Abstract
This study investigated the influence of aerobic capacity on the activation of the central serotonergic system and exercise fatigue in young men that ingested a selective serotonin reuptake inhibitor and were then subjected to moderate-intensity physical exercise. The maximal oxygen consumption of sixteen volunteers was measured during an incremental test. The volunteers were divided into two groups: subjects with higher (HAC) and lower (LAC) aerobic capacities. The volunteers were subjected to four experimental trials in which they ingested either placebo or paroxetine (10, 20 or 40 mg) and, 4.5 h later, cycled at 60% of their maximal power output until reaching fatigue. None of the three paroxetine doses influenced the total exercise time in the LAC group. However, for the HAC group, the time to fatigue in the 20 mg paroxetine condition was 15% less than that in the placebo condition (76.3 ± 5.1 min vs. 90.0 ± 7.9 min; p < 0.05). The time to fatigue was higher in the HAC group than in the LAC group for all treatments. Our results provide additional evidence that aerobic capacity modulates the activity of the serotonergic system. However, contrary to what would be expected considering previous reports, the activation of the serotonergic system in exercising subjects in the HAC group was not less than that in the LAC group.
Key points.
The physical performance of the higher aerobic capacity group after administration of 20 mg of paroxetine decreased relative to that after administration of the placebo, whereas the same dose of paroxetine had no effect in the lower aerobic capacity group.
Our results provide additional evidence that aerobic capacity modulates the activity of the serotonergic system.
Contrary to what would be expected considering previous reports, the present findings suggest that the activity of the serotonergic system during exercise is not attenuated in individuals with a higher aerobic capacity relative to those that have a lower aerobic capacity.
A dose-dependent effect of paroxetine on physical performance was not observed in either group; for example, in the subjects with higher aerobic capacity, 40 mg of paroxetine did not enhance or even reproduce the ergolytic effect caused by 20 mg of paroxetine.
None of the peripheral variables measured explain the reduced total exercise time after administration of 20 mg of paroxetine in the subjects with higher aerobic capacity.
Key words: Metabolism, performance, fatigue, serotonin, lactate, thermoregulation
Introduction
Fatigue is a common sensation experienced during exercise and is currently understood to be a protective mechanism that prevents severe disruption of an organism’s physical and physiological integrity (Ament and Verkerke, 2009; Noakes et al., 2004). Fatigue leads to either reduced power output or interruption of the exercise and can be modulated by, among other factors, exercise intensity/duration, nutritional status and physical training (Noakes et al., 2004; Blomstrand, 2006). The reduction in work capacity during prolonged physical exercise is considered to be a multi-factorial process (Noakes et al., 2004; Gandevia, 2001; Lambert et al., 2005) that includes a reduction of the central nervous system’s ability to recruit skeletal muscles (Nybo and Nielsen, 2001).
Fatigue during prolonged exercise has been associated with the activity of the monoaminergic system in the brain (Coimbra et al., 2012; Meeusen and Roelands, 2010). An elevated ratio of the concentration of serotonin (5-hydroxytryptamine; 5-HT) to that of dopamine in the brain may cause fatigue either by increasing lethargy or by inducing a loss in central drive/motivation (Meeusen et al., 2006). In running rats, a central injection of tryptophan, which is the amino acid precursor for 5-HT synthesis, increases the heat storage rate and decreases mechanical efficiency, thereby accelerating fatigue during submaximal exercise (Cordeiro et al., 2014; Soares et al., 2003; 2004). The contribution of serotonergic function to fatigue mechanisms has also been investigated using pharmacological tools in human studies (Bridge et al., 2003; Marvin et al., 1997; Meeusen et al. 1997; 2001; Roelands et al., 2009). However, some of the tested drugs, such as buspirone, are non-selective agonists of 5-HT receptors; therefore, caution is required when interpreting the outcomes from these studies based on exclusive changes in the serotonergic system.
In contrast to buspirone, paroxetine is a potent and selective inhibitor of the 5-HT transporter (Nemeroff and Owens, 2003). To date, only three studies (Struder et al., 1998; Strachan et al., 2004; Wilson and Maughan, 1992) have used paroxetine to first increase central serotonergic activity and then investigate the association between brain 5-HT and fatigue, and their conclusions are contradictory. Wilson and Maughan (1992) and Struder et al. (1998) showed that 20 mg of paroxetine reduced the total exercise time (TET) by 19% and 17%, respectively. In contrast, Strachan et al. (2004), using the same dose, did not observe any difference in the TET. Therefore, the evidence generated from human studies regarding the role of brain 5-HT in the modulation of fatigue is still limited and inconclusive. One of the hypotheses that we assessed in the present study was that the dose used in the published studies (i.e., 20 mg of paroxetine) was not sufficient to consistently influence the serotonergic system and, consequently, physical performance. Therefore, we investigated the effects that were induced on physical performance by a higher dose of paroxetine (40 mg).
Furthermore the response of 5-HT receptors may be influenced by the aerobic capacity of an individual. Previous studies have suggested that 5-HT receptors are down-regulated in endurance-trained individuals relative to non-endurance-trained individuals (Broocks et al., 1999; Jakeman et al., 1994). In contrast, Dywer and Flynn (2002) did not observe alterations in the sensitivity of 5-HT receptors in young men subjected to a short-term endurance training protocol. In addition to the contradictory findings, an important limitation of these studies (Broocks et al., 1999; Dywer and Flynn, 2002; Jakeman et al., 1994) is that the individuals were not exercising when they were challenged with the administration of 5-HT agonists. The sensitivity of 5-HT receptors during exercise was investigated in only one study (Dywer and Browning, 2000), which used experimental animals. Physical training decreased the ergolytic effect caused by the central administration of a 5-HT agonist, which suggests decreased sensitivity in trained animals. Because the evidence generated for a role of 5-HT in fatigue in rodents could not be consistently reproduced in human studies (Roelands et al., 2009), it is important to investigate whether the sensitivity of the 5-HT system is affected by aerobic capacity in exercising subjects.
Thus, in the present study, we investigated whether pre-existing differences in aerobic capacity modulate the response of the brain serotonergic system during cycling exercise. Our hypothesis was that individuals with higher aerobic capacities are less responsive to pharmacological activation of the serotonergic system and would, therefore, exhibit no changes on exercise performance induced by the ingestion of paroxetine. We also investigated whether the physical performance of young individuals subjected to moderate-intensity exercise is influenced by increasing doses of paroxetine.
Methods
Subjects
Sixteen healthy male young volunteers participated in this study. Although all the subjects were physically active, none of them had previous cycling experience or engaged in any training protocol for one year prior to the experiments. The volunteers were divided into two groups according their aerobic capacity: a group with eight volunteers who had the lowest aerobic capacity (LAC; 38 – 46 ml·kg-1·min-1 range) and another group with eight volunteers who had the highest aerobic capacity (HAC; 54 – 62 ml·kg-1.min-1 range). According to the Guidelines proposed by De Pauw et al. (2013), we could classify the LAC volunteers as Performance Level 1 subjects and the HAC volunteers as Performance Level 3 subjects. The age, anthropometric characteristics, maximal power output (MPO) and maximal oxygen uptake (VO2max) of the subjects are presented in Table 1.
