Abstract
In this issue of Blood, Kobayashi et al demonstrate that CD25, the α-chain of the interleukin-2 receptor (IL2RA), is expressed by certain cells that initiate chronic myeloid leukemia (CML) in a mouse model and that the IL-2/CD25 signaling axis supports CML development.1
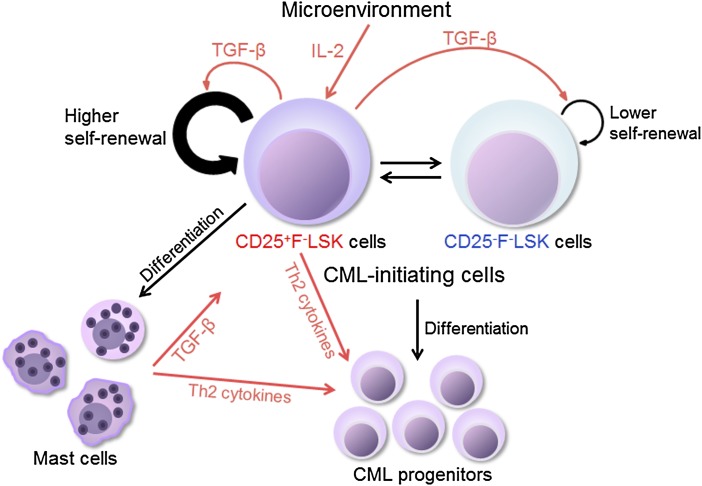
Proposed model indicating that distinct subsets of CML LICs are supported by the IL-2/CD25 axis. Both CD25+FcεRIα−Lin−Sca-1+c-Kit+ (F−LSK) cells and CD25−F−LSK cells possess CML LIC activities and can interconvert each other. CD25+F−LSK cells are also capable of differentiating into mast cells, and cycle faster than the CD25− counterpart. Microenvironment produced IL-2 can act on CD25+F−LSK cells, which further produce TGF-β to support self-renewal of both CD25+ and CD25− CML LICs. See supplemental Figure 16 in the article by Kobayashi et al that begins on page 2540.
CML is a hematopoietic malignancy that results from the t(9;22)(q34;q11) chromosome translocation, which produces the BCR/ABL fusion oncogene. Tyrosine kinase inhibitors (TKIs) effectively treat symptoms of CML patients, but the disease is not curable in most cases. It was proposed that leukemia-initiating cells (LICs) in these patients are insensitive to TKIs.2 Although CML LICs are thought to be counterparts of normal hematopoietic stem cells (HSCs),1 CML LICs have not yet been isolated as a homogenous population, and how these cancer stem cells interact with the microenvironment or niche is unclear.
Kobayashi et al have addressed these questions in a BCR/ABL-transducted CML-like myeloproliferative disease mouse model. They focused on study of the role of CD25 in CML LICs (see figure). CD25, as the α-chain of the IL-2R, forms a heterotrimeric high-affinity IL-2R together with the β- and γ-chains. CD25 was previously known to be mainly expressed on lymphocytes. An initial key finding of these authors is that CD25 is expressed on a subset of CML LICs but not on normal HSCs. Interestingly, they found that FcεRIα−Lin−Sca-1+c-Kit+ (F-LSK) cells that are positive or negative for CD25 possess CML LIC activities and that these CD25+ and CD25− populations can interconvert. Through gain-of-function and loss-of-function approaches, Kobayashi et al then demonstrated that IL-2, the ligand of CD25, acts together with CD25 to constitute a novel signaling axis to support CML LICs. Finally, the authors showed that CD25 is highly expressed on human CML CD34+CD38− cells and its expression positively correlated with disease progression based on an in silico analysis.
