Abstract
This study investigated the occurrence of core muscle fatigue during high-intensity running exercise and its limitation to exercise performance. A secondary aim was to investigate whether respiratory muscle work performed during intense running periods, would contribute to core muscle fatigue. Nine male recreational runners were recruited for two reasons; (1) to perform a continuous treadmill run at 85% VO2max with and without core muscle fatigue in the CR_F and CR trials, respectively; and (2) to mimic the treadmill run-induced respiratory response recorded in the CR trial while subjects were free of whole-body exercise (Mimic trial). The changes in global core muscle function with fatigue in this study were evaluated by performing a sport-specific endurance plank test (SEPT), and the associated influence on running performance was examined by comparing the time to exhaustion during the treadmill run between the CR and CR_F trials. Subsequent to the treadmill run in the CR trial, SEPT (255.7 ± 85.3 vs 177.3 ± 80.6 s) was reduced from baseline in all runners. The reduction correlated (r = 0.67) with the concomitant decline in inspiratory muscle function revealed by maximal inspiratory mouth pressure (PImax: 151.3 ± 18.2 vs 133.3 ± 17.2 cmH2O, p < 0.05). In the Mimic trial, similar results in SEPT (212.3 ± 90.2 s), PImax (129.0 ± 26.7 cmH2O), and correlation (r = 0.77, p < 0.05) were observed following voluntary hyperpneic activity. With the preceded fatigued core muscle workout in the CR_F trial, the running capacity was impaired significantly (10.7 ± 4.5 vs 6.5 ± 2.0 min, p < 0.05). The impairment was correlated (r=0.72) to the SEPT reduction resulting from the workout. The results suggest that a high-intensity maximum run may induce core muscle fatigue in runners. The core muscle fatigue, which may be partly attributed to the corresponding respiratory work, may limit their running endurance. Inspiratory muscle function appears to be essential for core stabilization during the intense running.
Key points.
A high-intensity maximum run may induce core muscle fatigue in runners. The core muscle fatigue, which may be partly attributed to the corresponding respiratory work, may limit their running endurance.
In support of previous notion, inspiratory muscles may share the work of core stabilization during intense exercise, while simultaneously increasing the demand for breathing.
Inspiratory muscle training incorporated into a running specific-core training regime potentially enhances the training effect on the core muscles in a functional manner to deal with the challenges faced during intense exercise.
Key words: Core stability, muscle function, respiratory muscle, plank test
Introduction
It has been suggested that precise control of the trunk position and motion over the pelvis could optimize the energy transfer in the kinetic chains from torso to extremities for performing athletic activities composed of highly loaded movements (Kibler et al., 2006). Such specific bodily control in the exercising human depends on the outputs of the core muscles (CM) that mainly function at the lumbo-pelvic-hip region as well as the proximal lower limbs (e.g. external and internal obliques, rectus abdominis, erector spinae). The critical role of the CM in the kinetic chains had led to associations that CM function may limit the performance of athletes in sports activities, especially those performed with the body in an upright position, as observed in running. It had been further postulated that enhanced specific CM function could improve associated sports performance. However, such postulation has never been concluded well in previous studies (Stanton et al., 2004; Tse et al., 2005; Nesser et al., 2008; Sato and Mokha, 2009; Okada et al., 2011), partly due to unclearness of the roles that specific CM have in various sports performance (Hibbs et al. 2008). In fact, CM function has never been explored as a factor limiting performance capacity in runners. The fundamental questions (1) whether CM function would be changed with fatigue during intense running, and (2) whether the fatigue of the CM would impair the capacity of running exercise, have never been addressed. Such unclear scenarios have been ascribed to the lack of core test specifically designed for assessing sports-related core muscle function in athletes. Since core muscle load during various stability tests depends on the joint torques required to hold a specific posture, those core tests established in rehabilitation settings may not be accurate to reveal the functional capacity of the complex core anatomy that is specific to dynamic athletic performance (McGill et al., 2010). Recently, a sport-specific core muscle test developed by Mackenzie (2005) had been validated. The plank maneuver test interspersed with the alternate raising of the arms and legs challenges the trunk flexors and lumbar extensors in a manner that is similar to that occurring in performing sports movements. The test has been demonstrated to be a valid and reliable method, with adequate sensitivity to assess the change in global core muscle function with fatigue in athletes (Tong et al. 2014).