Table 1.
Anthropometric and physiological characteristics of the subjects from both groups. Data are means (±SEM).
| Groups | Age (yr) |
Weight (kg) |
Height (m) |
Body fat (%) |
MPO (watt) |
HRmax (bpm) |
VO2max (ml·kg-1·min-1) |
|---|---|---|---|---|---|---|---|
| LAC (n = 8) | 24 (1) | 74.8 (3.1) | 1.76 (.03) | 18.2 (1.7) | 258.3 (13.2) | 189 (5) | 42.8 (0.9) |
| HAC (n = 8) | 24 (1) | 71.2 (3.3) | 1.80 (.02) | 9.4 (1.5) * | 294.8 (14.9) | 194 (2) | 57.3 (1.0) * |
* denotes a significant difference relative to the LAC group (p < 0.05).
This study was approved by the Ethics Committee of the Universidade Federal de Minas Gerais and conducted according to the standards described by the Brazilian National Health Council (Resolution 196/96) and by the Declaration of Helsinki (2008). All subjects provided informed written consent to participate in the experiments.
Procedures
Initial testing occurred one week before the first experimental trial and included body composition assessment and VO2max testing. The VO2max was measured during a protocol with continuous gas-exchange measurements (Cosmed K4b2, Rome, Italy) on an incremental cycle ergometer (Monark, model 824-E, Varberg, Sweden). Maximal heart rate (HRmax) and MPO were also recorded. HRmax was considered to be the HR at the voluntary termination of exercise. MPO was calculated using the equation proposed by Kuipers et al. (1985).
The percent body fat was calculated from estimations of the corporal density (Heyward and Stolarczyk, 1996), which, in turn, was estimated from three measurements of skin-fold thickness (Jackson and Pollock, 1978). Before participating in the study, each volunteer received recommendations to do the following: 1) avoid taking any medication throughout the period of the experiments; 2) abstain from alcohol, caffeine or heavy physical exercise, particularly with the inferior limbs, for 24 h before any experimental trial; and 3) record the dietary intake on the day before the first trial and replicate this intake on the day prior to the subsequent experimental trials.
Experimental design
During the experiments, the ambient temperature and relative humidity were controlled at 21.40 ± 0.03°C and 64.9 ± 0.4%, respectively. The order of the experimental trials was randomized and counterbalanced, and the trials were separated by at least 1 week. All experiments were conducted at the same time of day in a double-blind manner. Each volunteer was subjected to four exercise trials with the following drug conditions: placebo (PLA) and various doses of paroxetine (10, 20 and 40 mg). The placebo consisted of 20 mg of cellulose.
On the day of the experiments, the volunteers were instructed to consume a standardized breakfast (≈ 620 kcal) at 8:00 a.m. at home. Between breakfast and arrival at the laboratory, the volunteers were asked to not eat. They arrived at the lab at 11:00 a.m., and their left forearm vein was catheterized. The volunteers’ left hand and forearm were immersed in water at 42–44°C for 8 min to allow for arterialization of the venous blood (Hadjicharalambous et al., 2008). Following this procedure, a 21 G cannula was introduced into a superficial vein on the dorsal surface of the heated hand. The indwelling catheter was kept patent by flushing it with a small volume of heparinized saline after sampling. Then, the volunteers were allowed to rest in a supine position for 30 min, and the first blood sample was collected (8 ml) as follows: 4 ml in a tube containing NaF/K3-EDTA and 4 ml in a tube containing a coagulation activator. After this procedure, the indwelling catheter was withdrawn, and the volunteers ingested a capsule containing placebo/paroxetine at 12:00 p.m. and were taken to a cafeteria where they had lunch (≈ 850 kcal). Placebo and paroxetine were prepared in gelatin capsules that had similar appearance and stored in generic packs identified with a code. All the capsules were previously prepared by a pharmacist from another lab to ensure the double blind fashion of the present study.
After lunch, at 2:30 p.m., the volunteers returned to the laboratory and drank 500 ml of water to ensure that they were well hydrated before commencing exercise. At 3:00 p.m., the volunteers ate a standardized snack (≈ 490 kcal) and were subjected to the same previously described procedure for the insertion of the venous catheter. All of the meals were prescribed by a nutritionist. The volunteers then put on the experimental clothes (shorts, socks and tennis shoes) and inserted a disposable rectal thermistor (Measurement Specialties – 401 Series Medical Reusable Autoclavable, Hampton, VA, USA) 10 cm beyond the anal sphincter for measurements of the colonic temperature, an index of internal body temperature (Tint). Surface skin temperature probes (YSI, Yellow Springs, OH, USA) were attached to three sites (chest, arm and thigh), and a heart rate telemetry band was attached to the proper position (Polar S 120, Oulu, Finland). The mean skin temperature (Tskin) was determined using the equation proposed by Roberts et al. (1977). Because the peak paroxetine plasma concentrations are achieved within 3 to 8 hours after ingestion (Kaye et al., 1989), the volunteers started to cycle at 4:30 p.m. The cycling exercise consisted of maintaining a constant pedaling rate of 50 rpm until voluntarily termination the exercise at an intensity corresponding to 60% of the MPO attained during the incremental protocol. This fatigue protocol was selected to reproduce the methods used in the experiments of Wilson and Maughan (1992) and Strachan et al. (2004). The exercise was terminated at the moment when the subjects reached one of the following outcomes: could no longer maintain the pre-established intensity, rated 20 on Borg’s RPE scale or asked to stop cycling. All the subjects related no discomfort throughout the exercise trials.
The HR, Tint and Tskin were registered every 2 minutes throughout the exercise period. The rate of perceived exertion (RPE) was registered every 10 minutes, whereas blood samples were collected before the beginning of the exercise and every 15 minutes during the exercise. At the voluntary interruption of exercise, all parameters were registered, and blood samples were collected.
Blood handling and analysis
After homogenization, blood samples from the NaF/K3-EDTA tubes were refrigerated until centrifugation (3,600 rpm for 10 min at 4°C). Plasma aliquots were stored at -20°C for later measurement of the glucose and lactate concentrations. The lactate and glucose measurements were performed using the oxidase method (glucose analyzer 2300 StatPlus, YSI). Blood samples from the tubes containing the coagulation factor were allowed to clot and then centrifuged at 3,000 rpm for 10 min. Serum samples were collected, aliquoted and stored at -20°C for later measurement of the prolactin (PRL) concentrations. Serum PRL concentrations were analyzed using the chemiluminescence method (Advia Centaur, Bayer, Tarrytown, NY, USA) and were measured as a peripheral marker of central serotonergic activation. Analyses of the blood hemoglobin and hematocrit were used to calculate alterations of plasma volume (Dill and Costill, 1974). The concentrations of glucose, lactate and PRL were corrected for changes in plasma volume.
Calculations
The body surface area was determined using the equation proposed by Dubois and Dubois (1916). The heat storage rate (HSR) during the whole exercise (until the subjects reached fatigue) was calculated using the following equation:
where ΔTint = difference in Tint between the end and beginning of exercise, in °C; TET = total exercise time until the subjects were fatigued, in min; BSA = body surface area, in m2; m = body mass, in kg; and c = human body specific heat (3.48 kJ·kg-1. °C-1).
