Abstract
Background:
The global health burden has faced toward non-communicable diseases (NCDs). It is suggested that adulthood blood pressure (BP) is tracked from childhood. This study aims to evaluate the mean BP and the prevalence of prehypertension and hypertension in the Iranian pediatric population.
Methods:
In a national survey as the 4th phase of Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable diseases study and through random multistage cluster sampling, a national sample of Iranian school students, aged 6-18 years, were recruited. Data gathered by means of modified World Health Organization Global school-based student health survey questionnaire, a weight disorders determinants questionnaire and anthropometric and BP measurements. Prehypertension (HTN) was defined as BP equal or greater than 90th age and sex specific percentile or ≥120/80 mmHg and HTN was defined as BP ≥95th percentile.
Results:
A total of 13486 students entered the study (49.2% girls, 75.6% urban). Mean age of participants was 11.47 ± 3.36 years. A total rate of 4.17% (3.84-4.52 95% CI) for high systolic BP (SBP), 4.33% (3.99-4.68) for high diastolic BP (DBP) and 6.88% (6.45-7.32) for high SBP and/or DBP was depicted.
Conclusions:
The prevalence rate of high BP (pre-HTN together with HTN) is substantially high in this population. It is needed to study the causative situations and implement relevant interventions.
Keywords: Adolescent, blood pressure, child, hypertension, Iran, pre-hypertension
INTRODUCTION
High blood pressure (HBP) is the top global disease burden risk factor.[1] The action plan of the World Health Organization (WHO) for prevention and control of non-communicable diseases (NCDs) in 2013-2020 has aimed a 25% relative reduction in the prevalence of HBP.[2]
Research derived evidences have proposed the idea of developmental origins of health and disease.[3] In addition to the effects of fetal programming, several factors determine the blood pressure (BP) level in childhood, which is associated with the adult BP level through the “tracking” phenomenon. Hence, the prevention of NCDs, namely cardiovascular diseases in adulthood should begin from childhood.[4,5,6,7] In addition, HBP in childhood may induce target organ damages, such as left ventricular hypertrophy, thickening of the carotid vessel wall, retinal vascular changes, subtle cognitive changes,[8] and even premature development of atherosclerosis.[9]
Prevalence of HBP is increasing in the pediatric population in accordance with the childhood obesity epidemic and life-style widespread changes.[8] Alarming data exist on the considerable prevalence of childhood overweight and its metabolic consequences, not only in industrialized countries, but also in developing countries.[10] The strong relationship of even early stages of HBP (pre-hypertension [HTN], pre HTN) with obesity and environmental factors, as air pollution, noise pollution and passive smoking suggest that its prevalence will be escalating.[11] In the transitional state communities with rapid urbanization, most risk factors identified for pediatric HBP are modifiable by life-style change, moreover monitoring of their time-trend is of significant importance.[12,13]
Iran is a developing country of the Middle East and North Africa region. The country health burden is facing from communicable diseases to NCDs and road accidents.[14] NCDs have a remarkable prevalence among Iranian adults,[15,16] and the risk factors of NCDs are quite prevalent in Iranian children and adolescents.[17,18,19] High systolic blood pressure (SBP) has been recognized responsible for most deaths in all regions of Iran.[20] Limited experience exists on evaluating NCDs risk factors such as HBP in Iranian pediatric population at national level. Considering the aforementioned importance of pediatric health status and inspired by WHO recommendations, a national ongoing school based surveillance system for health risk and protective factors named Childhood and Adolescence Surveillance and PreventIon of Adult Non-communicable diseases (CASPIAN) study was established in Iran. The current study aims to determine the BP mean and percentiles, as well as and the prevalence of HBP in a nationally representative sample of Iranian children and adolescents.
METHODS
Study design, population and sampling
CASPIAN study is a school-based surveillance program.[21,22] It has been conducted in four succeeding phases from 2003 to 2012.[17,23,24,25] We have previously described the methods of the 4th phase in details,[25] and here we present it in brief.
CASPIAN-IV study is a national cross-sectional survey conducted during the year 2011-2012 in 30 provinces of Iran. The study population comprised of 19 to 18-year old school students equally from both genders. A total sample size of 14,880 was determined to cover all study objectives and then was divided proportionally to population size of urban and rural areas and equally in three educational levels (elementary, intermediate and high school). Multistage random cluster sampling was used to recruit subjects. Clusters were the individual schools and it was scheduled to obtain 10 sampling units (students with their parents) in each of 48 randomly selected schools for every province.
