Abstract
The objective of the study was to investigate the influence of serial administration of a carbohydrate (CHO) mouth rinse on performance, metabolic and perceptual responses during a cycle sprint. Twelve physically active males (mean (± SD) age: 23.1 (3.0) years, height: 1.83 (0.07) m, body mass (BM): 86.3 (13.5) kg) completed the following mouth rinse trials in a randomized, counterbalanced, double-blind fashion; 1. 8 x 5 second rinses with a 25 ml CHO (6% w/v maltodextrin) solution, 2. 8 x 5 second rinses with a 25 ml placebo (PLA) solution. Following mouth rinse administration, participants completed a 30 second sprint on a cycle ergometer against a 0.075 g·kg-1 BM resistance. Eight participants achieved a greater peak power output (PPO) in the CHO trial, resulting in a significantly greater PPO compared with PLA (13.51 ± 2.19 vs. 13.20 ± 2.14 W·kg-1, p < 0.05). Magnitude inference analysis reported a likely benefit (81% likelihood) of the CHO mouth rinse on PPO. In the CHO trial, mean power output (MPO) showed a trend for being greater in the first 5 seconds of the sprint and lower for the remainder of the sprint compared with the PLA trial (p > 0.05). No significant between-trials difference was reported for fatigue index, perceived exertion, arousal and nausea levels, or blood lactate and glucose concentrations. Serial administration of a CHO mouth rinse may significantly improve PPO during a cycle sprint. This improvement appears confined to the first 5 seconds of the sprint, and may come at a greater relative cost for the remainder of the sprint.
Key points.
The paper demonstrates that repeated administration of a carbohydrate mouth rinse can significantly improve peak power output during a single 30 second cycle sprint.
The ergogenic effect of the carbohydrate mouth rinse may relate to the duration of exposure of the oral cavity to the mouth rinse, and associated greater stimulation of oral carbohydrate receptors.
The significant increase in peak power output with the carbohydrate mouth rinse may come at a relative cost for the remainder of the sprint, evidenced by non-significantly lower mean power output and a greater fatigue index in the carbohydrate vs. placebo trial.
Serial administration of a carbohydrate mouth rinse may be beneficial for sprint athletes as a method of performance enhancement that minimizes the risk of performance decrement through body mass increase and gastrointestinal disturbances associated with ingesting carbohydrate solutions.
Key words: Anaerobic, exercise performance, exercise physiology, nutrition, physical performance
Introduction
The performance-enhancing effects of carbohydrate (CHO) ingestion during prolonged (≥1 hour) exercise are well documented (Jeukendrup, 2004). Mechanisms behind these effects have traditionally been attributed to metabolic influences such as sparing of endogenous muscle glycogen stores (Stellingwerff et al., 2007), and maintenance of blood glucose concentration as well as the rate of CHO oxidation in the later stages of exercise (Wilber and Moffatt, 1992). However, studies showing the efficacy of CHO ingestion during shorter duration exercise (≤ 1 hour; Anantaraman et al., 1995; Ball et al., 1995) indicate that CHO may also exert its effects via central mechanisms (Pottier et al., 2010), as endogenous CHO availability is generally not a limiting metabolic factor during exercise of this duration (Rollo et al., 2008).
The use of a CHO mouth rinse (rinsing a small volume of CHO solution around the oral cavity before expectorating it) has been shown to enhance performance during running and cycling lasting ~30-60 minutes (Carter et al., 2004; Chambers et al., 2009; Rollo et al., 2010). Functional magnetic resonance imagery (fMRI) studies demonstrate that introducing sweet and non-sweet carbohydrate into the oral cavity activates the primary and putative secondary taste cortices in the orbitofrontal cortex (Chambers et al., 2009; O’Doherty et al., 2001). Stimulation of these regions may also activate the dorsolateral prefrontal cortex, anterior cingulate cortex, ventral striatum, and anterior insula/frontal operculum (Chambers et al., 2009). These brain regions may control behavioural and autonomic responses to rewarding stimuli (Chambers et al., 2009; Rolls, 2007). In particular, the dorsolateral prefrontal cortex and the ventral striatum are thought to have a role in cognitive and attentional processing and motivation, respectively (Chambers et al., 2009; Kelley et al., 2002). These findings are suggestive of a non-metabolic mechanism of CHO efficacy, as CHO is not made systemically available when using a mouth rinse (Rollo et al., 2010). A non-metabolic mechanism is supported in a more ecologically valid way by observation of a significant increase in motor output and muscle force production immediately following the introduction of CHO into the oral cavity (Gant et al., 2010). Together, this work suggests that the presence of CHO in the mouth may stimulate oral CHO receptors that facilitate increased central drive and/or motivation, potentially improving exercise performance (Chambers et al., 2009). However, while oral taste receptors for sweet stimuli such as glucose have been identified (Rollo and Williams, 2011), potential receptors that may detect non-sweet CHO such as maltodextrin have not yet been documented (Chambers et al., 2009).