The sweat rate (SR) was calculated using the following equation:
where Δm = the difference between the final and initial body mass, in kg.
Statistical analyses
To evaluate the differences in HR, Tint, Tskin and plasma and serum parameters, three-way analyses of variance (ANOVAs; sources of variance: aerobic capacity, treatments and exercise time points) with repeated measures were used, followed by the post hoc Tukey test (coefficient of variation < 15%) or the Student-Newman-Keuls test (> 15%). The values of these parameters at the voluntary interruption of exercise were assessed using two-way ANOVAs (sources of variance: aerobic capacities and treatments) with repeated measures, followed by Tukey’s test. For comparing TET, HSR and SR between aerobic capacities and among treatments, two-way ANOVAs with repeated measures were used again, followed by Tukey’s test. For RPE, the only not-normally distributed data, a Friedman ranked test with repeated measures was used to compare intra-group differences, and a Mann-Whitney ranked test was used to compare inter-group differences. Pearson’s correlation coefficient was used to evaluate the relationship between PRL and TET. The effect size regarding the paroxetine-mediated decrease in physical performance (i.e., 20 mg of the drug in higher aerobic capacity individuals) was calculated by subtracting the TET after ingestion of paroxetine from the TET after ingestion of placebo and then dividing the result by a standard deviation for the data. All data are expressed as the mean ± S.E.M., except for the RPE data, which are expressed as the median. The significance level was set at p < 0.05.
Results
As shown in Table 1, no differences were observed between the two groups with regard to the subjects’ age, body mass, height, MPO and HRmax. As expected, the subjects from the HAC group presented VO2max values that were 34% higher than those in the LAC group. The volunteers with higher aerobic capacity also had lower estimated body fat content.
The TET after administration of paroxetine (any of the three doses) was not different from the placebo trial in the LAC group (Figure 1A). However, in the HAC group, 20 mg of paroxetine decreased the TET by 15% relative to that in the placebo trial (PLA = 90.0 ± 7.9 min versus 20 mg = 76.2 ± 5.1 min; p < 0.05), whereas the other two doses of paroxetine (i.e., 10 and 40 mg) did not affect physical performance (Figure 1B). Moreover, the TET was, on average, 80% higher in the HAC group than in the LAC group for each of the experimental trials (HAC: 83.5 ± 7.2 min versus LAC = 46.6 ± 5.7 min, pooled data, p < 0.001).
Figure 1.
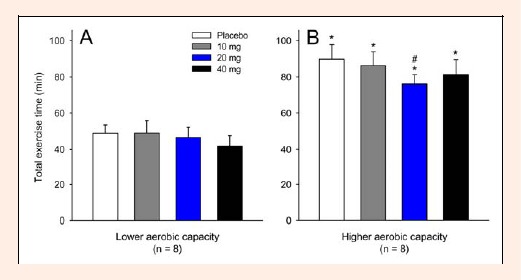
Total exercise time tolerated by the subjects with lower (A) and higher (B) aerobic capacity during cycling at 60% of their maximal power output. Each subject participated in four experimental trials with the following drug conditions: placebo and 10, 20 and 40 mg of paroxetine. The data are expressed as the mean ± SEM. * denotes significant differences relative to the respective control trial in the subjects with lower aerobic capacity (p < 0.05). # denotes a significant difference relative to the placebo trial (p < 0.05).
The Figure 2 shows the change in physical performance (%) caused by 20 mg of paroxetine relative to the placebo trial in each of the eight volunteers with higher aerobic capacities. Six from the eight subjects presented reduced performance, whereas only two presented increased performance after the ingestion of paroxetine. The effect size for the paroxetine-mediated decrease in TET corresponded to 0.73, which means a medium to large effect (Cohen, 1992). Taken together, these data indicate that the observed change in physical performance was not an experimental artifact.
Figure 2.
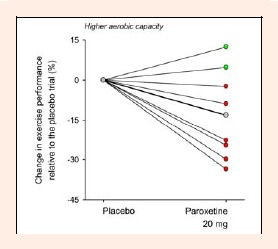
Individual changes in exercise performance between the placebo and 20 mg of paroxetine trials (expressed as percentage) in the volunteers with higher aerobic capacity. The thicker line and the gray symbols represent the mean change in exercise performance. The red symbols represent the volunteers who exhibited reduced physical performance, whereas the green symbols represent those who exhibited increased performance after the ingestion of paroxetine.
Slight and transient changes in the exercise-induced increase in Tint were observed after the treatment with paroxetine in both groups. In the LAC group, the volunteers had higher Tint after receiving 40 mg of paroxetine from the 2nd until the 16th min relative to the corresponding values for the 10 mg paroxetine trial (40 mg = 37.71 ± 0.13°C versus 10 mg = 37.59 ± 0.11°C at the 16th min; p < 0.05) and from the 8th until the 20th min relative to the corresponding values for the placebo trial (40 mg = 37.82 ± 0.12°C versus PLA = 37.68 ± 0.11°C at the 20th min; p < 0.05; Figure 3A). For the HAC group, the Tint in the 40 mg paroxetine trial was higher than that in the placebo trial from the 4th until the 20th min (40 mg = 37.88 ± 0.05°C versus PLA = 37.75 ± 0.07°C at the 20th min; p < 0.05; Figure 3B). At the end of exercise, the volunteers with higher aerobic capacity had higher Tint after receiving 10 and 40 mg of paroxetine than subjects with lower aerobic capacity (p < 0.05 for both comparisons). For both the placebo and 20 mg trials, the Tint values in the HAC group tended to be higher than those of the LAC group (p = 0.08 and 0.06 when the subjects reached fatigue in the placebo and 20 mg paroxetine trials, respectively).
Figure 3.
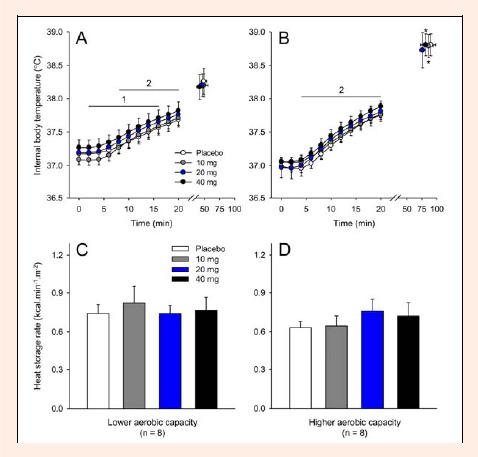
Internal body temperature recorded at rest, at 2-min intervals during exercise and at the voluntary termination of exercise by subjects with lower (A) and higher (B) aerobic capacity. During exercise, the internal temperature is presented until the 20th min, which represents the last time point at which all subjects were still cycling. The panels C and D show the cycling exercise-induced heat storage rate recorded in the subjects with lower and higher aerobic capacity, respectively. Each subject participated in four experimental trials with the following drug conditions: placebo and 10, 20 and 40 mg of paroxetine. The data are expressed as the mean ±SEM. 1 denotes that the results in the 40 mg paroxetine trial were significantly different from the results in the 10 mg paroxetine trial (p < 0.05). 2 denotes that the results in the 40 mg paroxetine trial were significantly different from the results in the placebo trial (p < 0.05). * denotes significant differences relative to the respective control trial in subjects with lower aerobic capacity (p < 0.05).