It has been reported that the tremendous ventilatory demand corresponding to ≥85% VO2max during continuous exercise would cause inspiratory muscle (IM) fatigue and associated exercise intolerance, while concomitant expiratory muscle fatigue is less likely to occur (Chevrolet et al., 1993; Dempsey et al., 2008; Ross et al., 2008; McConnell, 2011). From an anatomical view, both inspiratory and expiratory muscles in humans play a dual role in breathing and core stability during exercise. It was not known if the respiratory work during intense continuous running would contribute to the potential change in global CM function. The diaphragm, the major IM, has been shown to be activated during non-respiratory activities including power lifting and bicep curls (Al-bilbeisi and McCool, 2000). However, the interaction of CM and IM functions in core stabilization during intense continuous running is not clear. The purpose of this study were to investigate (1) the occurrence of CM fatigue and its limitation to exercise performance during continuous high-intensity running to exhaustion; and (2) whether the respiratory muscle work performed during intense running would contribute to the potential occurrence of CM fatigue. In this study, the new validated sport-specific endurance plank test would be used for examining the changes in global core muscle function with fatigue.
Methods
Subjects
Nine male recreational long-distance runners, who were asymptomatic for cardiorespiratory disease or lower back disorder, and engaged in regular long-distance running training with a lack of experience of regular CM / IM training, voluntarily participated in this study. Physical characteristics and athletic training background of the runners are shown in Table 1. After being fully informed of the experimental procedures and possible discomfort associated with the exercise test, subjects gave their written consent to participate. The local Ethical Committee for the Use of Human & Animal Subjects in Research provided ethical approval of the study.
Table 1.
Physical characteristics and sports training background of the runners (n=9).
| Variables | Mean (±SD) |
|---|---|
| Age (year) | 23.2 (6.4) |
| Height (m) | 1.70 (.03) |
| Weight (kg) | 58.9 (4.6) |
| FVC (l) | 4.2 (.4) |
| FEV1 (l) | 3.8 (.3) |
| FEV1 / FVC (%) | 90.9 (6.3) |
| 12-s MVV (l·min-1) | 184.6 (24.4) |
| VO2 max (ml·kg-1·min-1) | 65.0 (4.7) |
| VE max (l·min-1) | 145.2 (9.6) |
| HR max (beat·min-1) | 187.4 (9.7) |
| Experience of endurance running (years) | 6.9 (2.8) |
| Training frequency (sessions·week-1) | 4.4 (1.7) |
| Training duration (hrs·session-1) | 2.3 (.7) |
| Training distance (km·week-1) | 57.8 (17.2) |
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; 12-s
MVV, maximum voluntary ventilation measured in 12 s; VO2max, VEmax and HRmax, maximum oxygen uptake, minute ventilation and heart rate, respectively, recorded in maximum graded treadmill test.
Experimental design
Subjects were required to perform two trials of continuous runs on a treadmill at an intensity corresponding to 85% VO2max until volitional exhaustion. The first trial (CR trial) was to detect the occurrence of global CM and IM fatigue subsequent to intense running by comparing the results of post-exercise sport-specific endurance plank test (SEPT) and maximum inspiratory mouth pressure (PImax) measurement, respectively, with corresponding baseline values measured on separate days. The change in handgrip strength (HG), non-exercising muscle function, was also measured to evaluate if the potential decline in post-exercise SEPT and PImax was the result of reduced motivation or general whole-body fatigue (Ozkaplan et al., 2005). The measurements of HG, PImax and SEPT were performed in sequential order, and the sequence was identical in all trials. The second trial (CR_F trial) was to investigate if CM fatigue would limit running performance. The running time to exhaustion subsequent to a specific CM fatigued workout in the CR_F trial was compared with that without a preceded fatigued workout in the CR trial. The order of CR and CR_F trials were randomly assigned to subjects, with five performing the CR trial followed by the CR_F trial, with the rest of the subjects performing the trials in reverse order. To examine the independent contribution of respiratory muscle work, during intense running, to the occurrence of CM fatigue, a trial of voluntary isocapnic hyperpnea was performed. This was made possible by mimicking the ventilatory response recorded during the CR trial while subjects were free from whole-body exercise (Mimic trial). Post-hyperpnea SEPT performance was then compared with baseline values.
All trials were performed in an air-conditioned laboratory. Before each trial, the subjects refrained from eating for at least two hours, and from participation in strenuous physical activity for at least one day. All trials were scheduled to occur at the same time of day and were separated by a minimum of 3 days.
Procedures: Preliminary testing and familiarization trials
Table 2 shows the timeline of preliminary testing, familiarization and experimental trials. Prior to the experimental trials, physical characteristics, including lung function were measured. Following this, subjects were familiarized with the measurements of CM function, IM function, HG strength, and the treadmill run. This familiarization period utilized, the testing equipment, protocols, and provided the subjects with the sensation of exercising to exhaustion.
Table 2.