Procedures and measurements
Questionnaires
The students’ questionnaires were based on the WHO Global School-based student Health Survey (GSHS) program[21] by adding some questions. A questionnaire was also designed for parents. Details regarding the questionnaires are described before.[22,24,25,26] These scales are comprehensive and can assess many different aspects of students’ health as demographic data, diet, physical activity, smoking, violence and other risky behaviors, medical history, family characteristics, relation with parents and peers, etc., Supervision on the whole project was considered by principal investigators at national and sub-national levels.
Physical measurements
Anthropometric measures consisting height, weight, hip, waist and wrist circumferences were measured according to the standard protocols. Then, body mass index, waist to hip and waist to height ratios were calculated.
BP was measured by expert health care professionals using standardized and calibrated mercury sphygmomanometers in a sitting position from the right arm. With the zero point of manometer at the level of the student's hearth, appropriate size cuffs were used considering cuff bladders covering 80-100% of the arm circumference and approximately two-thirds of the length of the upper arm without overlapping. After enough rest for the subject to calm down, BP readings were taken twice for each person with 5 min interval. The readings at the first Korotkoff sound were considered as the SBP and at the fifth sound as the diastolic blood pressure (DBP). The average of duplicate measurements was recorded and included in the analysis.[25]
Definitions
HBP was categorized as pre HTN and HTN. Pre HTN was considered as BP equal or greater than the age- and gender-specific 90th percentile after adjusting for weight and height or BP equal or more than 120/80 mmHg according to the Fourth Report of the working group (formerly task force) on Blood Pressure Control in Children, commissioned by the National Heart, Lung and Blood Institute of the National Institutes of Health of America. When BP was equal or over the age- and gender-specific 95th percentile value, it was considered as HTN.[27]
Ethical considerations
