Abstract
Human papillomavirus (HPV), a known etiology of a subset of head and neck squamous cell carcinomas (HNCs), causes numerous alterations in normal cellular functions. This article reviews the biology, detection and treatment of HPV-positive HNC. The role of HPV oncoproteins in tumor development, the natural history of HPV infection, and risk factors for and prevention of transmission of oral HPV are considered. Commonly used methods for detecting HPV infection including limitations of these methods are discussed to aid the practicing clinician in utilizing these tests in their clinical practice. Clinical characteristics of HPV-positive HNC including potential explanations for the improved outcomes seen in patients with HPV-positive HNC are assessed. Ongoing clinical trials specific for patients with HPV-positive HNC are described and areas in need of additional research are summarized. Until the results of ongoing trials are known, treatment of HPV-positive HNC should not differ in clinical practice from treatment of similar non-HPV related cancers.
Keywords: Head and neck cancer, human papillomavirus, radiation therapy
INTRODUCTION
Squamous cell carcinoma of the head and neck (HNC) is the sixth most common cancer worldwide, resulting in over 600,000 new diagnoses annually (1). Traditionally, HNC has been related to tobacco and alcohol exposure (2); however, over the past decade, a growing number of head and neck cancers are attributable to human papillomavirus (HPV) infection (3). HPV-positive cancers are now thought to account for 30-65% of all HNC and 50-80% of cancers arising in the oropharynx (3, 4). Patients with HPV-positive HNC represent a distinct patient subgroup with unique epidemiologic and prognostic characteristics (5-14), and now represent a growing public health concern that is projected to become the primary cause of HNC in the coming decades (15).
This article attempts to summarize the current understanding of how HPVs cause cancer; factors in the transmission, detection, and prevention of HPV infection relevant to the practicing clinician; how HPV status can be integrated into the care of patients with HNC; and, future directions for research.
Human papillomavirus and its role in tumor formation
Papillomaviruses were first identified from rabbits in 1933 when they were found to be a transmissible, filterable, cause of the growth of benign papillomas (16). Human papillomavirus (HPV), first identified in 1956, is associated with a variety of benign growths in humans (reviewed in (17)). HPV has subsequently been shown to be the cause of multiple types of human cancer, a discovery highlighted by the awarding of the 2008 Nobel Prize to Harald zur Hausen for elucidating the role of HPV in the development of uterine cervical cancer (18).
HPV is a double-stranded, non-enveloped, DNA virus of approximately 8,000 base pairs. Classified on the basis of their L1 protein, there are currently well over 100 unique subtypes of HPV that can be sub-classified into cutaneous or mucosal subtypes based on their specific tissue tropism (19). HPVs can be further separated into low-risk and high-risk types based on their ability to cause malignant transformation and induce cancer. About 20 HPV strains are considered high-risk (Table 1) and are known to cause cancers of the uterine cervix, anus, vagina, vulva, penis, and head and neck (20).
Table 1.
High risk HPV types
| Highest risk | 16, 18, 31, 45 |
| Other high risk | 33, 35, 39, 51, 52, 56, 58, 59 |
| Probably high risk | 26, 53, 66, 68, 73, 82 |
The HPV genome encodes eight viral proteins (Figure 1) that regulate the viral life cycle (reviewed in (21)). The HPV L1 and L2 genes encode the viral capsid proteins that encapsulate the viral DNA and play no known role in carcinogenesis, but are important targets of the immune response to HPV infection (22). The early viral genes E1 and E2 are important for viral genome replication and play a role in transcriptional regulation of other viral genes (21). The E4 protein, translated from a spliced E1^E4 mRNA transcript, is thought to facilitate viral particle release into the environment and may also play a role in G2 arrest in HPV infected cells (23). The three HPV oncogenes, E5, E6, and E7 promote unrestrained cellular proliferation to allow for viral amplification but also contribute to the initiation and progression of cancer via the same mechanism and by inducing genomic instability (24-26).
Figure 1.
The HPV genome is a double-stranded circular DNA containing approximately 8000 base pairs and encoding eight proteins (A). E6 (B) and E7 (C) are the predominant HPV oncogenes and target a variety of diverse cellular processes.
Appreciating the unique life cycle of HPV infection helps one to understand how the tumor-promoting roles of HPV oncogenes have evolved from their normal function in the viral life cycle. Whereas most viruses infect a target cell and produce progeny from that same cell, the HPV life-cycle requires the infected cell to undergo mitosis and differentiation (27). HPV infects basal cells in the stratified squamous epithelium. It is thought to be exposed to this, otherwise protected, layer through micro-abrasions in the epithelial surface. Since differentiating cells in the suprabasal layer have exited the cell cycle, the basal cells are the only proliferating cells in the normal epithelium. The HPV genome does not encode enzymes necessary for viral replication; instead HPVs utilize host cell proteins to replicate viral DNA. In HPV infections, suprabasal cells that contain HPV genomes remain active in the cell cycle as they differentiate. This results in these otherwise non-dividing cells re-entering S-phase and amplifying the HPV genome prior to capsid protein synthesis, viral assembly, and release (28).
The viral E7 protein plays a critical role in promoting the proliferation of HPV-infected cells by binding members of the pocket protein family and targeting them for degradation (25, 29). Rb, the most well known pocket protein family member functions to prevent excessive cell growth by inhibiting cell cycle progression(30). Degradation of Rb results in E2F transcription factors driving expression of S phase genes, promoting progression through the cell cycle. While essentially all HPV E7 proteins bind Rb family members, the avidity of this interaction is significantly greater in high-risk E7 proteins (25). p53-dependent growth inhibition and apoptosis can occur as a result of E7-mediated proliferation. To counteract this, HPV E6, via activation of the ubiquitin ligase E6AP, causes degradation of p53 leading to inhibition of apoptosis and consequently unrestrained cellular growth (17, 24). While their relative contributions vary by epithelial site, both E6 and E7 contribute to carcinogenesis (29). Additionally, the high risk E5 protein cooperates with E6 and E7 to promote proliferation in infected cells and is thought to play a minor role in transformation (26).