The new findings of Kobayashi et al have important biological implications. Consistent with the roles of other signaling pathways, including Wnt/β-catenin, Hedgehog, and IL-6,3,4 this study suggests that certain signalings that are dispensable in normal HSCs are activated in leukemia stem cells. Such signaling pathways may contribute to cancer initiation or progression and provide targets for therapeutics designed to eliminate cancer. Importantly, the study by Kobayashi et al revealed a novel, unique relationship between the niche and CML LICs mediated through the IL-2/CD25 signaling axis. Their analysis also indicates that the surface phenotype of CML LICs differs from that of normal HSCs, and suggests that CML LICs can be further purified. Furthermore, the authors showed that CML LICs contain both CD25+ and CD25− fractions. This is concordant with the suggestion that distinct populations of cancer stem cells may be able to exist in a single patient that enable the tumor to evolve in order to adapt to the dynamic tumor microenvironment.3
This study raises provocative questions regarding extrinsic signaling for leukemia stem cells. It was known that the signal transducer and activator of transcription (STAT) pathway is activated by BCR/ABL and that Janus kinase/STAT signaling is the pathway activated by cytokine receptors including IL-2R.5 Is IL-2/CD25 signaling in CML LICs independent of BCR/ABL-induced signaling? How does this axis, together with other extrinsic and intrinsic pathways (IL-6, Wnt, Hedgehog, bone morphogenetic proteins, selectin and ligands, transforming growth factor-β[TGF-β], Alox5, among others),3,4,6-8 regulate the cell fates of CML LICs such as self-renewal, differentiation, apoptosis, and migration? What are the IL-2+ niche cells and how do these cells contribute to the complex nature of the CML LIC niche? Given extensive aberrant signaling in CML cells, is it possible to cure CML by combined use of TKIs and inhibition of a limited number of key extrinsic pathways? Because CD25 is considered as a prognostic factor for the acute myeloid leukemia (AML) development,9,10 might the IL-2/CD25 axis be a niche signaling in other hematopoietic malignancies including AML?
An important clinically relevant question is whether CD25 is a marker for human CML LICs and what human population is the counterpart of mouse CD25+ F-LSK cells. The authors of the current study showed that CD25 expression is higher in the accelerated phase (AP) and even further increased in the blast crisis (BC) phase of human CML than in the chronic phase (Figure 6E, Kobayashi et al1). Because the myeloid BC in CML resembles AML, and CD25 is also considered a prognostic factor for AML development,9,10 an interesting hypothesis triggered by these observations is that CD25 may mark or promote human CML advancement in the AP or BC stage. If so, the IL2/CD25 axis may not regulate the initiation of human CML but may contribute to disease progression. Testing this hypothesis may shed light on the pathobiology of the currently intractable AP and early phase of BC of CML.6
Overall, Kobayashi et al demonstrate that the IL-2/CD25 signaling axis is a promising antileukemia target that is activated in CML LICs, but not in normal HSCs. Inhibition of the IL-2/CD25 axis may contribute to the development of future combination therapies that selectively target multiple key signaling pathways unique to CML LICs and ultimately eradicate CML.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Kobayashi CI, Takubo K, Kobayashi H, et al. The IL-2/CD25 axis maintains distinct subsets of chronic myeloid leukemia–initiating cells. Blood. 2014;123(16):2540–2549. doi: 10.1182/blood-2013-07-517847. [DOI] [PubMed] [Google Scholar]
- 2.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121(1):396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crews LA, Jamieson CH. Selective elimination of leukemia stem cells: hitting a moving target. Cancer Lett. 2013;338(1):15–22. doi: 10.1016/j.canlet.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Reynaud D, Pietras E, Barry-Holson K, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20(5):661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 6.O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer. 2012;12(8):513–526. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- 7.Krause DS, Lazarides K, Lewis JB, von Andrian UH, Van Etten RA. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood. 2014;123(9):1361–1371. doi: 10.1182/blood-2013-11-538694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laperrousaz B, Jeanpierre S, Sagorny K, et al. Primitive CML cell expansion relies on abnormal levels of BMPs provided by the niche and on BMPRIb overexpression. Blood. 2013;122(23):3767–3777. doi: 10.1182/blood-2013-05-501460. [DOI] [PubMed] [Google Scholar]
- 9.Gönen M, Sun Z, Figueroa ME, et al. CD25 expression status improves prognostic risk classification in AML independent of established biomarkers: ECOG phase 3 trial, E1900. Blood. 2012;120(11):2297–2306. doi: 10.1182/blood-2012-02-414425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerny J, Yu H, Ramanathan M, et al. Expression of CD25 independently predicts early treatment failure of acute myeloid leukaemia (AML). Br J Haematol. 2013;160(2):262–266. doi: 10.1111/bjh.12109. [DOI] [PubMed] [Google Scholar]