Increased central drive and/or motivation associated with CHO mouth rinses suggests their use may be beneficial in sports requiring high levels of central activation and motivation over a short time, such as sprinting. The only study to investigate the influence of a CHO mouth rinse on single sprint performance reported no significant influence of mouth rinsing on performance or metabolic responses to a 30 second cycle sprint (Chong et al., 2011). However, only a single administration of the mouth rinse was used immediately prior to the sprint, resulting in the oral cavity being exposed to the mouth rinse for ~10 seconds. Gant et al. (2010) reported significant increases in motor output and force production when the oral cavity was exposed to a CHO mouth rinse for 15-60 seconds. Other work reported a significant increase in plasma insulin concentration with an oral exposure time of 45 seconds (Just et al., 2008). Therefore, the duration of oral exposure to a CHO mouth rinse may significantly influence its efficacy (Rollo et al., 2010), perhaps by increasing stimulation of oral receptors (Sinclair et al., 2013). The influence of oral exposure time on CHO mouth rinse efficacy is supported by recent work suggesting a dose-response relationship to the duration of CHO mouth rinse exposure on 30 minutes self-paced cycling performance (Sinclair et al., 2013).
The aim of this study is to investigate the influence of serial administration of a CHO mouth rinse (cumulative oral exposure time 40 seconds) on performance, metabolic and perceptual responses to a 30 second cycle sprint. We hypothesized that serial administration of a CHO mouth rinse would significantly improve sprint performance compared with a placebo (PLA) mouth rinse.
Methods
Participants
Twelve physically active males volunteered for the study (mean (± SD) age: 23.1 (3.0) years, height: 1.83 (0.07) m, body mass (BM): 86.3 (13.5) kg). Participants were physically active and regularly undertook cycle ergometer sprinting as part of their normal physical activity routine. Participants were informed of the experimental procedures prior to providing written consent. The Abertay University Research Ethics Committee granted ethical approval, in line with the Helsinki Declaration.
Design
The study employed a repeated measures, counterbalanced, cross-over design with simple randomization of trial orders and double-blind administration of mouth rinses. Each participant attended the laboratory on 3 occasions separated by 3-7 days. Sessions took place between 9-11 am. The first visit was a familiarization of the full protocol, in line with recommendations for improving the reliability of performance testing (Hopkins et al., 2001). The session began by describing and explaining the rating of perceived exertion (RPE), felt arousal (FA; Svebak and Murgatroyd, 1985), and nausea scales (Chong et al., 2011), and anchoring each end of those scales. Thereafter, BM in kg (Seca Scales, Hamburg, Germany) wearing shorts only and height in m (Seca Stadiometer, Hamburg, Germany) were recorded. Participants were then fitted with a heart rate (HR) monitor (Polar S610i, Finland) and sat quietly for 10 minutes. Halfway through this period FA and nausea ratings were taken along with a capillary blood sample from the index finger of the right hand for the immediate quantification of blood lactate (LactatePro, Arkray Factory Inc, Shiga, Japan) and glucose (Freestyle, Warwickshire, UK) concentrations. At 6 minutes, participants were provided with 25 ml of water and asked to rinse it around their mouth for 5 seconds, and then expectorate it into a beaker. Subsequently, every 2 minutes until the beginning of the cycle sprint participants repeated the mouth rinse procedure (6, 8, 10, 12, 14, 16, 18, and 20 minutes; 200 ml water).
At the end of the 10 minute seated period RPE, FA, and nausea were recorded and a capillary blood sample taken. The participant then mounted the cycle ergometer (Monark Ergomedic 894E, Sweden) for a standardised warm up of 5 minutes at 60 rpm against a 1.5 kg resistance. This was immediately followed by three practice starts where the investigator provided a 3 second countdown after which the participant cycled maximally. The participant was given ~2 seconds to overcome the inertia on the flywheel before the investigator introduced a resistance equivalent to 0.075 g·kg-1 of pre-exercise BM and the participant continued to cycle maximally for 3 seconds. Immediately after, the load was removed and the participant cycled at 60 rpm for 45 seconds. This practice start was repeated two more times. Administration of the mouth rinse continued every 2 minutes during the warm up. Total warm up duration was 7 minutes.