Despite the strong growth promoting effects of HPV oncogenes, additional oncogenic events are necessary for malignant transformation. Both high-risk E6 and E7 independently cause genomic instability (31). While the mutation rate in HPV-positive cancers appears to be lower than that in HPV-negative cancers (32, 33), expression of E6 and E7 can cooperate to cause errors in chromosomal segregation and the development of aneuploidy (34). Multiple rounds of centrosome synthesis can be induced by E7, resulting from the formation of multiple immature centrioles in a process that is linked to E7-mediated activation of CDK2 activity (35). Finally, E6 and E7 cooperate to allow cells with abnormal mitoses to accumulate by interfering with the G2-M checkpoint and inhibiting apoptosis (36). While their above roles are those that are best described, it has become clear that both E6 and E7 interact with a large number of additional cellular proteins (37, 38). The oncogenic role of these additional interactions remains the subject of further investigation.
While additional high-risk HPV strains have been identified in recent years (20), HPV-16 and HPV-18 remain the causative factor in the majority of HPV-associated cancers (20). For example, about 70% of cervical cancer cases are associated with either HPV-16 or HPV-18 (20). About 50% of penile cancers contain HPV DNA, mostly HPV-16 (20). HPV-16 or HPV-18 is responsible for a similar percentage of other male and female anogenital cancers. In the oropharynx, HPV-16 accounts for over 90% of all HPV-associated cancers (39, 40).
Risk factors for and natural history of oral HPV
The Centers for Disease Control and Prevention (CDC) classifies high-risk HPV as the most common sexually transmitted infection in the United States (41). The prevalence of oral HPV and of HPV-positive HNC has increased significantly during the last 20 years (3, 15), a change that is likely due to several overlapping factors: 1) increased awareness of the association of HPV and HNC, 2) improved identification of HPV within tumors cells, and 3) a true increase in the prevalence of HPV-associated cancers (particularly in the head and neck region). While the prevalence of oral HPV infection is 5-10 fold lower than that of genital HPV, transmission of both genital and oral high-risk HPVs is highly correlated with sexual activity (41-44). For example, the risk of having HPV isolated from the oral cavity is eight times greater among sexually experienced than in sexually inexperienced individuals; likewise, oral HPV infection is strongly associated with early sexual debut, multiple sexual partners, open-mouth kissing, and oral sex in both the oral-oral and oral-genital forms (42, 43, 45).
Additional risk factors that have been correlated with oral HPV transmission include current tobacco use, marijuana use, and alcohol consumption; all factors that may be linked to sexual activity (42, 43, 45). Although the reasons remain unclear, several studies have found that men are at 2-3 fold greater risk for becoming infected with oral HPV than are women (43, 45). At the present time, these gender differences in oral HPV infection cannot be explained by differences in sexual behavior suggesting that there are still poorly understood gender-based differences in the natural history of infection and/or exposure to co-factors of infection. Oral HPV prevalence shows a bimodal distribution with the largest peak in men and women 55-64 years of age and a second, smaller, peak at 30-34 years. (43, 45). Characteristics of non-malignant HPV infection vary significantly across patients. In many people with oral or genital HPV, infection is asymptomatic and is successfully eradicated by the normal immune system within a few months (46, 47). Recent data from a prospective trial in women suggested that low-risk HPV types are cleared from the oral cavity more quickly than high-risk HPVs (46). Consistent with the findings in HPV-positive HNC, several studies show the most common HPV type detected in the oral cavity is HPV-16 (48, 49). Unfortunately, the reasons for this HPV subtype-specific tropism remain unclear. Differences in individual immune-system responses to HPV infection likely underlie some of these differences. However, even in patients who develop HPV-positive HNC, significant differences in viral titers have been seen (8), suggesting that robust clearance of the incident infection may not prevent malignant transformation or that immune recognition occurs late in the oncogenic process.
Finally, why high-risk HPVs do not appear to infect all mucosal sites of the head and neck at the same rate remains unclear; an overwhelming proportion of HPV-positive HNC occurs in the mucosa lining the oropharynx (3, 4). One possibility is that the local microenvironment of the tonsil and base of tongue provide an optimal setting for infection to occur; the deep mucosal crypts trap HPV viral particles and prolong the contact time between the virus and the mucosa. Another possibility is that the local immune environment associated with these lymphatic rich sites results in a prolonged inflammatory state ultimately resulting in either clearance or malignant transformation. Finally, viral receptors (which have not been definitively identified to date) may be differentially expressed on the mucosa of these regions compared to that of the oral cavity or larynx. Ultimately, additional studies of the natural history of oral HPV infection and further delineation of the molecular targets involved in viral entry are needed to better define the transmission, incidence, clearance, persistence, and predictors of transformation for oral HPV infection.
Primary prevention of high risk HPV infection
The recognition that HPV causes cervical cancer, one of the most common cancers worldwide, led a number of groups to work to develop an HPV vaccine. In 2013, two separate vaccines are available in the US to decrease the burden of HPV-associated disease. Gardasil (Merck and Co.) and Cervarix (GlaxoSmithKline, Inc.) target four and two subtypes of HPV, respectively (50). Neither vaccine contains HPV DNA; rather they each make use of virus-like particles composed of the major capsid protein, L1, of the targeted HPV subtypes. The L1 protein constitutes 90-95% of the HPV outer covering (19), and immune-system surveillance for HPV-L1 results in acquired immunity. Both vaccines prevent infection with HPV-16 and HPV-18, the two most common strains linked to cancer. Unfortunately, use of the currently available vaccines following the development of cancer is unlikely to provide clinical benefit, as expression of the capsid proteins is usually lost during transformation. Current estimates in the US are that only 35% of the population completes the three injection series as currently recommended (51), meaning that we are likely to continue seeing a significant number of patients with HPV-associated cancers for the foreseeable future. Oncologists can play an important role in advocating for universal HPV vaccination of both boys and girls, a recommendation shared by the CDC and the American Academy of Pediatrics (50).