To compare the total thermal effects of exercise, the HSR was calculated. None of the three doses of paroxetine or the aerobic capacity influenced the exercise-induced HSR (Figure 3C and D). Similarly, no differences were observed in Tskin throughout the exercise among the experimental trials and between the groups with different aerobic capacities (Figure 4A and B). Regarding evaporative heat dissipation, none of the three doses of paroxetine changed the SR in either group. However, subjects with higher aerobic capacity presented higher SR after receiving 10 mg or 40 mg of paroxetine than subjects with lower aerobic capacity that received the same doses (10 mg: HAC = 8.0 ± 0.8 g·min-1·m-2 versus LAC = 4.8 ± 0.6 g·min-1·m-2, p < 0.01; 40 mg: HAC = 7.7 ± 0.5 g·min-1·m-2 versus LAC = 4.5 ± 0.4 g·min-1·m-2, p < 0.01; Figure 4C and D). For the placebo trial, the SR values in the HAC group tended to be higher than those in the LAC group (p = 0.06).
Figure 4.
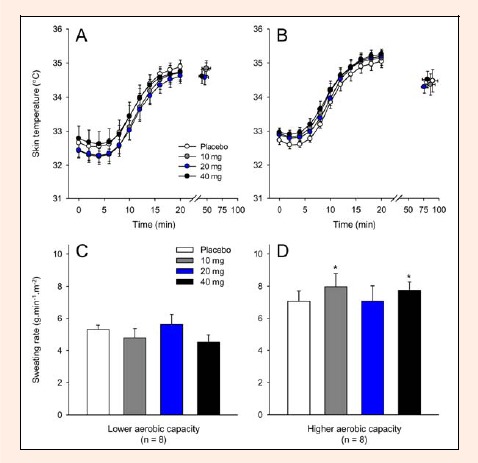
Skin temperature recorded at rest, at 2-min intervals during exercise and at the voluntary termination of exercise by subjects with lower (A) and higher (B) aerobic capacity. . The panels C and D show the cycling exercise-induced sweating rate recorded in the subjects with lower and higher aerobic capacity, respectively. Each subject participated in four experimental trials with the following drug conditions: placebo and 10, 20 and 40 mg of paroxetine. The data are expressed as the mean ±SEM. * denotes significant differences relative to the respective control trial in the subjects with lower aerobic capacity (p < 0.05).
Immediately before the exercise (at 0 min), the HR was higher in the 40 mg paroxetine trial than in the other experimental trials for both groups (PLA = 80 ± 5 bpm versus 10 mg = 85 ± 6 bpm versus 20 mg = 80 ± 4 bpm versus 40 mg = 91 ± 8 bpm; pooled data, p < 0.05). However, as the volunteers started cycling, no differences were observed in HR among the experimental trials or between the groups (Figure 5). When the volunteers fatigued, the average HR was 174 ± 4 beats·min-1.
Figure 5.
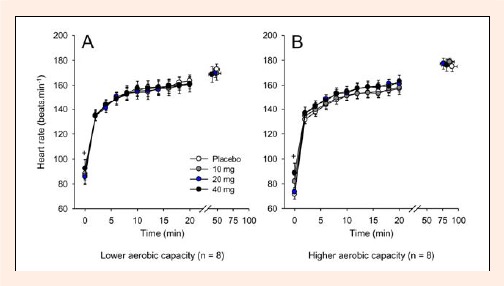
Heart rate recorded at rest, at 2-min intervals during exercise and at the voluntary termination of exercise by subjects with lower (A) and higher (B) aerobic capacity. Each subject participated in four experimental trials with the following drug conditions: placebo and 10, 20 and 40 mg of paroxetine. The data are expressed as the mean ±SEM. + denotes that the results of the 40 mg paroxetine trial were significantly different from those of the other experimental trials (p < 0.05).
In the LAC group, RPE was higher in the 40 mg paroxetine trial than in the 10 mg and placebo trials at the 10th and 20th min of cycling exercise (Figure 6A). In the HAC group, the administration of 20 mg of paroxetine reduced the RPE only at the 20th min relative to the corresponding value for the placebo trial (Figure 6B). Administration of 40 mg of paroxetine induced exaggerated increases in the RPE of subjects from the LAC group when compared to subjects in the HAC group, although the subjects were exercising at a similar intensity (Figure 6). When the volunteers fatigued, the median RPE values were 19 for every trial in both groups.
Figure 6.
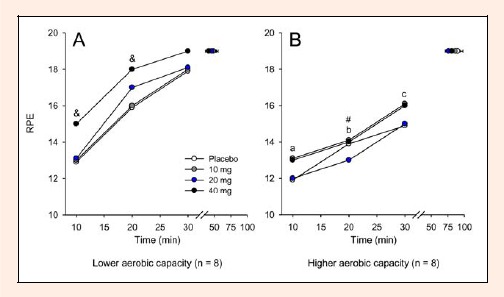
Rate of perceived exertion recorded at 10-min intervals during exercise and at the voluntary termination of exercise by subjects with lower (A) and higher (B) aerobic capacity. Each subject participated in four experimental trials with the following drug conditions: placebo and 10, 20 and 40 mg of paroxetine. The data are expressed as the median. & denotes that the results of the 40 mg paroxetine trial were significantly different from those of the 10 mg paroxetine and placebo trials (p < 0.05). # denotes that the results of the 20 mg paroxetine trial were significantly different from those of the placebo trial (p < 0.05). a denotes significant differences relative to the respective control trial in the subjects with lower aerobic capacity for the 40 mg paroxetine trial (p < 0.05). b denotes significant differences relative to the respective control trial in the subjects with lower aerobic capacity for the placebo, 20 mg paroxetine and 40 mg paroxetine trials (p < 0.05). c denotes significant differences relative to the respective control trial in subjects with lower aerobic capacity for all trials (p < 0.05).
Until the 30th min of cycling, none of the three paroxetine doses changed the exercise-induced increase in serum PRL concentration. Moreover, subjects from both groups had similar PRL concentrations during the first 30 minutes of exercise; however, at the interruption of exercise, the HAC group presented higher PRL concentrations than the LAC group for every experimental trial (PLA: HAC = 824.8 ± 84.5 ρmol·L-1 versus LAC = 664.0 ± 97.1 ρmol·L-1; 10 mg: HAC = 999.4 ± 153.2 ρmol·L-1 versus LAC = 477.6 ± 32.7 ρmol·L-1; 20 mg: HAC = 931.7 ± 192.6 ρmol.L-1 versus LAC = 445.9 ± 39.1 ρmol·L-1; 40 mg: HAC = 1026.1 ± 161.3 ρmol·L-1 versus LAC = 502.5 ± 54.7 ρmol·L-1, p < 0.05; Figure 7A and B). This enhanced prolactinemia most likely reflects the increased time to fatigue of the volunteers from the HAC group, as indicated by the correlation between serum PRL and TET (r = 0.52; p < 0.001; Figure 7C).