On completion of the warm-up participants sat quietly for 3 minutes. After administration of the final mouth rinse participants remounted the cycle and pedalled at 60 rpm unloaded for 1 minute. The investigator provided a 3 second count down after which the participant cycled maximally. Upon reaching a cadence of 110 rpm the 0.075 g·kg-1 BM resistance was automatically added to the flywheel and the 30 second sprint began. Vigorous verbal encouragement was provided. Immediately on completion of the sprint, the resistance was reduced to 1.5 kg and the participant continued cycling at 50-60 rpm to facilitate recovery. Rating of perceived exertion, FA, and a capillary blood sample were taken on completion of the sprint. The participant continued to cycle for 5 minutes, after which all perceptual and metabolic measurements were taken again. The participant then transferred to a chair and sat quietly for a further 5 minutes, at the end of which perceptual and metabolic measures were taken a final time. The beaker containing the mouth rinses expectorate was weighed to quantify its volume and determine if any of the solution had been ingested. A schematic of the experimental protocol is in Figure 1.
Figure 1.
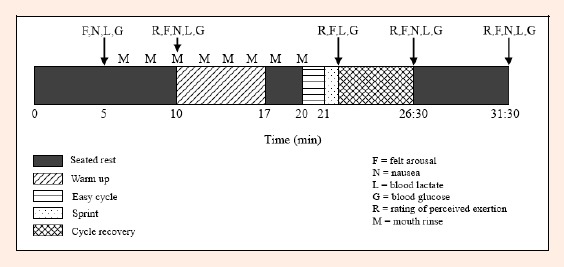
Schematic of the experimental protocol.
The cycle ergometer was attached to customised computer software (Monark Anaerobic Test v. 3.2.5.5, Sweden) allowing measurement of peak power output (PPO), mean power output (MPO) over the whole sprint (P0-30), 0-5 seconds (P0-5), 5-10 seconds (P5-10), 10-15 seconds (P10-15), 15-20 seconds (P15-20), 20-25 seconds (P20-25), and 25-30 seconds (P25-30) of the sprint, and fatigue index (FI), calculated as [(PPO-minimum power output) / PPO] x 100. Before leaving the laboratory, participants were provided with a food diary and asked to record all food and drink consumed, including portion sizes, for the 24 hour period before the next trial. They were requested to replicate this diet prior to their final trial. Participants were also requested to refrain from vigorous exercise or alcohol for at least 24 hours before each trial, to consume their breakfast meal 2 hours prior to the trial and then to consume only water for the 2 hour pre-trial period. Participants provided verbal confirmation of compliance to these procedures prior to each trial.
The experimental trials followed the same procedure as described above, the only difference being the mouth rinse that was provided. The experimental mouth rinse was a 6% (w/v) maltodextrin solution with a commercially available electrolyte tablet (HighFive, Bardon, Leicestershire) dissolved into the solution, yielding the following electrolyte composition per liter; sodium 250 mg, magnesium 60 mg, potassium 90 mg, calcium 20 mg. The electrolyte tablet contained a small amount of artificial sweetener (Saccharine) and was berry flavoured. The PLA solution comprised of one electrolyte tablet dissolved into 1 liter of water. Previous research using these solution formulations has demonstrated excellent blinding statistics (Phillips et al., 2010). An individual unrelated to the study prepared coded solutions, and only revealed the nature of the coding after data collection was completed.
Statistical analysis
The Shapiro-Wilk test assessed the distribution of all data sets. Learning effects for performance data were assessed using one-way repeated measures ANOVA. All sprint performance variables and analysis of expectorate volume were assessed using paired t-tests. Perceptual and metabolic measures were investigated using two-way (trial x time) repeated measures ANOVA. To examine significant main effects of time, planned repeated contrasts compared each time point with the previous time point. Mean ambient temperature and humidity were compared with a paired t-test and Wilcoxon matched-pairs test, respectively. Unless otherwise stated, data are mean (± SD) and significance is p = 0.05.
To investigate further the influence of the CHO mouth rinse on PPO, magnitude-based inference analysis was undertaken using published spreadsheets (Hopkins, 2003; Hopkins, 2007). Log transformation estimated the effect of the CHO mouth rinse as the difference in mean percentage change between the CHO and PLA trials (Hopkins, 2003). Inferences about the true (population) values of the effect of the CHO/ mouth rinse on PPO were made by expressing the uncertainty in the effect as 90% confidence limits and as likelihoods that the true value effect represents substantial change (harm or benefit; Hopkins, 2002; Stuart et al., 2005). The smallest worthwhile change in PPO was assumed to be 2% based on the observation that increases in performance with repeated testing generally do not substantially extend beyond the second trial (Hopkins et al., 2001), and that significant improvements in Wingate PPO of as little as 1.6% have been reported with experimental interventions in non-athletic samples (Bell et al., 2001). If the chance of benefit and harm were both >5% the true effect was reported as unclear. Otherwise, chances of benefit or harm were assessed as follows: <1%, almost certainly not; 1-5%, very unlikely; 5-25% unlikely; 25-75%, possible; 75-95%, likely; 95-99%, very likely; >99%, almost certain (Hopkins, 2007).