HPV Detection
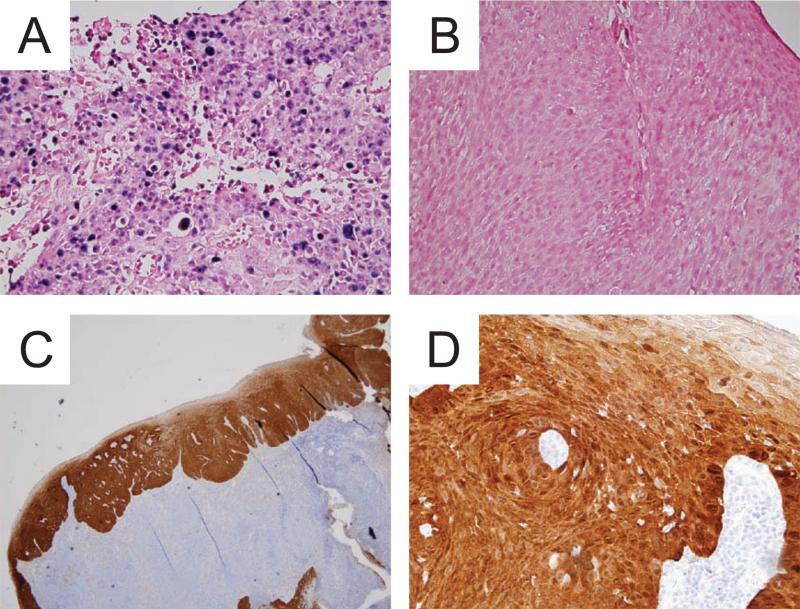
Accurate detection of HPV within tumor tissue is critical if we are to attempt personalizing therapy for patients with HPV-related cancers. Several potential techniques are available to detect the presence of HPV (Table 2 and reviewed in (52)). Southern blots are one of the oldest, most reliable methods still used to detect HPV DNA, giving low false positive rates, high sensitivity, and the ability to identify specific HPV subtypes (53). Southern blots utilize HPV subtype-specific probes that hybridize to total cellular DNA to identify specific HPV subtypes. Unfortunately, Southern blots are time intensive and require large quantities of cellular DNA, making them unsuited for routine clinical use. In situ hybridization (ISH, Figure 2A/B) is often used in diagnostic labs to detect a panel of HPV subtypes (54). ISH has lower specificity than Southern blots but it can be used on fixed or paraffin-embedded samples with very little additional processing (55). Polymerase chain reaction (PCR) and reverse transcriptase-PCR (RT-PCR) are other methods of detection that are highly sensitive at detecting HPV DNA or RNA within small amounts of tumor samples. However, they can lead to false positive results due to their high sensitivity to HPV genomes that may be present in oral tissues but that are unrelated to the malignancy (56, 57).
Table 2.
Methods of testing for HPV in cancer patients.
| Test | Comments |
|---|---|
| p16 IHC | commonly performed in clinical labs, high correlation with outcome, but may be elevated in HPV-negative cases |
| ISH | highly specific and can be performed on paraffin embedded samples, but low sensitivity for tumors with low numbers of copies of the HPV genome |
| PCR | highly specific and fast turnaround time but environmental contamination can lead to false positive results |
| RT-PCR | sensitive and specific because it detects actively HPV infection, but requires intact RNA so better results from fresh or frozen tissue than from paraffin-embedded tissue |
| serology | easy to perform, but no direct relationship to viral-associated cancers |
IHC, immunohistochemistry; ISH, in situ hybridization; PCR, polymerase chain reaction; RT-PCR, reverse transcripase polymerase chain reaction
Figure 2.
The only clinically used test that detects HPV DNA, in situ hybridization (ISH), utilizes oligonucleotide probes specific for the DNA of several high-risk HPV types (A, 40X magnification). Also shown is a slide stained as negative control for ISH (B). Immunohistochemistry for p16 (A, 4X magnification & B, 40X magnification) is another commonly accepted test to assess for HPV status with p16-positive tumors being deemed HPV-positive.
In many labs, the standard test for HPV involvement in a tumor (and for clinical trial enrollment) is detection of Cyclin-dependent kinase inhibitor 2A (a.k.a., p16Ink4A or p16) by immunohistochemistry (IHC) (Figure 2C/D) (58, 59). The HPV-16 E7 protein results in downregulation of Rb thus freeing E2F, its regulatory partner. Free E2F results in upregulation of p16. IHC detection of p16 is a quick, inexpensive, and readily available technique and has become the de facto standard for clinical assessment of HPV status (59). It should be noted that not all p16 positive tumors are HPV positive: across multiple studies, about 10-20% of p16 positive HNC show no evidence of HPV infection (6-8, 60, 61). While p16 status retains prognostic significance even in the absence of coincident HPV positivity (6-8, 60), the cause of p16 overexpression in these cases remains unclear, however other causes could include an alternative viral infection, carcinogen-induced mutations leading to p16 overexpression, or another etiology. In addition, whether HPV-negative, p16 positive tumors should be grouped with HPV-positive tumors in terms of personalized therapy and clinical trials is unclear and an issue that requires further study.
Tests adapted from cervical cancer screening programs can identify a group of high-risk HPV subtypes by using oligonucleotide probes and a variety of amplification chemistries (62, 63). ISH tests allow direct identification of HPV DNA within tumor cells, can be used for gross estimation of viral genome copy number (64), and can be designed to identify specific subytpes if sufficient tissue is available for testing. PCR-based tests can be designed to detect the most common high-risk HPV types and can be integrated with commercially available genotyping kits to identify the specific HPV type present in a PCR sample (65). Ultimately, next-generation sequencing technology can use high throughput methodology to rapidly examine tumor samples for HPV subtypes by sequencing the entire tumor genome, transcriptome, or a subset of pre-selected genes (66, 67). While not routinely used for clinical decision making due to high costs and challenges with data interpretation, next-generation sequencing will likely play a greater role in future trials and clinical decision making.