The timeline of preliminary testing, familiarization and experimental trials.
| 1st visit | Introduction and familiarization |
| ~ | |
| 2nd visit | Preliminary testing and familiarization |
| ~ | |
| 3rd visit | Graded treadmill running test |
| ~ | |
| 4th visit | Measurements of baseline HG, PImax and SEPT |
| ~ | |
| 5th visit | CR / CR_F trial |
| ~ | |
| 6th visit | CR / CR_F trial |
| ~ | |
| 7th visit | Mimic trial |
~ at least three days apart
The linear relationship between running speed and steady-state VO2 as well as aerobic capacity of the subjects were assessed by performing a standard graded treadmill testing protocol (Eston and Reilly, 2009) held on a separate day. Following the graded test, a running speed, which would elicit approximately 85% VO2max, was selected from the linear relationship of steady-state VO2 versus speed. The defined speed would be applied in the continuous intense running test during experimental trials.
Following the graded test, the measurements of baseline HG, PImax and SEPT were performed in sequential order in the laboratory on a separate day.
Experimental trials
CR trial: Following a standardized warm-up regime, subjects ran on the treadmill (h/p/cosmos, Pulsar, Germany) with gradient of 1% and at the speed corresponding to 85% VO2max until volitional exhaustion. During the test, respiratory responses were collected using Sensormedics metabolic measuring instrument (Vmax 229d, US). Heart rate (Polar HR monitor, Finland), and ratings of perceived exertion (Borg RPE scale 6-20) and of perceived breathlessness (Borg RPB scale 0-10), were collected every three minutes. Time to exhaustion during the test was interpreted as exercise capacity of the subjects. Immediately subsequent to the exercise, HG, PImax and SEPT were measured in sequential order.
CR_F trial: CM fatigue workout (Abt et al., 2007) was performed prior to the intense treadmill run. The workout involved four consecutive sets of exercise circuits consisting of (1) seated upper torso rotation with a 4-kg medicine ball, (2) static prone torso extension with a 4-kg medicine ball, (3) supine lower torso rotations with a 4-kg medicine ball, (4) sit-ups with a 10-lb dump-bell, (5) lateral side bends with a 10-lb dump-bell, and (6) rotating lumbar extensions with a 10-lb dump-bell. Each exercise was performed for 40 s followed by a 20-s recovery. Subsequent to the CM fatigue workout, HG, PImax and SEPT were measured immediately in sequential order. Afterwards, the subjects performed an intense treadmill run identical to that of CR trial until volitional exhaustion. During the test, respiratory and perceptual responses, as well as the time to exhaustion were recorded.
Mimic trial: In this trial, subjects performed voluntary isocapnic hyperpnea by mimicking the average VE, VT, fb and Ti/Ttot achieved over each minute of the running test in the CR trial while they were standing in an upright position, and free from whole-body exercise. The PETCO2 was closely supervised and maintained isocapnic (40 ± 2 mmHg) by adding CO2 to a flow-by stream of inspired air. The ventilatory data throughout the hyperpnea activity were monitored using the Sensormedics Vmax 229d instrument. HG, PImax and SEPT were measured in sequential order post-hyperpnea. Further, although expiratory muscle function is not likely to be changed with fatigue after endurance running (Ross et al. 2008), muscle function was evaluated by comparing the maximum static expiratory mouth pressure measured post-hyperpnea with their pre-exercise values.
Measurements: Sport-Specific Endurance Plank Test (SEPT)
The SEPT protocol, which has been shown to be valid and reliable in assessing athletes’ global CM function, has been previously described in detail (Tong et al., 2014). Briefly, subjects were required to maintain the prone bridge in a good form throughout the following stages with no rest in between: (1) hold the basic plank position for 60 sec; (2) lift the right arm off the ground and hold for 15 sec; (3) return the right arm to the ground and lift the left arm for 15 sec; (4) return the left arm to the ground and lift the right leg for 15 sec; (5) return the right leg to the ground and lift the left leg for 15 sec; (6) lift both the left leg and right arm from the ground and hold for 15 sec; (7) return the left leg and right arm to the ground, and lift both the right leg and left arm off the ground for 15 seconds; (8) return to the basic plank position for 30 seconds; (9) repeat the steps from (1) to (9) until the maintenance of the prone bridge failed.
In regard to the SEPT that subjects repeated with identical body posture, the distances between the left and right elbows (medial epicondyle), the left and right feet (1st metatarsal), and the elbow and feet on the left and right sides of the body were measured during the familiarization trial while the subjects were comfortably performing the prone bridge on a bench. Further, two elastic strings of ~80 cm length which were attached horizontally to a pair of vertical scales were placed beside the bench during the test. The two strings maintained at a distance of 10 cm were adjusted up and down until a height was reached that was at the same level as the subjects’ hip (the iliac crest was evenly in between the two strings). This setting acted as a reference for the objective monitoring of hip displacement during the test. The measured distances between elbows and feet, as well as the hip height, remained constant in subsequent experimental trials. During the test, the test administrator sat one meter away from the bench with the seat height adjusted to a level so that the hip displacement of the subjects could be monitored horizontally. The subjects were then asked to maintain the prone bridge throughout the test with maximum effort. For each time that the hip was beyond either of the reference lines, a warning would be given. The test would be terminated when the hip failed to be maintained at the required level after receiving two consecutive warnings. The measured time to exhaustion was used to reveal the subject’s global CM function.