Results
There was no order effect for PPO across the three trials (familiarization, CHO, PLA; p > 0.05). Eight of the 12 participants achieved a higher PPO in the CHO trial (Figure 2A), resulting in a significant 2.3% greater PPO compared to the PLA trial (13.51 ± 2.19 vs. 13.20 ± 2.14 W.kg, p < 0.05; Figure 2B). Table 1 shows mean changes in PPO for the CHO and PLA trials, statistics for difference in these changes, and the qualitative magnitude inference of the changes. There were no significant differences in MPO or FI between trials (Table 2).
Figure 2.
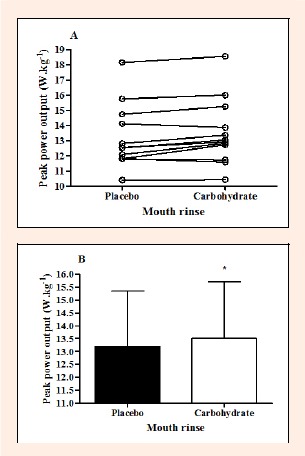
Individual peak power output (W·kg-1; A) and mean (± SD) peak power output (B) for the placebo and carbohydrate trials. * significantly greater than the placebo trial (p < 0.05). (n = 12).
Table 1.
Changes in peak power output in the carbohydrate (CHO) and placebo (PLA) trials and qualitative inferences about the effects on peak power output (n = 12).
| Change in Measure (%) | ||||
|---|---|---|---|---|
| CHO Trial (mean (± SD)) |
PLA Trial (mean (± SD)) |
Difference (±90% CL†) |
Qualitative Inference‡ |
|
| PPO | 55.6 (30.4) | 48.3 (29.3) | 7.3 ± 0.06 | Likely beneficial (81% likelihood) |
† 90% confidence limits.
‡ Based on the smallest worthwhile change in performance (2.0%)
Table 2.
Mean power output for the entire 30 second sprint, and for each 5 second segment, in the carbohydrate (CHO) and placebo (PLA) trials. Data are mean (± SD) (n = 12).
| Measurement | Trial | Mean Power Output (W·kg-1) |
|---|---|---|
| P0-30 |
CHO PLA |
8.74 (0.66) 8.78 (0.65) |
| P0-5 |
CHO PLA |
11.94 (1.52) 11.81 (1.47) |
| P5-10 |
CHO PLA |
9.60 (0.65) 9.67 (0.64) |
| P10-15 |
CHO PLA |
8.38 (0.73) 8.55 (0.66) |
| P15-20 |
CHO PLA |
7.63 (0.68) 7.69 (0.64) |
| P20-25 |
CHO PLA |
6.90 (0.69) 7.00 (0.61) |
| P25-30 |
CHO PLA |
6.22 (0.57) 6.29 (0.55) |
| Fatigue index (%) |
CHO PLA |
57.65 (6.65) 56.00 (6.33) |
All comparisons are non-significant (p > 0.05).
There were no significant between-trials differences for RPE, FA, nausea, blood lactate or glucose concentrations (p > 0.05; Table 3). However, RPE, FA, and blood lactate increased significantly with time (p < 0.05; Table 3). Nausea ratings and blood glucose concentration were not significantly different across time (p > 0.05; Table 3). Mean HR during the sprint was significantly greater in the PLA vs. CHO trial (147 (18) vs. 145 (18) bpm, p < 0.05).
Table 3.
Table 3. Perceptual and metabolic measures in the carbohydrate (CHO) and placebo (PLA) trials. Data are mean (± SD) (n = 12).
