I. 2.7–‡ months). There was no difference in median TTP between the two groups (p=0.41).
Figure 2.
A. Overall survival
Kaplan-Meier curve showing overall survival (OS) with 95% C.I. of 50 patients treated with bendamustine for relapsed SCLC. The median OS in this population was 4.8 months (95% C.I. 3.8–6.3 months).
B. Overall survival in chemosensitive and chemoresistant disease
Kaplan-Meier curves showing overall survival (OS) with 95% C.I. of 29 patients treated with bendamustine for relapsed SCLC with chemosensitive disease and OS with 95% C.I. of 21 patients treated with bendamustine for relapsed SCLC with chemoresistant disease. Patients with chemosensitive disease had a median OS of 5.7 months (95% C.I. 4.1–7.4 months) and patients with chemoresistant disease had a median OS of 4.1 months (95% C.I. 2.5–7.9 months). There was no difference in median OS between the two groups (p=0.53).
Toxicity
The most common adverse events (AE) from this study are listed in Table 2. There were no grade 5 AEs related to bendamustine. The most common grade 3/4 AEs were fatigue, dyspnea, and anemia experienced by 20%, 12%, and 12% respectively. Twelve patients experienced a serious AE that required 15 separate hospitalizations while on the study. The reasons for hospitalization included dyspnea (3 episodes), anemia (2), bronchitis (2), diarrhea (2), neutropenic fever (2), pneumonia (1), nausea/vomiting (1), pain (1), and hip fracture (1). The rate of grade 3/4 febrile neutropenia was 4%, sensory neuropathy was 2%, and 4% of patients experienced any grade alopecia.
Table 2.
Adverse events
| Toxicity | Grade 1/2 AE | Grade 3/4 AE |
|---|---|---|
| N (%) | N (%) | |
| Bone Marrow | ||
| Anemia | 22 (44%) | 6 (12%) |
| Neutropenia | 4 (8%) | 2 (4%) |
| Thrombocytopenia | 15 (30%) | 2 (4%) |
| Constitutional Symptoms | ||
| Fatigue | 17 (34%) | 10 (20%) |
| Weight Loss | 9 (18%) | 1 (2%) |
| Alopecia | 2 (4%) | 0 (0%) |
| Metabolic/Laboratory | ||
| Hyponatremia | 5 (10%) | 4 (8%) |
| Hyperglycemia | 7 (14%) | 1 (2%) |
| Pulmonary | ||
| Dyspnea | 10 (20%) | 6 (12%) |
| Cough | 7 (14%) | 1 (2%) |
| Gastrointestinal | ||
| Nausea | 20 (40%) | 3 (6%) |
| Vomiting | 15 (30%) | 0 (0%) |
| Diarrhea | 7 (14%) | 3 (6%) |
| Constipation | 9 (18%) | 0 (0%) |
| Infection | ||
| Febrile Neutropenia | 0 (0%) | 2 (4%) |
| Neurologic | ||
| Sensory Neuropathy | 2 (4%) | 1 (2%) |
Select toxicities of 50 patients treated with bendamustine for relapsed SCLC. Toxicities are listed by number of patients affected and grade is NCI grade by CTC version 2.0.
Legend: AE = Adverse Events
Discussion
In this single-arm phase II study, bendamustine had modest activity and was well tolerated in patients with relapsed chemotherapy sensitive and resistant SCLC. Treatment with bendamustine resulted in a median TTP of 4.0 months (95% C.I. 3.3–5.4 months) and did not meet the prespecified primary endpoint of a median TTP of 4.5 months. Although cross comparison studies are difficult, the response rate and survival in patients with relapsed chemosensitive disease appear similar to results from a previous study of bendamustine conducted in Europe8. This study, however, provides the first evidence that bendamustine has activity in patients with chemotherapy resistant disease. This study was conducted with patients that are likely to be representative of the majority of patients with relapsed SCLC as the study included those with ECOG PS of 2 and patients with up to 2 prior lines or therapy.
In the last twenty years median survival for patients with chemotherapy resistant SCLC has not appreciably changed.9 Multi-drug regimens studied in relapsed SCLC have generally shown improved response rates compared to single agents, but this has not translated into a survival benefit. In addition, a multitude of chemotherapy drugs and targeted agents tested as single agents in phase II studies in patients with relapsed SCLC have also not shown superiority to topotecan.
In the current study, bendamustine was generally well tolerated. Fatigue, anemia, and thrombocytopenia were the most common side effects and were found in similar numbers of patients compared to previous studies of bendamustine5–8. The rate of grade 3/4 drug-related hematological toxicity and febrile neutropenia were lower than in reported clinical trials of topotecan used in this disease.10 Importantly bendamustine does not commonly cause alopecia which can be a consideration for many lung cancer patients concerned about appearance.11,12 It also does not routinely cause either neuropathy or infusion reactions, and as such, does not require corticosteroids for premedication.
Bendamustine has shown efficacy in this population in two separate studies, and, due to its attractive side effect profile, may be an option for use in patients with relapsed and/or resistant SCLC. A larger phase III study will be required to prove improved efficacy and quality of life compared to topotecan.
Acknowledgements
We would like to thank all the patients and families who participated in this study. We would also like to acknowledge the members of the thoracic team at Vanderbilt Ingram Cancer Center and members of the Vanderbilt Ingram Cancer Center Affiliate Network who participated in this study. This study was supported by a grant from Teva pharmaceuticals.
Financial Support: Study was supported by a grant from Cephalon. Dr. Lammers received funding support from NIH K12CA90625.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Tiseo M, Ardizzoni A. Current status of second-line treatment and novel therapies for small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007 Aug;2(8):764–772. doi: 10.1097/JTO.0b013e3180986262. [DOI] [PubMed] [Google Scholar]
- 3.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999 Feb;17(2):658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Dec 1;24(34):5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 5.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Sep 10;27(26):4378–4384. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 6.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010 Jan 1;116(1):106–114. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin's lymphoma: results from a phase II multicenter, single-agent study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jan 10;26(2):204–210. doi: 10.1200/JCO.2007.12.5070. [DOI] [PubMed] [Google Scholar]
- 8.Schmittel A, Knodler M, Hortig P, Schulze K, Thiel E, Keilholz U. Phase II trial of second-line bendamustine chemotherapy in relapsed small cell lung cancer patients. Lung Cancer. 2007 Jan;55(1):109–113. doi: 10.1016/j.lungcan.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Ebi N, Kubota K, Nishiwaki Y, et al. Second-line chemotherapy for relapsed small cell lung cancer. Japanese journal of clinical oncology. 1997 Jun;27(3):166–169. doi: 10.1093/jjco/27.3.166. [DOI] [PubMed] [Google Scholar]
- 10.von Pawel J, Gatzemeier U, Pujol JL, et al. Phase ii comparator study of oral versus intravenous topotecan in patients with chemosensitive small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001 Mar 15;19(6):1743–1749. doi: 10.1200/JCO.2001.19.6.1743. [DOI] [PubMed] [Google Scholar]
- 11.Bernard M, Brignone M, Adehossi A, et al. Perception of alopecia by patients requiring chemotherapy for non-small-cell lung cancer: a willingness to pay study. Lung Cancer. 2011 Apr;72(1):114–118. doi: 10.1016/j.lungcan.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002 Jul 1;95(1):155–163. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]