While it is possible to determine the specific HPV-subtype within an individual tumor, in many hospitals, clinically used tests do not pursue this degree of specificity. In head and neck cancer, identification of the specific HPV type does not currently play a clinical role due both to the >90% incidence of HPV-16 and to the lack of knowledge regarding how to utilize this information if it were routinely obtained. However, in the cervix, two separate groups have reported evidence of clinically relevant differences between HPV-16 and HPV-18 infection that could be used to guide clinical decision making (68, 69). If similar data is found in the head and neck, tests to determine not only the presence of HPV but also the individual subtype will become more important.
Clinical characteristics of patients with HPV-positive HNC
Patients with HPV-positive HNC often present at a younger age and with a more advanced stage of cancer than those with HPV-negative HNC (4, 14, 70). HPV-positive HNC patients are less likely to use tobacco, less likely to develop a second malignancy, and equally likely to be male as patients with HPV-negative HNC. They present with a tumor arising in the oropharynx and often present with stage III or IV disease due to the presence of involved regional lymph nodes. Numerous clinical reports provide convincing evidence of improved outcomes in patients with HPV-positive HNC compared to those with HPV-negative HNC, a result that spans multiple continents and treatment approaches (5-12, 14). The true biologic basis for this improvement remains unclear; although, it has been postulated that enhanced sensitivity to radiation underlies much of the observed difference (13, 39) and recent preclinical data provides support for this hypothesis (71, 72).
Several prospective trials and retrospective reviews utilizing radiation alone have reported outcomes based on HPV status (reviewed in (13) and (73), respectively). Each of these studies support the hypothesis that patients with HPV-positive HNC have significantly better overall survival and local/regional control than those with HPV-negative disease. Patients with HPV-positive disease have a 60-80% reduction in mortality, a result that has been repeated across multiple trials. Consistent with this, in a retrospective analysis of the RTOG 0129 trial, 3 year progression free survival for patients who were found to be HPV-positive was better than those who were HPV-negative (74% vs. 43%, respectively) (4). Likewise, in the TAX 324 study, the proportion of HPV-positive patients free of progression was improved compared to HPV-negative patients (73% vs. 29%) (14).
A consistent theme across studies that utilize radiation is that the primary benefit to HPV-positivity appears to be an improvement in local/regional control; although small improvements in the rates of metastatic disease are also seen. For example, in RTOG 0129 fewer local/regional failures were seen in HPV-positive patients (14% vs. 35% at 3 years) (4). Similarly, in the TAX 324 study, local/regional failure was seen less frequently in HPV-positive than in HPV-negative patients (13% vs. 42%) (14). These data could be interpreted to suggest that HPV positivity may correlate with an intrinsic enhanced sensitivity to radiation but do not rule out alternative biologic factors such as improved immune surveillance of HPV-positive tumors as the cause of improved outcomes.
Multimodality therapy for HNC is associated with a high risk of both acute and long-term treatment-related toxicity. Based upon the improved outcomes seen in patients with HPV-positive HNC, recent discussions have focused on treatment de-intensification in an effort to decrease morbidity while maintaining favorable survival outcomes. To aid in this process, several groups have published retrospective analyses to help identify patients in whom deintensification of therapy is a reasonable risk (74-76). For example, O'Sullivan and colleagues recently reported upon a cohort of 500 patients with oropharyngeal carcinoma (both HPV-positive and HPV-negative) uniformly treated with either radiotherapy alone (usually with an accelerated schedule) or combined chemoradiotherapy in a single institution (74). Patients were analyzed based on their HPV status as well as their tumor and treatment characteristics. They found that advanced T or N stage were both predictors for distant metastatic recurrence, regardless of HPV association, with similar rates seen in both HPV-positive and HPV-negative cohorts. This implies that there could be a limit to the biologic favorability conferred by HPV-positivity. Thus, increased local/regional disease burden at diagnosis could be used to identify an HPV-positive subgroup that may benefit from the use of chemotherapy to treat early micrometastatic disease. Other groups have shown that tobacco use of greater than 10 pack years decreases the progression free survival rate in HPV-positive HNC patients (74-76), suggesting interplay between HPV-mediated and carcinogen-induced oncogenesis. These reports have led cooperative groups to embrace both HPV status and tobacco use as stratification factors to identify patients with an extremely good prognosis for inclusion in deintensification trials. However, they should also be taken as a cautionary tale that both known, and perhaps unknown, risk factors may influence disease control in these patients.
De-Intensified therapy for HPV-positive HNC patients
A number of large clinical trials that investigate therapeutic dose reduction and are specific for patients with HPV-positive HNC are currently underway (Table 3). In broad terms, these trials can be divided into two camps: 1) deintensification of local therapy via use of alternative chemotherapy, reduced dose radiation, or surgery; and, 2) use of induction therapy to identify good responding patients for subsequent dose reduction.
Table 3.