Maximum mouth pressure measurements
IM function was assessed by measuring the PImax at nearly zero flow. This was measured by performing maximal inhalations at residual volume against an occluded rubber-scuba-type mouthpiece with a 1 mm orifice. Maximum expiratory mouth pressure was measured by performing maximal exhalations at total lung capacity as described above. The change in the mouth pressure was detected by a differential pressure transducer coupled with a signal conditioner (Collins, Braintree, MA, USA) during the maximal respiratory maneuvers. The subjects were required to repeat each measurement at least 5 times until the results were stable, and the highest value was recorded for analysis (Tong et al., 2010).
Handgrip strength tTest
Subjects remained in standing position, holding the T.K.K.5001 grip dynamometer (Takei, Japan) in their dominant hand, and griped with maximal effort. The median of the three highest values within a 5-kg range was recorded.
Statistical analyses
The Kolmogorov-Smirnov normality test revealed the data of all variables were normally distributed. Paired t-test was applied to examine the difference in variables between baseline and post-exercise measurements, and between CR and CR_F trials. ANOVA with repeated measures was computed to examine the difference in variables between selected time points, and across different trials. Post-hoc analyses using Newman-Keuls were performed when the main effects of ANOVA were significant. Relationships between variables were assessed using simple regression. All tests for statistical significance were standardized at an alpha level of p < 0.05. Data were expressed as mean ±SD.
Results
The occurrence of CM fatigue during high-intensity running
In the CR trial, the group mean of the speed of intense running was 15.9 ± 1.5 km·hr-1. The corresponding VO2 measured at the 5th min of the exercise was 54.6 ± 3.9 ml·kg-1·min-1, equivalent to 84.0 ± 3.3% VO2max. The time to exhaustion during the high-intensity running was 10.7 ± 4.5 min.
Table 3 shows the performance of SEPT, PImax and HG at baseline, and those measured in the CR, CR_F and Mimic trials. In the CR trial, the post-exercise SEPT and PImax performances decreased significantly from corresponding baseline values (p < 0.05). In contrast, HG strength did not change post-exercise (p > 0.05).
Table 3.
Changes in the performances of SEPT, PImax and HG by comparing with corresponding baseline values in CR, CR_F and Mimic trials (n=9). Data are means (±SD).
| Baseline | CR Trial Post-exercise |
CR_F Trial Post-CM workout |
Mimic Trial Post-hyperpnea |
|
|---|---|---|---|---|
| SEPT (sec) | 255.7 (85.3) | 177.3 (80.6) * | 174.6 (85.0) * | 212.3 (90.2) * |
| PImax (cmH2O) | 151.3 (18.2) | 133.3 (17.2) * | 135.1 (14.8) * | 129.0 (26.7) * |
| HG (kg) | 39.2 (7.1) | 39.2 (6.2) | 39.4 (4.9) | 39.6 (5.3) |
SEPT, sport-specific endurance plank test; PImax, maximum inspiratory mouth pressure; HG, handgrip strength
* Significantly different from corresponding baseline value (p < 0.05)
The limitation of CM fatigue to the intense running performance
Subsequent to the CM fatigue workout in the CR_F trial, SEPT performance and PImax, but not HG strength (Table 2), decreased from corresponding baseline values (p < 0.05). The time to exhaustion during intense running (6.5 ± 2.0 min) was also reduced significantly (p < 0.05) from that of the CR trial. Associated changes in cardio-respiratory and perceptual responses during the exercise are shown in Table 4.
Table 4.
Respiratory, metabolic and perceptual responses during exercise in the CR_F trial at exhaustion, in the CR trial at the iso-time point, and in the CR trial at exhaustion (n=9). Data are means (±SD).
| CR_F Trial at Exhaustion | CR Trial at Iso-time Point | CR Trial at Exhaustion | |
|---|---|---|---|
| VE (l·min-1) | 123.9 (14.2) | 120.2 (14.7) | 127.3 (16.0) |
| VT (l) | 2.01 (.42 | 2.07 (.42) | 2.06 (.42) |
| fb (breath·min-1) | 63.8 (12.0 | 60.3 (13.4) | 64.2 (13.2) |
| Ti/Ttot (%) | 51.0 (2.9 | 50.0 (3.8) | 50.3 (3.2) |
| VO2 (ml·kg-1·min-1) | 58.2 (5.8 | 58.3 (5.8) | 59.6 (4.1) |
| HR (beats·min-1) | 177.4 (6.8) * | 179.2 (7.9) * | 182.6 (7.6) |
| RPB | 8.7 (1.4) | 6.9 (1.4) * | 9.1 (.8) |
| RPE | 18.9 (1.3) | 16.6 (1.8) *† | 18.8 (1.2) |
VE, minute ventilation; VT, tidal volume; fb, breathing frequency; Ti/Ttot, duty cycle; VO2, oxygen consumption; HR, heart rate; RPB, rating of perceived breathlessness; RPE, rating of perceived exertion.