The study was approved by national and discrete regulatory organizations of the ministry of education and institutional ethical and scientific review boards. Clear explanation of the study aims and methods anteceded taking written informed consent from parents and verbal assent from students. Data were handled confidentially and de-identified. Each subject had the right to withdraw his/her consent at any time. The project was completely free of charge for all participants
Statistical analysis
Statistical analysis was performed using survey data analysis methods by the Stata software: StataCorp. 2011. Stata statistical software: Release 12. College Station, TX: StataCorp LP. P < 0.05 was considered as the level of significance. For quantitative measures, mean and standard deviations (SD) with 95% confidence intervals (CIs) were calculated. For nominal or categorical variables, frequencies and prevalence rates with the same CI were recorded. Student t-test and Chi-square tests were used for comparing that data between relevant groups, where applicable. Missing data were not included in the analyses.
RESULTS
Overall, 13486 out of 14880 invited students with a response rate of 90.6% participated in the study. Participants consisted of 6640 girls (49.2%) and 6846 boys (50.8%) with one of their parents, 75.6% from urban areas. Nearly 46%of them were elementary school, 25.9% intermediate school and 28.1% high school students. Mean (SD) age of participants was 11.47 (3.36) years, without significant difference between boys (12.36 [3.39] years) and girls (12.58 [3.32] years).
As you can be seen in Table 1, the mean (SD) SBP of all participants was 101.52 (13.46) mmHg and their mean DBP was 64.88 (11.41) mmHg. The BP percentiles of participants are also presented in this table.
Table 1.
Blood pressure and anthropometric measures of participants: The CASPIAN-IV study
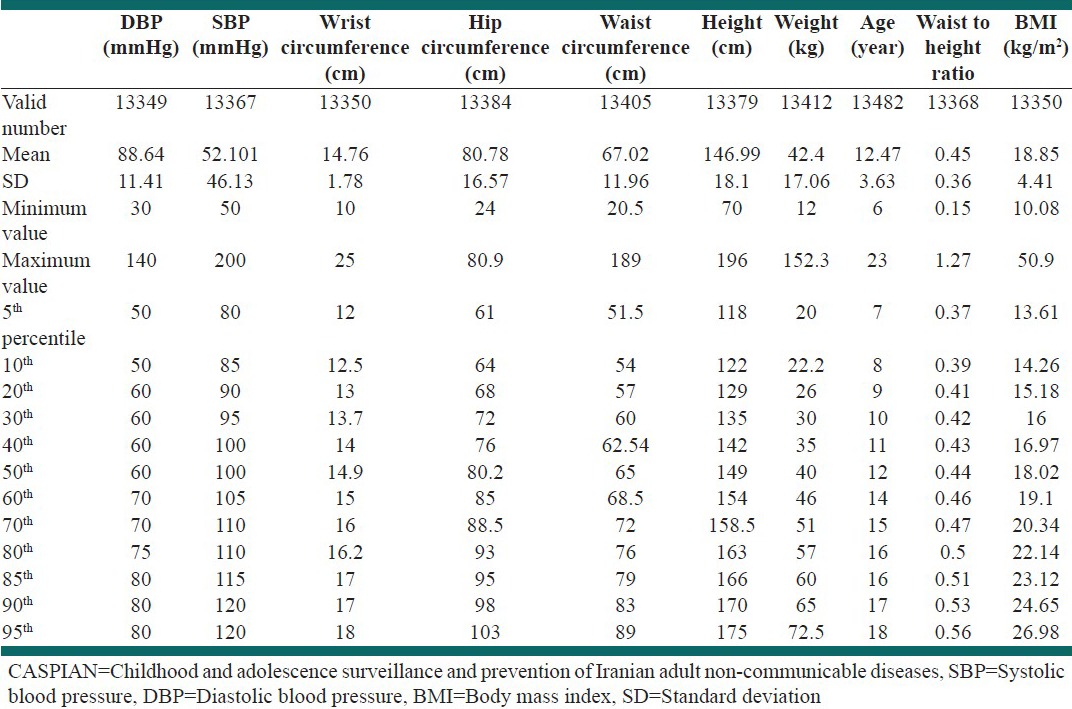
Overall, the prevalence rate (95% CI) of pre HTN was 3.25% (2.79-3.8) for SBP (in 13367 measures), 1.25% (0.98-1.6) for DBP (in 13349 measures) and 3.13% (2.71-3.62) for SBP and/or DBP (in 13350 measures). The corresponding figures for HTN were 0.92% (0.73-1.16), 3.08% (2.58-3.67) and 3.75% (3.21-4.36), respectively. These together yield a total rate of 4.17% (3.84-4.52) for elevated SBP, 4.33% (3.99-4.68) for elevated DBP and 6.88% (6.45-7.32) for elevated SBP and/or DBP.
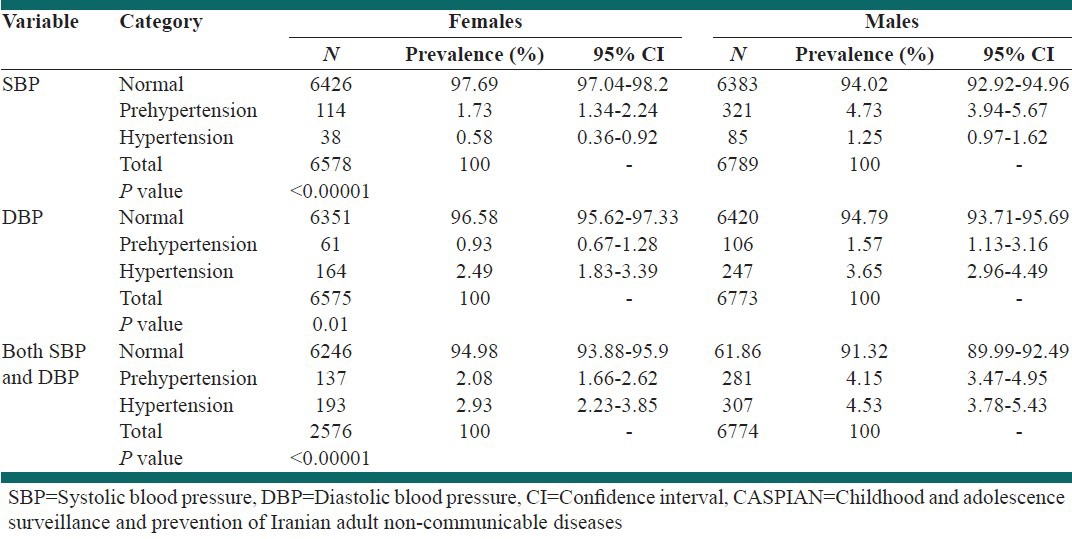
As shown in Table 2, Chi-square test showed that the prevalence rates of pre HTN and HTN were different in terms of gender and were significantly higher in boys than in girls.
Table 2.