Figure 7.
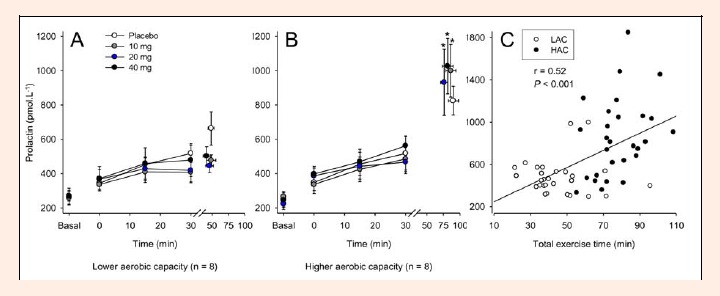
Serum prolactin concentrations recorded at rest, at 15-min intervals during exercise and at the voluntary termination of exercise by subjects with lower (A) and higher (B) aerobic capacity. Each subject participated in four experimental trials with the following drug conditions: placebo and 10, 20 and 40 mg of paroxetine. Panel C shows the correlation between serum prolactin concentrations and total exercise time. The data are expressed as the mean ±SEM (panels A and B) and as individual values (panel C). * denotes significant differences relative to the respective control trial in the subjects with lower aerobic capacity (p < 0.05). The reduced number of observations for the serum prolactin concentrations (n = 7) relative to the other analyses (n = 8) was the result of the removal of an outlier value in each group. This removal did not affect our major conclusions regarding the prolactin data.
Exercise induced a ≈10% reduction in plasma volume in all experimental trials, irrespective of the aerobic capacity of the volunteers (15th min = 9.82% ± 0.13; 30th min = 10.09% ± 0.02; fatigue = 8.82% ± 0.41, pooled data, p < 0.05). As expected, the plasma lactate concentrations increased (Figure 8A and B), whereas the glucose concentrations decreased during exercise (Figure 8C and D). Again, no differences for these metabolic parameters were observed between groups or among experimental trials while the subjects were exercising. Immediately before the exercise, the subjects from both groups had increased plasmatic glucose concentrations after receiving 40 mg of paroxetine relative to the corresponding values for the placebo and 10 mg paroxetine treatments (40 mg = 6.8 ± 0.6 mmol·L-1 versus PLA = 5.9 ± 0.3 mmol·L-1 versus 10 mg = 6.0 ± 0.5 mmol·L-1, pooled data, p < 0.05, Figure 8C and D).
Figure 8.
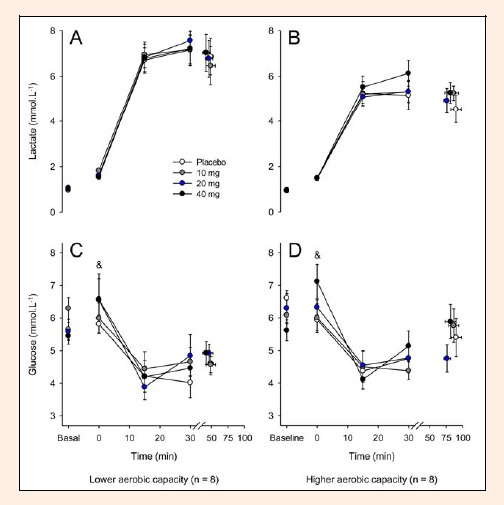
Plasma lactate and glucose concentrations recorded at rest, at 15-min intervals during exercise and at the voluntary termination of exercise by subjects with lower (A and C) and higher (B and D) aerobic capacity. Each subject participated in four experimental trials with the following drug conditions: placebo and 10, 20 and 40 mg of paroxetine. The data are expressed as the mean ±SEM. & denotes that the results of the 40 mg paroxetine trial were significantly different from those of the 10 mg of paroxetine and placebo trials (p < 0.05).
Discussion
The main finding of the present study was that the physical performance of the HAC group after administration of 20 mg of paroxetine decreased relative to that after administration of the placebo, whereas the same dose of paroxetine had no effect in the LAC group (Figure 1). Contradicting our hypothesis, these results suggest that individuals with higher aerobic capacity are more responsive to pharmacological activation of the serotonergic system during exercise. Another intriguing finding was that a dose-dependent effect of paroxetine on physical performance was not observed in either group; for example, in the subjects with higher aerobic capacity, 40 mg of paroxetine did not enhance or even reproduce the ergolytic effect caused by 20 mg of paroxetine.
In the present study, the reduced performance of subjects in the HAC group after administration of 20 mg of paroxetine was not associated with an exaggerated exercise-induced HSR or higher Tint at the end of exercise (Figure 3). Only the higher dose of paroxetine exaggerated exercise hyperthermia; however, this effect was short-lived, and no changes in the TET were evoked by 40 mg of paroxetine. In agreement with this notion that thermoregulation was not the reason for the decreased performance, autonomic thermoeffectors (i.e., cutaneous vasodilation, as estimated by Tskin, and sweating) were not different in the 20 mg paroxetine trial relative to the placebo trial (Figure 4).
As indicated by the inter-group comparisons, thermoregulation does not explain the differences in performance when comparing subjects with different aerobic capacities. Volunteers with higher aerobic capacity cycled, on average, 80% more during each trial, although their HSR and Tskin were not different from those of the volunteers with lower aerobic capacity (Figure 1 and 4). In both groups, the average Tint was below 39°C when the exercise was voluntarily interrupted (Figure 3), which suggests that the tested exercise intensity, combined with the ambient temperature conditions, may not have represented a significant strain on the thermoregulatory system. When the exercise was interrupted, Tint tended to be higher in the HAC group than in the LAC group for every experimental trial, which most likely reflects the greater time and distance covered by the volunteers with higher VO2max.
The responses of physiological systems other than the thermoregulatory system could explain the reduced performance after ingestion of 20 mg of paroxetine in the HAC group. Therefore, we indirectly investigated the availability of carbohydrates and the acid-basic balance during exercise by evaluating the plasma concentrations of glucose and lactate, respectively. No differences were observed among experimental trials or between groups in the plasma concentrations of glucose and lactate at fatigue (Figure 8), indicating that the differences in exercise performance cannot be attributed to changes in carbohydrate availability or acid-basic balance. Similarly, previous reports (Struder et al., 1998; Strachan et al., 2004; Wilson and Maughan, 1992) did not show any differences in glucose and lactate concentrations at the moment of fatigue for subjects who received 20 mg of paroxetine. These results support the hypothesis that the effects of paroxetine on performance are mainly associated with central mechanisms, such as sleepiness, fatigue, lethargy, drowsiness and loss of motivation.