| Time | ||||||
|---|---|---|---|---|---|---|
| Pre-Exercise Seated Period (min) | Sprint | Post-Exercise Recovery Period (min) | ||||
| Variable | Trial | 5 | 10 | End | 5 | 10 |
| Rating of perceived exertion § |
CHO PLA |
--- --- |
6.0 (0) 6.0 (0) |
17.2 (2.4)ǁ 17.0 (2.4)ǁ |
8.4 (2.3)ǁ 8.2 (2.7)ǁ |
6.9 (1.6)ǁ 7.2 (2.1)ǁ |
| Felt arousal § |
CHO PLA |
1.4 (0.5) 1.4 (0.7) |
1.6 (0.7)ǁ 1.6 (0.7)ǁ |
4.5 (1.4)ǁ 4.7 (1.1)ǁ |
2.2 (1.3)ǁ 2.1 (1.2)ǁ |
1.8 (0.9)ǁ 1.8 (1.0)ǁ |
| Nausea |
CHO PLA |
5.0 (6.0) 7.0( 9.0) |
6.0 (9.0) 7.0 (9.0) |
--- --- |
13.0 (15.0) 16.0 (19.0) |
11.0 (12.0) 16.0 (26.0) |
| Blood lactate (mmol·L-1) § |
CHO PLA |
1.5 (0.5) 1.4 (0.4) |
1.4 (0.4) 1.5 (0.4) |
7.2 (3.6)ǁ 5.0 (1.4)ǁ |
11.1 (1.7)ǁ 10.9 (1.8)ǁ |
10.4 (2.3) 10.5 (2.5) |
| Blood glucose (mmol·L-1) |
CHO PLA |
4.6 (0.7) 5.0 (1.1) |
4.7 (0.5) 4.6 (0.7) |
4.4 (0.6) 4.6 (0.7) |
5.0 (0.8) 4.9 (0.6) |
4.9 (1.0) 4.8 (0.6) |
--- indicates data was not collected at that time point.
§ Significant main effect of time (p < 0.01).
ǁ Significantly different from prior time point (p < 0.01)
The mean volume of solution ingested in the CHO and PLA trials was 12 ± 6 and 12 ± 7 ml, respectively (p > 0.05). Mean ambient temperature and humidity were not significantly different between the CHO and PLA trials (19.2 (0.4) vs. 19.2 (0.4)°C and 31.0 (4.4) vs. 30.6 (3.9)%, respectively, p > 0.05).
Discussion
The results of this study suggest that serial administration of a CHO mouth rinse may significantly improve PPO during a maximal cycle sprint. No significant influence of the CHO mouth rinse was found for MPO, FI, or any physiological or perceptual measurements.
The significant increase in PPO in the current study contrasts with the finding of Chong et al. (2011). The current study employed a thorough familiarization trial, in line with published recommendations (Hopkins et al., 2001), and double-blinded solution administrations to minimise the possibility of a PLA effect, which has been a concern in previous mouth rinse studies (Carter et al., 2004). The absence of a learning effect for any performance variable supports the veracity of the familiarization. Furthermore, participants in the current study were experienced at cycle ergometer sprinting, and Hopkins et al. (2001) reported that learning/practice effects are similar for athletes and non-athletes. Therefore, it is unlikely that a learning/practice effect explains the results, even in this non-athletic sample.
The current study used the same Wingate protocol as Chong et al. (2011). The primary difference between the two studies is the mouth rinse administration. The serial administration paradigm was developed in pilot work as one that could be utilised by individuals in an externally-valid setting but that was also employable alongside the protocol used in this study. Repeated exposure of the oral cavity to the CHO mouth rinse may indicate a cumulative effect of a CHO mouth rinse on processes related to central drive, motivation, or motor output (Chambers et al., 2009; Gant et al., 2010) that enabled a significant increase in PPO in contrast to the single administration of Chong et al. (2011). This cumulative effect could be explained by the increased exposure time of the oral cavity to the mouth rinse (40 seconds in the current study compared with 10 seconds in Chong et al., 2011), and would support the contention that increased oral exposure time can facilitate the ergogenic effect of a CHO mouth rinse (Rollo et al., 2010; Sinclair et al., 2013). However, if a cumulative effect of CHO mouth rinsing did occur it did not manifest via increased sensations of arousal in the participants. It may be that the cumulative effect resided in other central alterations or because the FAS was not sensitive enough to detect alterations in arousal that may have occurred. Future work should test the veracity of mouth rinse oral exposure times using more objective measures of central activation such as electromyography.
Despite a statistically significant improvement in PPO with a CHO mouth rinse and magnitude inference analysis reinforcing the potential of the performance benefit, the observed improvement (2.3%) only just exceeded the smallest worthwhile improvement (2%). Therefore, while this study demonstrates the potential for an ergogenic effect of CHO mouth rinsing during sprinting, further research into optimising this effect is warranted. It would be interesting to conduct similar work with trained cyclists, as a performance enhancement of ~2% in that population would be meaningful. Based on the findings of this study and of Sinclair et al. (2013) during a 30 minute self-paced cycle, future work should consider focussing on the combined influence of duration of oral exposure to a CHO mouth rinse and the timing of mouth rinse use prior to sprinting in an attempt to develop the optimal pre-sprint mouth rinse strategy.