Ongoing trials for patients with HPV-positive HNC.
| Type | # of patients | Group/institution | ClinicalTrials. gov identifier | Trial Design |
|---|---|---|---|---|
| Phase 2 | 83* | ECOG | NCT01084083 | neoadjuvant chemotherapy and response adapted radiation (54 or 66-70 Gy) + cetuximab |
| Phase 2 | 50 | North Shore Long Island Jewish Health System | NCT01525927 | neoadjuvant TPF and response adapted radiation (60 Gy) +/− concurrent chemotherapy |
| Phase 2 | 50 | Univ. of California Davis | NCT01716195 | neoadjuvant chemotherapy followed by paclitaxel + response adapted radiation (50 or 60 Gy) |
| Phase 2 | 36 | Univ. of Michigan | NCT01663259 | weekly cetuximab + radiation (70 Gy) |
| Phase 2 | 40 | Univ. of North Carolina | NCT01530997 | radiation with weekly cisplatin followed by supra-selective neck dissection |
| Phase 3 | 706 | RTOG | NCT01302834 | randomized to cetuximab vs. cisplatin with concurrent radiation (70 Gy in 6 weeks) |
| Phase 3 | 365 | Mount Sinai School of Medicine | NCT01706939 | weekly carboplatin/cetuximab + 56 Gy vs. weekly carboplatin + 70 Gy |
| Phase 2 | 337 | ECOG | pending | transoral resection - risk adapted postop RT (0 vs. 50 vs. 60 vs. 66 Gy with weekly cisplatin) |
| Phase 3 | 496 | Washington Univ. | NCT01687413 | postoperative radiation (60 Gy) +/− weekly cisplatin |
RTOG: Radiation Therapy Oncology Group; ECOG: Eastern Cooperative Oncology Group; TPF: docetaxel, cisplatin, 5-fluorouracil
trial closed to accrual
Several trials investigating alternative chemotherapy are underway. Accrual is nearly completed in Radiation Therapy Oncology Group (RTOG) 1016, a phase III trial randomizing HPV-positive HNC patients to cisplatin vs. cetuximab given concurrent with 70 Gy radiation. This study hopes to definitively answer the question of whether cetuximab, with its favorable toxicity profile, can be safely substituted for cisplatin in patients with HPV-positive HNC. In addition, the University of Michigan and the University of North Carolina (UNC) are pursuing Phase 2 trials investigating dose de-intensification without induction therapy using either cetuximab + standard dose radiation or cisplatin + reduced dose radiation, respectively. Both of these trials are based upon the premise that the experimental treatment is less toxic than standard of care therapy. While still preliminary, the UNC trial has shown promising results in HPV-positive patients treated with reduced dose radiation (60 Gy), less toxic weekly cisplatin (30 mg/m2), and undergoing a mandatory post treatment biopsy of the primary site and a supra-selective neck dissection of the pre-therapy involved neck (Personal Communication, Bhishamjit Chera, UNC) Mount Sinai is leading a group of several institutions randomizing patients with HPV-positive HNC to one of two regimens: either carboplatin + cetuximab with 56 Gy radiation or carboplatin alone with 70 Gy radiation.
Two additional randomized trials investigating whether surgery can be incorporated into a radiation dose reduction paradigm for patients with HPV-positive HNC are currently underway in the US (Table 3). The Eastern Cooperative Oncology Group (ECOG) 3311 trial is a recently approved randomized phase 2 study investigating whether upfront surgical excision and pathologic staging of all evident disease can permit reduced dose adjuvant therapy. Several surgical approaches are permitted and patients receive either observation (low risk pathologic stage I-II); trimodality therapy with 66 Gy and weekly cisplatin (high risk cohort); or, are randomized to 50 Gy vs. 60 Gy (intermediate risk cohort). Using a similar approach, Washington University School of Medicine is leading a group of institutions studying patients undergoing minimally invasive surgery with removal of all known disease and with lymph nodes showing extracapsular spread who are then randomized to postoperative radiation only vs. radiation plus weekly cisplatin.
Several other groups are proceeding with phase II studies investigating the role of induction chemotherapy and response-adapted radiation in patients with HPV-positive HNC (Table 3). Use of induction therapy is based on data suggesting that initial response to therapy can predict overall tumor control (reviewed in (77)). It is hypothesized that the use of induction therapy will deliver successful cytotoxic therapy to micrometaststic disease at the earliest time point, thus improving distant disease control without compromising local/regional control. The recently closed ECOG 1308 phase II trial utilized induction chemotherapy to select patients for radiation dose modification (from 66-70 Gy to 54 Gy) based on whether or not they achieved a complete response to induction therapy. Results from this study are currently pending. Both North Shore Long Island Jewish Health System and the Univ. of California Davis are pursuing this approach, albeit with the use of different induction and concurrent chemotherapy regimens (Table 3). If these studies provide promising results, it may be time to pursue studies comparing induction therapy with upfront definitive therapy for patients with HPV-positive HNC.
Several important caveats to these approaches should be mentioned and are being considered as the next generation of trials is being developed. First, there is significant uncertainty regarding the value of therapies targeting the epidermal growth factor receptor (EGFR) in HPV-positive HNC patients. There is no preclinical data currently published that examines the role of EGFR inhibition in HPV-positive HNC. Two large clinical studies have demonstrated contrasting results regarding the role of EGFR inhibitors in HPV-positive HNC. In the landmark study demonstrating an overall survival benefit with the addition of cetuximab to radiation in patients with locally advanced head and neck cancer, the greatest apparent benefit was in patients whose tumor originated in the oropharynx (78). While no tumor blocks were available for HPV testing in this study, oropharyngeal tumors are thought to be the most likely to be HPV-positive, suggesting a potential interplay between HPV-positivity and EGFR inhibition. Alternatively, in a study investigating the role of panitumumab, an alternative anti-EGFR antibody, there was no apparent benefit to EGFR-directed therapy in HPV-positive patients with recurrent or metastatic HNC (79). Thus, defining the role of EGFR inhibitors in HPV-positive HNC awaits the completion of ongoing clinical studies such as the RTOG 1016 study described above. Finally, several groups have now reported preclinical data supporting increased radiation sensitivity in HPV-positive HNC (71, 72). Thus, one potential concern regarding the use of induction chemotherapy is that it may result in the delay of what could potentially be the most effective therapy available for these tumors (i.e., radiation).
Overall, each of the studies described varies in terms of radiation dose, chemotherapy, and specific selection criteria but are similar in that they enroll only patients with HPV-positive HNC. Until we have results from these, and other studies, it is our opinion that at this time, no treatment decisions should be made outside the setting of a clinical trial based solely upon HPV status for patients with HNC. As 3 year overall survival for patients with HPV-positive HNC in recently completed studies still hovers around 70-75%, we urge caution in pursuing dose reduction regimens outside the setting of a clinical trial due to the possibility of increases in treatment failures.