Our hypothesis was that the administration of 20 mg of paroxetine would increase serotonergic activity, thereby reducing motivation and inducing lethargy in subjects from the HAC group. Nevertheless, despite the different TET between the 20 mg paroxetine and placebo trials in the HAC group, the RPE values were similar throughout the exercise period (Figure 6). A lack of association of changes in exercise performance with changes in the Borg RPE scale was previously observed (Roelands et al., 2008b, 2012; Watson et al., 2005). Taken together, these findings suggest that a given score on the Borg scale may also reflect changes in the motivation / drive to exercise induced by a pharmacological treatment (Roelands et al., 2012). Struder et al. (1998) used another scale designed to assess the momentary self-perceived state of the subjects during their investigation. This scale consisted of a list of 40 adjectives that could be categorized as “drive”, “confidence”, “mood” and “fatigue”. Despite the ergolytic effects of 20 mg of paroxetine, the self-perceived state measured immediately after the volitional fatigue was not influenced by the drug.
To date, few studies have investigated the relationship between the serotonergic system and fatigue by administering paroxetine, which is a pharmacological tool that increases the extracellular concentrations of 5-HT. Wilson and Maughan (1992) observed that the administration 20 mg of paroxetine decreased the TET in a temperate environment, whereas Strachan et al. (2004) reported that the same treatment failed to decrease performance in a warm environment. Struder et al. (1998), whose findings also demonstrated an ergolytic effect of 20 mg of paroxetine, did not describe the environmental condition at which their experiments were conducted. Because the subjects from the studies of Wilson and Maughan (1992) and Strachan et al. (2004) had average maximal oxygen consumption equivalent to that in our group with higher aerobic capacity, it is tempting to speculate that the effect of decreasing performance caused by paroxetine is dependent on the aerobic capacity and ambient temperature because the effect only occurred in subjects with high aerobic capacity tested under temperate conditions. A plausible hypothesis to explain the dependence of the paroxetine effects on ambient temperature is that in warm environments, other neurotransmitters, such as dopamine and noradrenaline, may play a more important role in fatigue than serotonin (Roelands et al., 2008a; 2008b; 2009; Coimbra et al., 2012).
We also addressed the hypothesis that the dose of paroxetine used in previous reports (Struder et al., 1998; Strachan et al., 2004; Wilson and Maughan, 1992) was not sufficiently high to clearly demonstrate the influence of the serotonergic system on fatigue. Our results show that the TET of the subjects from both groups did not change between the 40 mg paroxetine and placebo trials (Figure 1). The inability of the high dose of paroxetine to affect physical performance may be explained by the pharmacological profile of this agent. Selective serotonin reuptake inhibitors, including paroxetine, increase the extracellular concentrations of brain 5-HT, which can promote the release of dopamine through the stimulation of the 5-HT3 receptors (Nakayama, 2002). Considering that the paroxetine-mediated release of dopamine is dose-dependent (Nakayama, 2002), it is possible that the 5-HT3 receptors experienced greater stimulation from the 40 mg dose of paroxetine than from the 20 mg dose, thus increasing dopamine release into the synaptic cleft. Therefore, the ergogenic effect of increased brain dopaminergic activity (Roelands et al., 2008a) may have compensated for the ergolytic effects of increased serotonergic activity when the subjects were treated with 40 mg of paroxetine.
Paroxetine doses as high as 40 mg inhibit both 5-HT and norepinephrine transporters (Nemeroff and Owens, 2003). In the present study, there was evidence of enhanced noradrenergic activity after administration of 40 mg of paroxetine in both groups: immediately before exercise, the HR was higher than that in the other experimental trials (Figure 5), whereas the plasma glucose concentration was increased compared with that of the placebo and 10 mg paroxetine trials (Figure 7). Whether this increased noradrenergic activity has prevented the ergolytic effects of 5-HT is still questionable, because there is no consensus about the effects of noradrenergic activity on exercise performance: previous reports have report either no changes (Piacentini et al., 2002) or decreases in physical performance of trained cyclists at temperate ambient temperatures (Roelands et al., 2008b). Considering that 5-HT, dopamine and noradrenaline have different effects on physical performance, the TET after administration of 40 mg of paroxetine was most likely determined by a complex and, so far, not understandable interplay among these three brain monoamines.
The serum PRL was measured as a peripheral marker of brain serotonergic system activity. In the present study, administration of the three doses of paroxetine increased the serum PRL concentrations only in the HAC group and at the end of exercise (Figure 6A and B). However, caution is necessary when interpreting the serotonergic activity based on peripheral PRL measurements, because PRL release from the pituitary gland depends on a complex interaction among several neurotransmitters (Meeusen et al., 2006). PRL release can also be stimulated by many types of stress, including physical exercise (Freeman et al., 2000) and/or exercise-induced hyperthermia (Melin et al., 1988; Radomski et al., 1998). Our data showed that, when all of the experimental trials were analyzed together, the serum concentrations of PRL were directly associated with the TET (Figure 7C). Thus, we suggest that prolonged exertion of subjects with higher aerobic capacity led to increased secretion of PRL.
Administration of 20 mg of paroxetine reduced physical performance in subjects with higher aerobic capacity but not in those with lower aerobic capacity (Figure 1 and 2). Moreover, after administration of 10 mg, 20 mg or 40 mg of paroxetine but not after administration of the placebo, the LAC group had lower serum PRL concentrations than the HAC group when exercise was interrupted (Figure 7). This investigation is the first to show that aerobic capacity modulates the activity of the serotonergic system in exercising subjects. Thus, our results contradict previous findings that, under resting conditions, endurance-trained athletes present lower serotonergic sensitivity than non-endurance-trained controls, as indicated by the attenuated release of pituitary hormones when these subjects were challenged by the application of serotonergic agonists (Broocks et al., 1999; Jakeman et al., 1994). These contradictory findings may reflect the different methods used to evaluate the sensitivity of the 5-HT receptors (i.e., resting vs. exercising conditions and administration of agonists vs. reuptake inhibitors) and suggest that inferences regarding the activity of the serotonergic system during exercise should not be extrapolated from experiments performed under resting conditions.
In the present study, the subjects in the HAC group had a lower body fat content than those in the LAC group (Table 1). Because body composition can influence the biodistribution of drugs (Hanley et al., 2010), the higher body fat content may partially explain the lower sensitivity to paroxetine observed in volunteers from the LAC group. However, this hypothesis seems unlikely because more than 95% of circulating paroxetine and other selective serotonin reuptake inhibitors is bound to plasmatic proteins (Kaye et al., 1989). Therefore, less than 5% of the circulating paroxetine could be taken up by adipose tissue; this amount is likely insufficient to explain the different effects of this drug in both groups.
Although all subjects exercised at the same intensity [as evidenced by similar exercise-induced increases in the HR (Figure 5)], the TET was more prolonged in the HAC than in the LAC group during the placebo trial and for each of the paroxetine doses tested (Figure 1). Although we did not measure the lactate threshold, it is plausible that the intensity selected in our experiments was at or even above the lactate threshold in the LAC group, whereas the intensity was likely below the lactate threshold in the HAC group. This hypothesis is supported by the finding that the RPE at the 20th min was 2-3 points higher in the LAC group than in the HAC group (Figure 6).