There was no significant effect of the CHO mouth rinse on MPO. However, it is interesting to note that MPO showed a trend for being greater in the CHO trial over the first 5 seconds of the sprint, but a trend for being greater in the PLA trial at all other time intervals. Similarly, FI was greater in the CHO vs. the PLA trial, although this was not significant. It therefore appears that the greater initial power in the CHO trial may come at a relative cost for the remainder of the sprint (Beaven et al., 2013). As a result, a CHO mouth rinse may have a shorter duration of influence during a maximal effort compared with prolonged submaximal exercise. In support, Beaven et al. (2013) reported that a CHO mouth rinse improved PPO only in the first of five 6 second cycle sprints, despite use of the mouth rinse prior to each sprint. A CHO mouth rinse may therefore exert a comparatively greater benefit during a shorter sprint than that used in the current study. The current study also agrees with the suggestion of Beaven et al. (2013) that the mechanisms behind improvements with CHO mouth rinsing during sprinting are likely central, as PPO was greater early in the sprint in the CHO trial, suggesting an ability of the CHO mouth rinse to improve performance when participants are in a non-fatigued state (the absence of significant phosphocreatine depletion).
The present study used a maltodextrin mouth rinse. There is no clear evidence for the presence of maltodextrin receptors in the oral cavity (Feigin et al., 1987). However, in their non-exercising fMRI studies Chambers et al. (2009) reported similar activation of brain regions associated with reward and motor control in response to glucose and maltodextrin mouth rinses. This suggests that the anatomy and function of human oral CHO receptors is not fully understood (Chambers et al., 2009) and that, based on the current study’s findings, maltodextrin CHO mouth rinses have the potential to promote central ergogenic responses. The digestion and absorption of CHO begins with salivary amylase secretion from salivary glands in the oral cavity (Butterworth et al., 2011), and it has been shown that within 5 minutes following introduction of a CHO solution into the oral cavity, a cephalic phase insulin release is observed (Just et al., 2008). Therefore, while oral CHO receptors may play a role in CHO mouth rinse efficacy, it is also worth considering the potential role of sublingual CHO absorption.
The glucose and maltodextrin solutions used in the imaging studies of Chambers et al. (2009) were 18% (w/v) concentration, and the fMRI scans were conducted at rest. Therefore, it is not possible to state that the central responses to these solutions would be replicated with a lower CHO concentration ([CHO]) during exercise. An avenue of future research is to investigate the possibility of a dose-response relationship between the [CHO] of a mouth rinse and sprint performance.
Participants took part in the study in a controlled post-prandial state. The influence of a CHO mouth rinse on performance may be reduced when participants are fed (Beelen et al., 2009; Fares and Kayser, 2011). Therefore, the ergogenic effect of the CHO mouth rinse may have been larger if participants had fasted longer than 2 hours prior to testing. However, participants in the study of Chong et al. (2011) performed an overnight fast yet no significant influence of the CHO mouth rinse was found. Furthermore, fasting prior to training or competition is not common practice; therefore the finding of the current study has greater external validity. However, it would be of benefit for future work to standardise further pre-exercise nutrition between participants to fully account for the potential influence of this variable on performance.
It has been suggested that mouth rinse research should incorporate a no mouth rinse trial along with a PLA trial, due to observation of a longer time to complete a cycle time trial when a water mouth rinse was used compared to no mouth rinse (Gam et al., 2013). Longer time to completion with use of the mouth rinse was attributed to interruption of the natural breathing cycle during exercise. A no mouth rinse trial was not used in the current study; however, participants did not use the mouth rinse during exercise. Therefore, the issues raised by Gam et al. (2013) are not relevant to this study.
Practical applications
The current study provides support for the use of a CHO mouth rinse prior to maximal-intensity cycle sprinting as a potential method for improving PPO. Sprint cycle athletes and coaches should be aware of this as a possible avenue of performance improvement. Use of a mouth rinse would also be of benefit to sprint athletes as it represents a possible method of performance enhancement without the drawback of ingesting a solution, which can lead to small increases in BM (potentially reducing power to weight ratio) and gastrointestinal disturbances, particularly during high-intensity exercise (Leiper et al., 2001).
Conclusion
We have demonstrated that serial administration of a CHO mouth rinse significantly improves PPO when performing a 30 second cycle sprint. The mouth rinse exerted no significant effect on MPO, FI or any of the physiological or perceptual measurements made. However, MPO was lower in the CHO trial from 5 seconds into the sprint, and FI was greater in the CHO trial. It therefore appears that the ergogenic effect of the CHO mouth rinse was confined to the first 5 seconds of the sprint, and that this effect came at a relative cost for the remainder of the sprint.