Potential explanations for improved outcomes
There are several potential explanations for the improved outcomes seen in patients with HPV-positive HNC (Figure 3). Over the last several years, it has become accepted that factors intrinsic to individual tumors (e.g., specific mutations or, potentially, HPV status) can play a major role in modulating the tumor microenvironment (reviewed in (80)). These alterations can affect immune cell infiltration, stromal architecture, and tumor vasculature, among other factors. In addition, the viral oncogenes may alter tumor cell regulation of genomic instability and remove the selective pressure for tumors to develop mutations in TP53.
Figure 3.
Potential factors contributing to improved outcomes in patients with HPV-positive HNC include the presence of viral-specific anti-tumor immunity, wild-type p53, HPV oncogene modulated genomic instability, and alterations in tumor microenvironment leading to improved tumor oxygenation.
It has been hypothesized that the immune system plays a more important role in clearance of HPV-positive cancers due to the expression of viral proteins within HPV-positive HNC. Growing data over the last several years appear to support this hypothesis. Using mouse models, Spanos and colleagues demonstrated better tumor control in HPV-positive cell lines implanted into immune competent (as opposed to immunocompromised) mice (81). In patients with HPV-positive HNC, HPV-specific T-cells have been described (82). Another group demonstrated a shift from naïve to effector and memory T cells in patients with HNC when compared to healthy donors (83). This shift was greater in HPV-positive, than in HPV-negative patients suggesting a greater immune response to HPV-positive tumors (83). The presence of programmed death-1 (PD-1) positive T-cells has been correlated with improved survival in HPV-positive HNC (84). Finally, radiation can induce loss of CD47 (a cell surface marker that plays a role in identification of self) expression in HPV-positive cell lines, providing a potential explanation for the proposed interaction between the immune system and radiation therapy (85).
Several studies have utilized lab correlates of hypoxia to examine its relationship to HPV status; the results from these studies have been mixed. For example, in the Danish Head and Neck Cancer Group (DAHANCA) 5 study, a subset of patients underwent testing for plasma osteopontin (a marker of hypoxia). The proportion of patients with high osteopontin levels was greater in HPV-negative than in HPV-positive tumors, a result suggesting more hypoxia in HPV-negative tumors (86). On the other hand, in a different group of patients, neither carbonic anhydrase IX (upregulated in hypoxic tissues) or tumor pO2 were found to correlate with tumor HPV status (87). Two prospective trials have investigated the effect of hypoxic modification (with nimorazole or tirapazamine) in HNC. In both studies, the use of a hypoxic modifier resulted in a trend toward improved local-regional control in HPV-negative tumors, but had no measurable effect in HPV-positive tumors (9, 88). These results suggest that hypoxia, and hence resistance to radiation, may play a more important role in HPV-negative tumors. While these studies largely measured surrogates of tumor oxygenation, several groups have examined imaging markers of hypoxia (e.g., dynamic contrast enhanced-MRI (DCE-MRI), proton magnetic resonance spectroscopy ((1)H-MRS), and (18)F-fluoroazomycin arabinoside positron emission tomography/computed tomography (FAZA PET/CT)) and have failed to show any correlation between imaging measured intra-tumoral hypoxia and HPV status (89, 90).
Finally, several groups have hypothesized that viral oncogenes play an important role in increased sensitivity to therapy. They have systematically investigated HPV-positive HNC cell lines and demonstrated enhanced sensitivity to radiation in HPV-positive HNC cells (71, 72). We have shown that low levels of residual wild-type p53 present in HPV-positive cells can become activated following radiation resulting in cell death (72), while Rieckmann and colleagues have suggested that HPV-positive cells have a defect in DNA damage repair capacity (71). Each of these pathways may contribute to increased intrinsic sensitivity to DNA damage induced by radiation. Unfortunately, there is essentially no data in the literature addressing whether HPV-positive HNC is more sensitive to chemotherapy than HPV-negative HNC. In our own work we see a significant overlap in the concentration of drug required to achieve 50% growth inhibition between HPV-positive and HPV-negative cells treated with cisplatin, a result that suggests little difference in sensitivity, at least in vivo. Clearly, further work is needed to better define the molecular determinants of increased sensitivity to both radiation and chemotherapy in HPV-positive HNC cells and in HPV-positive HNC patients.
Future Directions
A number of groups are attempting to better understand the biology of HPV-positive HNC using in vitro and in vivo model systems to improve prevention, screening, diagnosis, and to identify and validate novel therapeutic targets. Unfortunately the limited number of available HPV-positive cell lines has hampered progress in understanding the sensitivity of these cancers to current and investigational therapeutics. While thousands of patients with HPV-positive HNC have been treated worldwide, there are only a handful of documented, HPV-positive HNC cell lines available. The lack of established HPV-positive HNC cell lines raises interesting questions regarding the biology of this unique disease and why it has been so challenging to establish HNC cell lines from patients with HPV-positive tumors. In light of this limitation, we have spent the last several years developing a patient-derived xenograft system that utilizes fresh tumor obtained by our surgical colleagues and immediately implanted into mice (91). We are using this system to compare the sensitivity of HPV-positive and HPV-negative HNC to a variety of therapies and to probe the mechanisms underlying differential responses between HPV-positive and HPV-negative HNC in the hopes of identifying improved therapeutic approaches.