* Significantly different from the CR trial at exhaustion (p < 0.05).
† Significantly different from the CR_F trial at exhaustion (p < 0.05).
The contribution of respiratory work to the exercise-induced CM fatigue
The selected respiratory data at 50 and 100% exercise duration in the CR trial did not differ (p > 0.05) from those obtained at the iso-time points during the voluntary hyperpnea in the Mimic trial (Table 5), indicating that the respiratory responses during the CR trial were well mimicked in the Mimic trial. Subsequent to the hyperpnea activity, the SEPT performance and PImax (Table 2) decreased from corresponding baseline values with no change in the HG strength. For the maximum expiratory mouth pressure, the values of baseline (161.9 ± 28.3 cmH2O) and post-hyperpnea (155.0 ± 16.8 cmH2O) were not different.
Table 5.
Respiratory data recorded at 50% and 100% exercise duration in the CR trial and those recorded at the iso-time points in the Mimic trial (n = 9). Data are means (±SD).
| 50% | 100% | |||
|---|---|---|---|---|
| CR | Mimic | CR | Mimic | |
| VE (l.min-1) | 102.9 (10.1) | 101.4 (13.1) | 127.3 (16.0) | 122.0 (16.4) |
| VT (l) | 2.02 (.41) | 1.99 (.34) | 2.06 (.42) | 1.87 (.26) |
| fb (breath.min-1) | 53.4 (13.5) | 52.9 (11.7) | 64.2 (13.2) | 66.1 (8.6) |
| Ti/Ttot (%) | 49.8 (2.8) | 48.4 (5.4) | 50.3 (3.2) | 49.9 (2.7) |
VE, minute ventilation; VT, tidal volume; fb, breathing frequency; Ti/Ttot, duty cycle
The difference in all variables between CR and Mimic are not significant (p > 0.05)
Correlations
Significant inter-individual correlations among the changes (Δ), when expressed as percentage of either baseline or CR value, in time to exhaustion, SEPT, PImax, RPE, and RPB in different trials are shown in Table 6.
Table 6.
Significant correlations (p < 0.05) among the percentage change (Δ) in the variables (n = 9).
| ΔSEPT in CR Trial |
ΔSEPT in CR_F Trial |
ΔSEPT in Mimic Trial |
ΔRPE in CR_F Trial |
|
|---|---|---|---|---|
| Δ time to exhaustion in CR_F Trial | .79 | .72 | - | -.74 |
| ΔPImax in CR Trial | .67 | - | - | - |
| ΔPImax in Mimic Trial | .77 | - | ||
| ΔSEPT in CR_F Trial | -.69 | |||
| ΔRPB in CR_F Trial | .75 |
Discussion
CM fatigue and its limitation to the running performance
In the CR trial, the maximum SEPT performance was reduced by 30.7% from baseline after intense running, suggesting that exercise had led to CM fatigue in the runners. The fatigue was possibly local in nature, as there was no concurrent reduction observed in HG strength - the maximum force output of non-exercising muscles. Nevertheless, further interpretation of the change in SEPT performance may have limitations. In SEPT, CM function is assessed globally in a prone position, rather than the function of isolated CM, such as rectus abdominis, in an upright body position. Moreover, it is not clear whether the CM groups mainly loaded during the SEPT were identical to those recruited dominantly during the intense exercise in the CR trial for stabilizing the trunk and maintaining the running form. For evaluating the changes in the CM function resulting from the running exercise with the altered SEPT performance, the potential existing contradictions including the discrepancies in CM recruitment, and that in the trunk configuration and associated muscle length and angle of pull of various CM groups between these two maneuvers should be taken into account. Although the change in CM function as a consequence of intense running may not be quantified precisely with the altered SEPT performance, the current findings agree with the previous notion that CM is actively involved in providing core stiffness that helps stabilize running form, and maximize the kinetic chains of upper and lower extremity function during running activities (Borghuis et al., 2008; Kibler, 2006). It provides a clear answer to the very fundamental question of whether global CM function is being reduced with fatigue during intense running, and hence, suggests that the occurrence of CM fatigue may be a potential limiting factor in relation to running capacity.