Perspective
Identification of the mechanisms by which monoaminergic systems in the brain modulate fatigue during prolonged physical exercise may reveal important information regarding the optimization of physical training loads and regarding the athlete’s health because there is evidence that brain monoamines are involved in overtraining syndrome and in many degenerative diseases associated with aging. An interesting finding of the present study was that the individuals with a higher aerobic capacity demonstrated exaggerated activity of the serotonergic system during exercise relative to individuals with a lower aerobic capacity. Our experiments provide the first measurements of the sensitivity of the brain serotonergic system in exercising humans, in contrast to the measurements obtained during resting conditions in past investigations (Jakeman et al., 1994; Broocks et al., 1999). Considering that brain 5-HT is usually associated with decreased physical performance (Wilson and Maughan, 1992) or no changes at all (Roelands et al., 2009), our results are counterintuitive because they suggest increased sensitivity to 5-HT in individuals with higher conditioning. An interesting hypothesis to be tested in future studies is whether the sensitivity of the dopaminergic/noradrenergic systems also differs in exercising subjects with different aerobic capacities.
Conclusion
We therefore conclude that administration of paroxetine influences human exercise capacity, at least in subjects with a higher aerobic capacity. This result provides evidence for a role of the serotonergic system in the modulation of fatigue; however, the mechanisms underlying this relationship remain unclear. None of the peripheral variables measured explain the reduced TET after administration of 20 mg of paroxetine in the HAC group. Our findings also suggest that the activity of the serotonergic system during exercise is not attenuated in individuals with a higher aerobic capacity relative to those that have a lower aerobic capacity.
Acknowledgments
The authors are thankful to the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), to the National Council for Scientific and Technological Development (CNPq/Brazil) and to the Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais for financial support. Francisco Teixeira-Coelho was a recipient of a fellowship from the Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES / Brazil).
Biographies

Francisco TEIXEIRA-COELHO
Employment
Universidade Federal do Recôncavo da Bahia, Amargosa (BA), Brazil
Degree
MSc
Research interests
Exercise physiology, Aerobic training, Fatigue, Systemic inflammation and Thermoregulation
E-mail: coelhoft@gmail.com

João Paulo UENDELES-PINTO
Employment
Universidade Federal de Minas Gerais, Belo Horizonte (MG), Brazil
Degree
Bachelor in Physical Education
Research interests
Exercise physiology, Hydration, Oxidative stress, Physical performance and Thermoregulation
E-mail: jaoup@yahoo.com.br

Ana Cláudia Alves SERAFIM
Employment
Universidade Federal de Minas Gerais, Belo Horizonte (MG), Brazil
Degree
MSc
Research interests
Exercise physiology, Neuromuscular physiology and Resistance training
E-mail: anaclaudiaufmg@hotmail.com

Samuel Penna WANNER
Employment
Universidade Federal de Minas Gerais, Belo Horizonte (MG), Brazil
Degree
PhD
Research interests
Exercise physiology, Cardiovascular system, Fatigue, Systemic inflammation and Thermoregulation
E-mail: samuelwanner@eeffto.ufmg.br
Márcio Matos COELHO
Employment
Universidade Federal de Minas Gerais, Belo Horizonte (MG), Brazil
Degree
PhD
Research interests
Edema, Fever, Inflammation and Pain
E-mail: marciocoelhobh@yahoo.com.br

Danusa Dias SOARES
Employment
Universidade Federal de Minas Gerais, Belo Horizonte (MG), Brazil
Degree
PhD
Research interests
Exercise physiology, Mechanisms underlying exercise fatigue, Neurotransmitters and Thermoregulation
E-mail: danusa56@gmail.com
References
- Ament W., Verkerke G.J. (2009) Exercise and fatigue. Sports Medicine 39, 389-422 [DOI] [PubMed] [Google Scholar]
- Blomstrand E. (2006) A role for branched-chain amino acids in reducing central fatigue. The Journal of Nutrition 136, 544S-547S [DOI] [PubMed] [Google Scholar]
- Bridge M.W., Weller A.S., Rayson M., Jones D.A. (2003) Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. European Journal Applied Physiology 89,451-459 [DOI] [PubMed] [Google Scholar]
- Broocks A., Meyer T., George A., Hillmer-Vogel U., Meyer D., Bandelow B., Hajak G., Bartmann U., Gleiter C.H., Ruther E. (1999) Decreased neuroendocrine responses to meta-chlorophenylpiperazine (m-CPP) but normal responses to ipsapirone in marathon runners. Neuropsychopharmacology 20, 150-161 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1992) A power prime. Psychological Bulletin 112, 155-159 [DOI] [PubMed] [Google Scholar]
- Coimbra C.C., Soares D.D., Leite L.H.R. (2012) Involvement of brain monoamines on the onset of hyperthermic central fatigue.: An international perspective on topics in sports medicine and sports injury.: Kenneth R.Zaslav; Rijeka: Intech: 275-306 [Google Scholar]
- Cordeiro L.M.S., Guimarães J.B., Wanner S.P., La Guardia R.B., Miranda R.M., Marubayashi U., Soares D.D. (2014) Inhibition of tryptophan-hydroxylase abolishes fatigue induced by central tryptophan in exercising rats. Scandinavian Journal of Medicine and Science in Sports 24(1),80-88 [DOI] [PubMed] [Google Scholar]
- De Pauw K., Roelands B., Cheung S.S., de Geus B., Rietjens G., Meeusen R. (2013) Guidelines to Classify Subjects Groups in Sport-Science Research. International Journal of Sports Physiology and Performance 8, 111,122 [DOI] [PubMed] [Google Scholar]
- Dill D.B., Costill D.L. (1974) Calculation of percentage changes in volumes of blood, plasma and red cells in dehydration. Journal of Applied Physiology 37, 247-248 [DOI] [PubMed] [Google Scholar]
- Dubois D., Dubois E.F. (1916) A formula to estimate the approximate surface area if height and weight be known. Archives of Internal Medicine 17, 863-871 [Google Scholar]
- Dwyer D., Browning J. (2000) Endurance training in Wistar rats decreases receptor sensitivity to a serotonin agonist. Acta Physiologica Scandinavica 170, 211-216 [DOI] [PubMed] [Google Scholar]
- Dwyer D., Flynn J. (2002) Short term aerobic exercise training in young males does not alter sensitivity to a central serotonin agonist. Experimental Physiology 87, 83-89 [DOI] [PubMed] [Google Scholar]
- Freeman M.E., Kanyicska B., Lerant A., György N. (2000) Prolactin: structure, function, and regulation of secretion. Physiological Reviews 80, 1523-1631 [DOI] [PubMed] [Google Scholar]
- Gandevia S.C. (2001) Spinal and supraspinal factors in human muscle fatigue. Physiological Reviews 81, 1725-89 [DOI] [PubMed] [Google Scholar]
- Hadjicharalambous M., Kilduff L.P., Pitsiladis Y.P. (2008) Brain serotonin and dopamine modulators, perceptual responses and endurance performance during exercise in the heat following creatine supplementation. Journal of the International Society of Sports Nutrition 5:1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley M.J., Abemethy D.R., Greenblatt DJ. (2010) Effect of obesity on the pharmacokinetics of drugs in humans. Clinical Pharmacokinetics 49: 71-87 [DOI] [PubMed] [Google Scholar]