Conclusions
The improved outcomes seen in patients with HPV-positive HNC are, at this time, thought to be independent of treatment approach. Thus, until sufficient data accumulates to state otherwise, patients with HPV-positive HNC should be treated using standard of care approaches unless they are enrolled on a prospective clinical trial. Successful treatment of HNC carries significant risks for treatment-related toxicities that may adversely impact on patient quality of life. The improved outcomes seen in patients with HPV-positive HNC have made many in our field hopeful that the intensity of treatment can be decreased while maintaining good tumor control. However, care must be taken so as to not adversely affect outcomes by trying to decrease toxicity without sufficient supporting data. In much the same way we differentiate estrogen receptor positive from estrogen receptor negative breast cancer, one day soon we may employ significantly different treatments for patients with HPV-positive as compared to HPV-negative HNC. Within the next few years, we will likely use tumor HPV-status not only to aid in prognostic discussions with patients, but also to aid in the selection of treatment approaches. Understanding the biology of HPV-positive cancers, how HPV modulates the way in which tumors respond to specific therapies, and the clinical implications of HPV in head and neck cancer should provide patients and providers with the ability to rationally personalize therapy to improve therapeutic outcomes for those with this previously overwhelming disease.
Acknowledgments
Financial support and disclosures: Supported by R00 CA160639 (RK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010 doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Decker J, Goldstein JC. Risk factors in head and neck cancer. N Engl J Med. 1982;306:1151–1155. doi: 10.1056/NEJM198205133061905. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, et al. Improved Survival of Patients With Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Shah NG, Trivedi TI, Tankshali RA, et al. Prognostic significance of molecular markers in oral squamous cell carcinoma: A multivariate analysis. Head & Neck. 2009;31:1544–1556. doi: 10.1002/hed.21126. [DOI] [PubMed] [Google Scholar]
- 7.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 9.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 11.Lindel K, Beer KT, Laissue J, et al. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 13.Lassen P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol. 2010;95:371–380. doi: 10.1016/j.radonc.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shope RE, Hurst EW. Infectious Papillomatosis of Rabbits : With a Note on the Histopathology. J Exp Med. 1933;58:607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durst M, Gissmann L, Ikenberg H, et al. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzich JA, Ghim SJ, Palmer-Hill FJ, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humans IWGotEoCRt Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 21.Hebner CM, Laimins LA. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16:83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- 22.Horvath CA, Boulet GA, Renoux VM, et al. Mechanisms of cell entry by human papillomaviruses: an overview. Virol J. 2010;7:11. doi: 10.1186/1743-422X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight GL, Pugh AG, Yates E, et al. A cyclin-binding motif in human papillomavirus type 18 (HPV18) E1^E4 is necessary for association with CDK-cyclin complexes and G2/M cell cycle arrest of keratinocytes, but is not required for differentiation-dependent viral genome amplification or L1 capsid protein expression. Virology. 2011;412:196–210. doi: 10.1016/j.virol.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 25.Munger K, Werness BA, Dyson N, et al. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiMaio D, Mattoon D. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene. 2001;20:7866–7873. doi: 10.1038/sj.onc.1204915. [DOI] [PubMed] [Google Scholar]
- 27.Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010;118:422–449. doi: 10.1111/j.1600-0463.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng S, Schmidt-Grimminger DC, Murant T, et al. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9:2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- 29.Song S, Liem A, Miller JA, et al. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267:141–150. doi: 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- 30.Indovina P, Marcelli E, Casini N, et al. Emerging roles of RB family: new defense mechanisms against tumor progression. J Cell Physiol. 2013;228:525–535. doi: 10.1002/jcp.24170. [DOI] [PubMed] [Google Scholar]
- 31.Fan X, Liu Y, Heilman SA, et al. Human papillomavirus E7 induces rereplication in response to DNA damage. J Virol. 2013;87:1200–1210. doi: 10.1128/JVI.02038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000;97:10002–10007. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duensing A, Liu Y, Tseng M, et al. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25:2943–2949. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finzer P, Aguilar-Lemarroy A, Rosl F. The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 2002;188:15–24. doi: 10.1016/s0304-3835(02)00431-7. [DOI] [PubMed] [Google Scholar]
- 37.White EA, Kramer RE, Tan MJ, et al. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J Virol. 2012;86:13174–13186. doi: 10.1128/JVI.02172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huh KW, DeMasi J, Ogawa H, et al. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102:11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 40.Schache AG, Liloglou T, Risk JM, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17:6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satterwhite CL, Torrone E, Meites E, et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2008. Sex Transm Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 42.Pickard RK, Xiao W, Broutian TR, et al. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18-30 years. Sex Transm Dis. 2012;39:559–566. doi: 10.1097/OLQ.0b013e31824f1c65. [DOI] [PubMed] [Google Scholar]
- 43.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Souza G, Agrawal Y, Halpern J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders AE, Slade GD, Patton LL. National prevalence of oral HPV infection and related risk factors in the U.S. adult population. Oral Dis. 2012;18:430–441. doi: 10.1111/j.1601-0825.2011.01892.x. [DOI] [PubMed] [Google Scholar]
- 46.Louvanto K, Rautava J, Willberg J, et al. Genotype-specific incidence and clearance of human papillomavirus in oral mucosa of women: a six-year follow-up study. PLoS One. 2013;8:e53413. doi: 10.1371/journal.pone.0053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mooij SH, Boot HJ, Speksnijder AG, et al. Oral human papillomavirus infection in HIV-negative and HIV-infected men who have sex with men: the HIV & HPV in MSM (H2M) study. AIDS. 2013 doi: 10.1097/QAD.0b013e328362395c. [DOI] [PubMed] [Google Scholar]