Acknowledgments
No financial support was provided for the completion of this project. The authors gratefully acknowledge Mr Rodrigo Aspe (University of Gloucester) for his assistance with participant recruitment and the participants for their time and effort.
Biographies

Shaun PHILLIPS
Employment
Abertay University.
Degree
PhD
Research interests
Fatigue mechanisms during exercise; perceptual regulation of exercise performance; nutritional interventions for improving performance.
E-mail: S.Phillips@abertay.ac.uk

Scott FINDLAY
Employment
Abertay University.
Degree
BSc (Hons)
Research interests
High intensity training and long distance endurance sports
E-mail: 1000766@live.abertay.ac.uk

Mykolas KAVALIAUSKAS
Employment
Abertay University.
Degree
MSc
Research interests
Psycho-physiological performance regulation in team sports; monitoring of training responses
E-mail: m.kavaliauskas@abertay.ac.uk

Marie Clare GRANT
Employment
Abertay University.
Degree
PhD
Research interests
Mechanisms of high intensity exercise performance; metabolic energy systems
E-mail: marieclare.grant@abertay.ac.uk
References
- Anantaraman R., Carmines A.A., Gaesser G.A., Weltman A. (1995) Effects of carbohydrate supplementation on performance during 1 hour of high-intensity exercise. International Journal of Sports Medicine 16, 461-465 [DOI] [PubMed] [Google Scholar]
- Ball T.C., Headley S.A., Vanderburgh P.M., Smith J.C. (1995) Periodic carbohydrate replacement during 50 min of high-intensity cycling improves subsequent sprint performance. International Journal of Sport Nutrition 5, 151-158 [DOI] [PubMed] [Google Scholar]
- Beaven C.M., Maulder P., Pooley A., Kilduff L., Cook C. (2013) Effects of caffeine and carbohydrate mouth rinses on repeated sprint performance. Applied Physiology, Nutrition and Metabolism 38, 633-637 [DOI] [PubMed] [Google Scholar]
- Beelen M., Berghuis J., Bonaparte B., Ballak S.B., Jeukendrup A.E., van Loon L.J. (2009) Carbohydrate mouth rinsing in the fed state: lacking of enhancement of time-trial performance. International Journal of Sport Nutrition and Exercise Metabolism 19, 400-409 [DOI] [PubMed] [Google Scholar]
- Bell D.G., Jacobs I., Ellerington K. (2001) Effect of caffeine and ephedrine ingestion on anaerobic exercise performance. Medicine and Science of Sports and Exercise 33, 1399-1403 [DOI] [PubMed] [Google Scholar]
- Butterworth P.J., Warren F.J., Ellis P.R. (2011) Human α-amylase and starch digestion: an interesting marriage. Starch 63, 395-405 [Google Scholar]
- Carter J.M., Jeukendrup AE., Jones D.A. (2004) The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Medicine and Science in Sports and Exercise 36, 2107-2111 [DOI] [PubMed] [Google Scholar]
- Chambers E.S., Bridge M.W., Jones D.A. (2009) Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. Journal of Physiology 587, 1779-1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong E., Guelfi K.J., Fournier P.A. (2011) Effect of a carbohydrate mouth rinse on maximal sprint performance in competitive male cyclists. Journal of Science and Medicine in Sport 14, 162-167 [DOI] [PubMed] [Google Scholar]
- Fares E.J.M., Kayser B. (2011) Carbohydrate mouth rinse effects on exercise capacity in pre- and postprandial states. Journal of Nutrition and Metabolism 385962, 1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin M.B., Sclafani A., Sunday S.R. (1987) Species differences in polysaccharide and sugar taste preferences. Neuroscience and Biobehavioural Reviews 11, 231-240 [DOI] [PubMed] [Google Scholar]
- Gam S., Guelfi K.J., Fournier P.A. (2013) Opposition of carbohydrate in a mouth-rinse solution to the detrimental effect of mouth rinsing during cycling time trials. International Journal of Sport Nutrition and Exercise Metabolism 23, 48-56 [DOI] [PubMed] [Google Scholar]
- Gant N., Stinear C.M., Byblow W.D. (2010) Carbohydrate in the mouth immediately facilitates motor output. Brain Research 1350, 151-158 [DOI] [PubMed] [Google Scholar]
- Hopkins W.G. (2002) Probabilities of clinical or practical significance. Sportscience 6. Available from URL: http://www.sportsci.org/jour/0201/wghprob.htm.