- Heyward V.H., Stolarczyk L.M. (1996) Applied body composition assessment. Champaign, IL: Human Kinetics [Google Scholar]
- Jackson A.S., Pollock M.L. (1978) Generalized equations for predicting body density for men. British Journal of Nutrition 40, 497-504 [DOI] [PubMed] [Google Scholar]
- Jakeman P.M., Hawthorne J.E., Maxwell S.R.J., Kendall M.J., Holder G. (1994) Evidence for downregulation of hypothalamic 5-hydroxytryptamine receptor function in endurance-trained athletes. Experimental Physiology 79, 461-464 [DOI] [PubMed] [Google Scholar]
- Kaye C.M., Haddock R.E., Langley P.F., Mellows G., Tasker T.C., Zussman B.D., Greb W.H. (1989) A review of the metabolism and pharmacokinetics of paroxetine in man. Acta Psychiatrica Scandinavica Supplementum 350,60-75 [DOI] [PubMed] [Google Scholar]
- Kuipers H., Verstappen F.T.J., Keizer H.A., Geurten P., Vankranenburg G. (1985) Variability of aerobic performance in the laboratory and its physiological correlates. International Journal of Sports Medicine 6, 197-201 [DOI] [PubMed] [Google Scholar]
- Lambert E.V., St Clair Gibson A., Noakes T.D. (2005) Complex systems model of fatigue: integrative homoeostatic control of peripheral physiological systems during exercise in humans. British Journal of Sports Medicine 39, 52-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin G., Sharma A., Aston W., Field C., Kendall M.J., Jones D.A. (1997) The effects of buspirone on perceived exertion and time to fatigue in man. Experimental Physiology 82, 1057-1060 [DOI] [PubMed] [Google Scholar]
- Melin B., Curé M., Pequignot J.M., Bittel J. (1988) Body temperature and plasma prolactin and norepinephrine relationships during exercise in a warm environment: effect of dehydration. European Journal of Applied Physiology Occupational Physiology 58,146-151 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Roeykens J., Magnus L., Keizer H., De Meirleir K. (1997) Endurance performance in humans: the effect of a dopamine precursor or a specific serotonin (5-HT2A/2C) antagonist. International Journal of Sports Medicine 18, 571-577 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Piacentini M.F., Van Den Eynde D., Magnus L., De Meirleir K. (2001) Exercise performance is not influenced by a 5-HT reuptake inhibitor. International Journal of Sports Medicine 22, 329-336 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Watson P., Hasegawa H., Roelands B., Piacentini M.F. (2006) Central fatigue – The serotonin hypothesis and beyond. Sports Medicine 36, 881-909 [DOI] [PubMed] [Google Scholar]
- Meuseen R., Roelands B. (2010) Central fatigue and neurotransmitters, can thermoregulation be manipuled? Scandinavian Journal of Medicine and Science in Sports 20, (Suppl.3), 19-28 [DOI] [PubMed] [Google Scholar]
- Nakayama K. (2002) Effect of paroxetine on extracellular serotonin and dopamine levels in the prefrontal cortex. Naunyn-Schmiedebergs Archives of Pharmacology 365, 102-105 [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B., Owens M.J. (2003) Neuropharmacology of Paroxetine. Psychopharmacology Bulletin 37(Suppl. 1), 8-18 [PubMed] [Google Scholar]
- Noakes T.D., St Clair Gibson A., Lambert E.V. (2004) From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans. British Journal of Sports Medicine 38, 511-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L., Nielsen B. (2001) Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. Journal of Applied Physiology 91, 2017-2023 [DOI] [PubMed] [Google Scholar]
- Piacentini M.F., Meeusen R., Buyse L., De Schutter G., Kempenaers F., Van Niivel J., De Meirleir K. (2002) No effect of a noradrenergic reuptake inhibitor on performance in trained cyclists. Medicine and Science in Sports and Exercise 34, 1189-1193 [DOI] [PubMed] [Google Scholar]
- Radomski M.W., Cross M., Buguet A. (1998) Exercise-induced hyperthermia and hormonal responses to exercise. Canadian Journal of Physiology and Pharmacology 76, 547-552 [DOI] [PubMed] [Google Scholar]
- Roberts M.F., Wenger C.B., Stolwijk J.A.J., Nadel E.R. (1977) Skin blood flow and sweating changes following exercise training and heat acclimatization. Journal of Applied Physiology 43, 133-137 [DOI] [PubMed] [Google Scholar]
- Roelands B., Hasegawa H., Watson P., Piacentini M.F., Buyse L., De Schutter G., Meeusen R. (2008a). The effects of acute dopamine reuptake inhibition on performance. Medicine and Science in Sports and Exercise 40, 879-885 [DOI] [PubMed] [Google Scholar]
- Roelands B., Goekint M., Heyman E., Piacentini M.F., Watson P., Hasegawa H., Buyse L., Pauwels F., De Schutter G., Meeusen R. (2008b) Acute norepinephrine reuptake inhibition decreases performance in normal and high ambient temperature. Journal of Applied Physiology 105, 206-212 [DOI] [PubMed] [Google Scholar]
- Roelands B., Goekint M., Buyse L., Pauwels F., De Schutter G., Piacentini M.F., Hasegawa H., Watson P., Meeusen R. (2009) Time trial performance in normal and high ambient temperature: is there a role for 5-HT? European Journal of Applied Physiology 107, 119-26 [DOI] [PubMed] [Google Scholar]
- Roelands B., Watson P., Cordery P., Decoster S., Debaste E., Maughan R., Meeusen R. (2012) A dopamine/noradrenaline reuptake inhibitor improves performance in the heat, but only at the maximum therapeutic dose. Scandinavian Journal of Medicine and Science in Sports 22, e93-e98. [DOI] [PubMed] [Google Scholar]
- Soares DD, Lima NRV, Coimbra CC, Marubayashi U. (2003). Evidence that tryptophan reduces mechanical efficiency and running performance in rats. Pharmacol Biochem Behav 74, 357-362 [DOI] [PubMed] [Google Scholar]
- Soares D.D., Lima N.R.V., Coimbra C.C., Marubayashi U. (2004) Intracerebroventricular tryptophan increases heating and heat storage rate in exercising rats. Pharmacology, Biochemistry, and Behavior 78, 255-261 [DOI] [PubMed] [Google Scholar]
- Strachan A.T., Leiper J.B., Maughan R.J. (2004) Paroxetine administration (failed) to influence human exercise capacity, perceived effort or hormone responses during prolonged exercise in a warm environment. Experimental Physiology 89, 657-664 [DOI] [PubMed] [Google Scholar]
- Struder H.K., Hollmann W., Platen P., Donike M., Gotzmann A., Weber K. (1998) Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Hormone and Metabolic Research 30, 188-194 [DOI] [PubMed] [Google Scholar]
- Watson P., Hasegawa H., Roelands B., Piacentini M.F., Looverie R., Meeusen R. (2005) Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. The Journal of Physiology 565, 873-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W.M., Maughan R.J. (1992) Evidence for a possible role of 5 hydroxytryptamine in the genesis of fatigue in man: administration of paroxetine, a 5-HT re-uptake inhibitor, reduces the capacity to perform prolonged exercise. Experimental Physiology 77, 921-924 [DOI] [PubMed] [Google Scholar]