To explore this further, we examined the influence of CM fatigue on intense running performance in CR_F trial. The application of fixed-intensity exercise undertaken to the limit of tolerance, rather than time trial which is more close to racing situation, in CR and CR_F trials is due to its good sensitivity to small performance change (McConnell, 2011). It also allows physiological and perceptual responses to be compared under identical conditions. Subsequent to a specific CM fatigue workout, SEPT performance was reduced by 31.8% with no change in HG strength. Regarding the running performance, a 39.2% decline was observed (Figure 1). The comparable VO2 and HR between the CR_F trial at exhaustion and the CR trial at the iso-time point (Table 4) indicated that the decline in exercise capacity was not the result of a flawed exercise intensity control. With CM fatigue, RPB and fb appeared to increase while VT reduced. Such changes in breathing pattern and breathlessness sensation have been shown to be associated with IM fatigue (Boutellier, 1998; Tong et al., 2001), and are associated with the reduction in PImax noted post-core workout. Despite the fact that IM fatigue could impair exercise capacity independently (McConnell, 2009), the mild changes in respiratory and perceptual variables inferred that IM fatigue may not be the only factor associated with the severe reduction in running performance in the CR_F trial.
Figure 1.
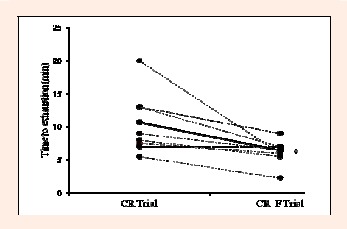
The changes in time to exhaustion of the intense running from CR trial to CR_F trial in all subjects (n = 9). Solid line indicates the average change. * Significantly different from the CR trial (p < 0.05)
Although the absolute exercise load between the CR_F and CR trials were identical, the rate of increase in the RPE in the CR_F trial was higher than that in the CR trial. Moreover, the changes in RPE and time to exhaustion during the intense running are inter-individual correlated to the reduction in SEPT resulting from the CM fatigue workout (r2 = 47.6-54.8%, Table 6). It is reasonable to postulate that the greater physical exertion perceived during exercise in the CR_F trial following the preceded CM fatigue might be, at least in part, due to the additional recruitment of motor units in active muscles compensating for the loss of CM output responsible for the maintenance of stable running form during intense running. As a result, neuromuscular input increased in active muscles, and eventually, early-onset of fatigue emerged. Based on the current findings, the occurrence of CM fatigue during intense running in the CR trial might have been involved in limiting the runners’ running capacity. However, it does not imply that CM function is one of the factors limiting the exercise capacity. To explore this, further research on the interrelationship between enhancement of CM function and the running capacity is required.
The contribution of respiratory work to exercise-induced CM fatigue
Table 5 shows that the respiratory responses in the CR trial were successfully simulated in the Mimic trial. Although the corresponding respiratory work during the two trials was not measured, similar work done by the respiratory muscles was assumed. Such isocapnic hyperpnea performed voluntarily by following the respiratory variables of VE, VT, fb and Ti/Ttot recorded during exercise has been applied in previous studies (Tong et al., 2003; 2004) to allow the participants to perform respiratory work equivalent to that of high-intensity intermittent exercise while free from whole-body exercise. Subsequent to the hyperpnea activity, IM fatigue revealed by the decreased PImax occurred, and was in line with the reduction in PImax post-exercise in the CR trial. On the other hand, the unaltered maximum expiratory pressure was in agreement with previous findings that intense endurance exercise was less likely to hinder muscles performing expiratory work (Ross et al., 2008). In fact, expiratory muscle fatigue seems to occur under conditions where exercise intensity is maximal, and where the propulsive force transmissions of the muscles are required, such as in rowing (McConnell, 2011).
Regarding CM function, the SEPT performance was reduced significantly post-hyperpnea, suggesting that the heavy respiratory work which induced IM fatigue during the intense running could independently lead to global CM fatigue in the runners. Moreover, the correlations between the ΔPImax and ΔSEPT (Figure 2, r2 > 45%) found in both CR and Mimic trials support the previous notion that IM plays a dual role in breathing and core stabilization during exercise (McConnell, 2011). It further implies that the limits in exercise capacity in association with heavy respiratory work, which have been well defined (McConnell, 2011), may be partly associated with the impairment of core stability. This suggestion has not been considered in previous work.
Figure 2.
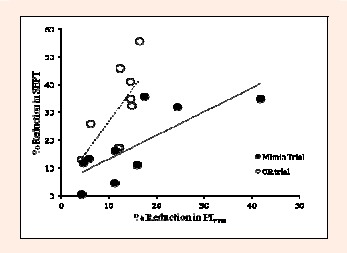
The % reduction in the sport-specific endurance plank test performance (SEPT) plotted against that in the maximum inspiratory mouth pressure (PImax) during the CR and Mimic trials are shown. Solid line is the regression of Mimic trial. Dotted line is the regression of CR trial.