- 49.Kero K, Rautava J, Syrjanen K, et al. Oral mucosa as a reservoir of human papillomavirus: point prevalence, genotype distribution, and incident infections among males in a 7-year prospective study. Eur Urol. 2012;62:1063–1070. doi: 10.1016/j.eururo.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 50.Committee on Infectious D HPV vaccine recommendations. Pediatrics. 2012;129:602–605. doi: 10.1542/peds.2011-3865. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease C Prevention. National and state vaccination coverage among adolescents aged 13-17 years--United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–677. [PubMed] [Google Scholar]
- 52.Kimple AJ, Torres AD, Yang RZ, et al. HPV-associated head and neck cancer: molecular and nano-scale markers for prognosis and therapeutic stratification. Sensors (Basel) 2012;12:5159–5169. doi: 10.3390/s120405159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiffman MH, Bauer HM, Lorincz AT, et al. Comparison of Southern blot hybridization and polymerase chain reaction methods for the detection of human papillomavirus DNA. J Clin Microbiol. 1991;29:573–577. doi: 10.1128/jcm.29.3.573-577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 55.Caussy D, Orr W, Daya AD, et al. Evaluation of methods for detecting human papillomavirus deoxyribonucleotide sequences in clinical specimens. J Clin Microbiol. 1988;26:236–243. doi: 10.1128/jcm.26.2.236-243.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim CJ, Jeong JK, Park M, et al. HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol Oncol. 2003;89:210–217. doi: 10.1016/s0090-8258(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 57.Seo SS, Song YS, Kim JW, et al. Good correlation of HPV DNA test between self-collected vaginal and clinician-collected cervical samples by the oligonucleotide microarray. Gynecol Oncol. 2006;102:67–73. doi: 10.1016/j.ygyno.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 58.Thomas J, Primeaux T. Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol. 2011 doi: 10.1016/j.anndiagpath.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Fischer CA, Kampmann M, Zlobec I, et al. p16 expression in oropharyngeal cancer: its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol. 2010;21:1961–1966. doi: 10.1093/annonc/mdq210. [DOI] [PubMed] [Google Scholar]
- 60.Harris SL, Thorne LB, Seaman WT, et al. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2011;33:1622–1627. doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]
- 61.Chau NG, Perez-Ordonez B, Zhang K, et al. The association between EGFR variant III, HPV, p16, c-MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol. 2011;3:11. doi: 10.1186/1758-3284-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ko V, Tambouret RH, Kuebler DL, et al. Human papillomavirus testing using hybrid capture II with SurePath collection: initial evaluation and longitudinal data provide clinical validation for this method. Cancer. 2006;108:468–474. doi: 10.1002/cncr.22285. [DOI] [PubMed] [Google Scholar]
- 63.Ginocchio CC, Barth D, Zhang F. Comparison of the Third Wave Invader human papillomavirus (HPV) assay and the digene HPV hybrid capture 2 assay for detection of high-risk HPV DNA. J Clin Microbiol. 2008;46:1641–1646. doi: 10.1128/JCM.01824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurtycz DF, Smith M, He R, et al. Comparison of methods trial for high-risk HPV. Diagn Cytopathol. 2010;38:104–108. doi: 10.1002/dc.21161. [DOI] [PubMed] [Google Scholar]
- 65.Steinau M, Swan DC, Unger ER. Type-specific reproducibility of the Roche linear array HPV genotyping test. J Clin Virol. 2008;42:412–414. doi: 10.1016/j.jcv.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Barzon L, Militello V, Lavezzo E, et al. Human papillomavirus genotyping by 454 next generation sequencing technology. J Clin Virol. 2011;52:93–97. doi: 10.1016/j.jcv.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Conway C, Chalkley R, High A, et al. Next-generation sequencing for simultaneous determination of human papillomavirus load, subtype, and associated genomic copy number changes in tumors. J Mol Diagn. 2012;14:104–111. doi: 10.1016/j.jmoldx.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Wang CC, Lai CH, Huang YT, et al. HPV genotypes predict survival benefits from concurrent chemotherapy and radiation therapy in advanced squamous cell carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2012;84:e499–506. doi: 10.1016/j.ijrobp.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 69.Lai CH, Chang CJ, Huang HJ, et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J Clin Oncol. 2007;25:3628–3634. doi: 10.1200/JCO.2007.11.2995. [DOI] [PubMed] [Google Scholar]
- 70.Goon PK, Stanley MA, Ebmeyer J, et al. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rieckmann T, Tribius S, Grob TJ, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 74.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 75.Gillison ML, Zhang Q, Jordan R, et al. Tobacco Smoking and Increased Risk of Death and Progression for Patients With p16-Positive and p16-Negative Oropharyngeal Cancer. Journal of Clinical Oncology. 2012 doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanna GJ, Haddad RI, Lorch JH. Induction chemotherapy for locoregionally advanced head and neck cancer: past, present, future? Oncologist. 2013;18:288–293. doi: 10.1634/theoncologist.2012-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 79.Vermorken J, Stohlmacher J, Oliner K, et al. 2011 Eurpoean Multidisciplinary Cancer Congress. Vol 47S2. European Journal of Cancer; Stockholm, Sweden: 2011. Safety and Efficacy of Panitumumab (pmab) in HPV Positive (+) and HPV Negative (−) Recurrent/metastatic (R/M) Squamous Cell Carcinoma of the Head and Neck (SCCHN): Analysis of the Phase 3 SPECTRUM Trial. p. 13. [Google Scholar]
- 80.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 82.Heusinkveld M, Goedemans R, Briet RJ, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. 2012;131:E74–85. doi: 10.1002/ijc.26497. [DOI] [PubMed] [Google Scholar]
- 83.Turksma A, Bontkes H, van den Heuvel H, et al. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2012 doi: 10.1111/odi.12037. [DOI] [PubMed] [Google Scholar]
- 84.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 85.Vermeer DW, Spanos WC, Vermeer PD, et al. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int J Cancer. 2013;133:120–129. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Overgaard J, Eriksen JG, Nordsmark M, et al. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005;6:757–764. doi: 10.1016/S1470-2045(05)70292-8. [DOI] [PubMed] [Google Scholar]
- 87.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol. 2010;94:30–35. doi: 10.1016/j.radonc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Mortensen LS, Johansen J, Kallehauge J, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 90.Jansen JF, Carlson DL, Lu Y, et al. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncol. 2012;48:717–722. doi: 10.1016/j.oraloncology.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]