- Hopkins W.G. (2003) A spreadsheet for analysis of straightforward controlled trial. Sportscience 7. Available from URL: http://www.sportsci.org/jour/03/wghtrials.htm
- Hopkins W.G. (2007) A spreadsheet for deriving a confidence interval, mechanistic inference and clinical inference from a p value. Sportscience 11. Available from URL: http://www.sportsci.org/2007/wghinf.htm
- Hopkins W.G., Schabort E.J., Hawley J.A. (2001) Reliability of power in physical performance tests. Sports Medicine 31, 211-234 [DOI] [PubMed] [Google Scholar]
- Jeukendrup A.E. (2004) Carbohydrate intake during exercise and performance. Nutrition 20, 669-677 [DOI] [PubMed] [Google Scholar]
- Just T., Pau H.W., Engel U., Hummel T. (2008) Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 51, 622-627 [DOI] [PubMed] [Google Scholar]
- Kelley A.E., Bakshi V.P., Haber S.N., Steininger T.L., Will M.J., Zhang M. (2002) Opioid modulation of taste hedonics within the ventral striatum. Physiological Behaviour 76, 365-377 [DOI] [PubMed] [Google Scholar]
- Leiper J.B., Broad N.P., Maughan R.J. (2001) Effect of intermittent high-intensity exercise on gastric emptying in man. Medicine and Science in Sports and Exercise 33, 1270-1278 [DOI] [PubMed] [Google Scholar]
- O’Doherty J., Rolls E.T., Francis S., Bowtell R., and McGlone F. (2001) Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology 85, 1315-1321 [DOI] [PubMed] [Google Scholar]
- Phillips S.M., Turner A.P., Gray S., Sanderson M.F., Sproule J. (2010) Ingesting a 6% carbohydrate-electrolyte solution improves endurance capacity, but not sprint performance, during intermittent, high-intensity shuttle running in adolescent team games players aged 12–14 years. European Journal of Applied Physiology 109, 811-821 [DOI] [PubMed] [Google Scholar]
- Pottier A., Bouckaert J., Gilis W., Roels T., Derave W. (2010) Mouth rinse but not ingestion of a carbohydrate solution improves 1-h cycle time trial performance. Scandinavian Journal of Medicine and Science in Sports 20, 105-111 [DOI] [PubMed] [Google Scholar]
- Rollo I., Cole M., Miller R., Williams C. (2010) Influence of mouth rinsing a carbohydrate solution on 1-h running performance. Medicine and Science in Sports and Exercise 42, 798-804 [DOI] [PubMed] [Google Scholar]
- Rollo I, Williams C. (2011) Effect of mouth-rinsing carbohydrate solutions on endurance performance. Sports Medicine 41, 449-461 [DOI] [PubMed] [Google Scholar]
- Rollo I., Williams C., Gant N., Nute M. (2008) The influence of carbohydrate mouth rinse on self-selected speeds during a 30-min treadmill run. International Journal of Sport Nutrition and Exercise Metabolism 18, 585-600 [DOI] [PubMed] [Google Scholar]
- Rolls E.T. (2007) Sensory processing in the brain related to the control of food intake. Proceedings of the Nutrition Society 66, 96-112 [DOI] [PubMed] [Google Scholar]
- Sinclair J., Bottoms L., Flynn C., Bradley E., Alexander G., McCullagh S., Finn T., Hust T.H. (2013) The effect of different durations of carbohydrate mouth rinse on cycling performance. European Journal of Sport Science, DOI: 10.1080/17461391.2013.785599. [DOI] [PubMed] [Google Scholar]
- Stellingwerff T., Boon H., Gijsen A.P., Stegen J.H.C.H., Kuipers H., van Loon L.J.C. (2007) Carbohydrate supplementation during prolonged cycling spares muscle glycogen but does not affect intramyocellular lipid use. European Journal of Physiology 454, 635-647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G.R., Hopkins W.G., Cook C., Cairns S.P. (2005) Multiple Effects of Caffeine on Simulated High-Intensity Team-Sport Performance. Medicine and Science in Sports and Exercise 37, 1998-2005 [DOI] [PubMed] [Google Scholar]
- Svebak S., Murgatroyd S. (1985) Metamotivational dominance: a multimethod validation of reversal theory constructs. Journal of Personality and Social Psychology 48, 107-116 [Google Scholar]
- Wilber R.L., Moffatt R. (1992) Influence of carbohydrate ingestion on blood glucose and performance in runners. International Journal of Sport Nutrition 2, 317-327 [DOI] [PubMed] [Google Scholar]