Increase in IM function via specific IM training has been well-demonstrated to enhance one’s capacity in the performance of various types of exercise including swimming, cycling, endurance and high-intensity intermittent running (Romer, & Polkey, 2008; McConnell, 2009; Tong et al., 2008; 2010). The underlying mechanisms for the ergogenic effects include the attenuation of the IM metaboreflex-induced vasoconstriction in exercising limb muscles and the resultant reduced perception of limb discomfort (Romer and Polkey, 2008). It was also attributed to the direct attenuation of the exercise-induced breathless sensation (Tong et al., 2008; 2010). During intense exercise, IM may share the work of core stabilization that is required to overcome and control the potential posture perturbation, while simultaneously increasing the demand for breathing. Whether the ergogenic effects of specific IM training on the capacity of various exercise modalities could be attributed to the collateral enhancements of CM function and, in turn, core stabilization awaits further investigation.
In this study, we have demonstrated that intense running to exhaustion induces CM fatigue in endurance runners. The occurrence of CM fatigue during running exercise may be partly attributed to exercise-induced increase in respiratory work. Moreover, the reduction in CM function with fatigue in runners may limit their capacity to perform intense running. Although we did not demonstrate directly the limitations of CM function on endurance runners’ performance, the current findings outlining the occurrence of CM fatigue during intense running and its negative influence on runners’ endurance capacity provide a strong rationale of the essential role of core training in running performance. Moreover, the available evidence of the dual role of IM in breathing and core stabilization during intense running in the present study suggests that IM training incorporated into a running specific-core training regime potentially enhances the effectiveness of the core training in a functional manner to deal with the challenge faced during intense exercise. Further evidences of the synergetic effect of combined training on running performance await thorough experimental investigation.
Conclusion
In conclusion, CM function in endurance runners subsequent to intense running to exhaustion was impaired with fatigue. With the preceded CM fatigue workout, the endurance capacity for performing intense running was reduced. In mimicking the respiratory responses recorded during intense running while the runners were standing upright and free from whole-body exercise, CM function decreased.
Biographies
Tomas K. TONG
Employment
Department of Physical Education, Hong Kong Baptist University
Degree
PhD
Research interests
Exercise physiology, training
E-mail: tongkk@hkbu.edu.hk
Shing WU
Employment
Department of Physical Education, Hong Kong Baptist University
Degree
MPhil
Research interests
Exercise physiology
E-mail: kennyshingshing@gmail.com
Jinlei NIE
Employment
School of Physical Education and Sports, Macao Polytechnic Institute
Degree
PhD
Research interests
Exercise physiology, cardiovascular health
E-mail: jnie@ipm.edu.mo
Julien S. BAKER
Employment
Institute of Clinical Exercise and Health Sciences, University of the West of Scotland
Degree
PhD
Research interests
Exercise physiology, training
E-mail: jsbaker@uws.ac.uk
Hua LIN
Employment
Physical Education Department, Liaoning Normal University
Degree
PhD
Research interests
Exercise physiology, Public health
E-mail: wam3627459@163.com
References
- Abt J.P., Smoliga J.M., Brick M.J., Jolly J.T., Lephart S.M., Fu F.H. (2007) Relationship between cycling mechanics and core stability. Journal of Strength and Conditioning Research 21(4), 1300-1304 [DOI] [PubMed] [Google Scholar]
- Al-bilbeisi F., McCool F.D. (2000) Diaphragm recruitment during nonrespiratory activities. American Journal of Respiratory and Critical Care Medicine 162(2 Pt 1), 456-459 [DOI] [PubMed] [Google Scholar]
- Borghuis J., Hof A.L., Lemmink K.A.P.M. (2008) The importance of sensory-motor control in providing core stability. Sports Medicine 38(11), 893-916 [DOI] [PubMed] [Google Scholar]
- Boutellier U. (1998) Respiratory muscle fitness and exercise endurance in healthy humans. Medicine and Science in Sports & Exercise 30(7), 1169-1172 [DOI] [PubMed] [Google Scholar]
- Chevrolet J.C., Tschopp J.M., Blanc Y., Rochat T., Junod A.F. (1993) Alterations in inspiratory and leg muscle force and recovery pattern after a marathon. Medicine and Science in Sports and Exercise 25(4), 501-507 [PubMed] [Google Scholar]
- Dempsey J.A., McKenzie D.C., Haverkamp H.C., Eldridge M.W. (2008) Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest 134(3), 613-622 [DOI] [PubMed] [Google Scholar]
- Eston R., and Reilly T. (2009) Kinanthropometry and Exercise Physiology Laboratory Manual: Tests, Procedures and Data: Vol. 2. Physiology. 3rd edition Routledge, London [Google Scholar]
- Hibbs A.E., Thompson K.G., French D., Wrigley A., and Spears I. (2008) Optimizing performance by improving core stability and core strength. Sports Medicine 38(12), 995-1006 [DOI] [PubMed] [Google Scholar]
- Kibler W.B., Press J., and Sciascia A. (2006) The role of core stability in athletic function. Sports Medicine 36(3), 189-198 [DOI] [PubMed] [Google Scholar]
- Mackenzie B. (2005) 101 Performance Evaluation Tests. Electric Word plc, London [Google Scholar]
- McConnell A.K. (2011) Breathe Stronger, Perform Better. Human Kinetics, Champaign, IL [Google Scholar]
- McConnell A.K. (2009) Respiratory muscle training as an ergogenic aid. Journal of Exercise Science and Fitness 7(2, Suppl), 18-27 [Google Scholar]
- McGill S.M., Belore M., Crosby I., Russell C. (2010) Clinical tools to quantify torso flexion endurance: Normative data from student and firefighter populations. Occupational Ergonomics 9(1), 55-61 [Google Scholar]
- Nesser T.W., Huxel K.C., Tincher J.L., Okada T. (2008) The relationship between core stability and performance in division I football players. Journal of Strength and Conditioning Research 22(6), 1750-1754 [DOI] [PubMed] [Google Scholar]
- Okada T., Huxel K.C., Nesser T.W. (2011) Relationship between core stability, functional movement, and performance. Journal of Strength and Conditioning Research 25(1), 252-261 [DOI] [PubMed] [Google Scholar]
- Ozkaplan A., Rhodes E.C., Sheel A.W., Taunton J.E. (2005) A comparison of inspiratory muscle fatigue following maximal exercise in moderately trained males and females. European Journal of Applied Physiology 95(1), 52-56 [DOI] [PubMed] [Google Scholar]
- Romer L.M., Polkey M.I. (2008) Exercise-induced respiratory muscle fatigue: implications for performance. Journal of Applied Physiology 104(3), 879-888 [DOI] [PubMed] [Google Scholar]
- Ross E., Middleton N., Shave R., George K., McConnell A.K. (2008) Changes in respiratory muscle and lung function following marathon running in man. Journal of Sports Sciences 26(12), 1295-1301 [DOI] [PubMed] [Google Scholar]
- Sato K., Mokha M. (2009) Does core strength training influence running kinetics, lower-extremity stability, and 5000-m performance in runners? Journal of Strength and Conditioning Research 23(1), 133-140 [DOI] [PubMed] [Google Scholar]
- Stanton R., Reaburn P.R., Humphries B. (2004) The effect of short-term Swiss ball training on core stability and running economy. Journal of Strength and Conditioning Research 18(3), 522-528 [DOI] [PubMed] [Google Scholar]
- Tong T.K., Fu F.H., Chow B.C., Quach B., Lu K. (2003) Increased sensations of intensity of breathlessness impairs maintenance of intense intermittent exercise. European Journal of Applied Physiology 88(4-5), 370-379 [DOI] [PubMed] [Google Scholar]
- Tong T.K., Fu F.H., Chow B.C. (2001) Nostril dilatation increases capacity to sustain moderate exercise under nasal breathing condition. The Journal of Sports Medicine and Physical Fitness 41(4), 470-478 [PubMed] [Google Scholar]
- Tong T.K., Fu F.H., Chung P.K., Eston R., Lu K., Quach B., So R. (2008) The effect of inspiratory muscle training on high-intensity, intermittent running performance to exhaustion. Applied Physiology, Nutrition, and Metabolism 33(4), 671-681 [DOI] [PubMed] [Google Scholar]
- Tong T.K., Fu F.H., Eston R., Chung P.K., Quach B., Lu K. (2010) Chronic and acute inspiratory muscle loading augment the effect of a 6-week interval program on tolerance of high-intensity intermittent bouts of running. Journal of Strength and Conditioning Research 24(11), 3041-3048 [DOI] [PubMed] [Google Scholar]
- Tong T.K., Fu F.H., Quach B., Lu K. (2004) Reduced sensations of intensity of breathlessness enhances maintenance of intense intermittent exercise. European Journal of Applied Physiology 92(3), 275-284 [DOI] [PubMed] [Google Scholar]
- Tong T.K., Wu S., Nie J. (2014) Sport-specific endurance plank test for evaluation of global core muscle function. Physical Therapy in Sport 15(1), 58-63 [DOI] [PubMed] [Google Scholar]
- Tse M.A., McManus A.M., Masters R.S.W. (2005) Development and validation of a core endurance intervention program: Implications for performance in college-age rowers. Journal of Strength and Conditioning Research 19(3), 547-552 [DOI] [PubMed] [Google Scholar]