Prevalence of different categories of blood pressure according to gender: the CASPIAN-IV study
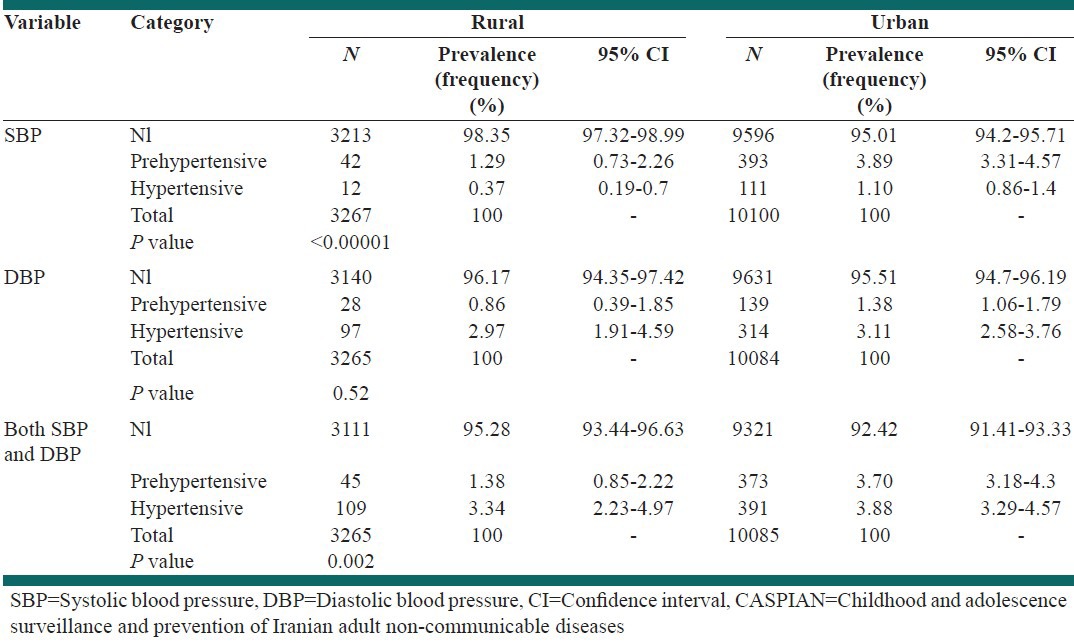
The prevalence rates of elevated levels of SBP and SBP and/or DBP were significantly higher in urban than in rural residents too [Table 3].
Table 3.
Prevalence of different categories of blood pressure according to the living area: The CASPIAN-IV study
DISCUSSION
The current nationwide survey revealed a substantially high prevalence rate for HBP among Iranian children and adolescents. By considering the additive prevalence of pre HTN and HTN, the prevalence rate of high SBP and/or DBP was 6.88%; and isolated high SBP and DBP were documented in 4.17% and 4.33% of participants, respectively. In the previous phase of this nationwide study, 5.35% of children and adolescents had BP levels equal or more than the 90th percentile values.[18] The increased rate in the current phase of this study may be attributable to unhealthy life-style and nutritional patterns and low physical activity levels in the pediatric age group.
Other studies about HBP in Iranian children and adolescents have been conducted in some cities and no other nationwide study has been performed on pediatric cardio-metabolic risk factors. A series of nationwide studies conducted among Iranian adult population, entitled the Survey of Risk Factors of NCDs included individuals with 15-65 years of age.[28] Their results are reported in 10 years age groups, e.g., 15-24 year-old group and according to adults’ definitions, therefore their results are not comparable with the CASPIAN studies.
By considering the cut point ≥95th percentile to define HTN, the prevalence of HTN would be expected to be around 5% and in fact 1-3% following the recommended three separate measurements in children with an initial BP measurement ≥95th percentile.[8] However, different prevalence rates in children and adolescents are reported around the world. For instance, the prevalence of systolic and diastolic hypertension in Sousse, Tunisia, was reported as 6.4% and 4.5%, respectively.[29] In Germany, the prevalence of HBP was 13% according to national standard curves and 2% according to the definition proposed by the International Diabetes Federation for metabolic syndrome.[30] In Portugal, pediatric HTN had prevalence of 2-5%.[31] In adolescents of Texas, United States of America (USA), the prevalence rates of HTN and pre HTN are reported as 3.2% and 15.7%, respectively.[32] In another study in the USA, a remarkable proportion of youths (6.9%) had HTN or nearly met its definition.[33] Two other studies in USA reported different prevalence rates, in one of them, 12.7% of youths had pre HTN and 5.4% had HTN,[34] whereas in another study, these rates were 3.4% and 3.6%, respectively.[35] These differences between studies can be explained by racial, ethnic[36] and genetic issues, different socio-economic states, environmental circumstances, as well as dietary and physical activity habits of participants.[37]
In the current study, significant gender difference was documented in the frequency distribution of HBP, with higher prevalence rates of pre HTN and HTN in boys than in girls. This finding is consistent with a longitudinal study that showed the risk of developing persistent HTN is higher in boys than it is for girls.[38] However, in the previous survey of the CASPIAN study, the prevalence of HBP was higher in girls than in boys.[18] Another survey did not detect any difference in terms of gender for the prevalence of HBP in the pediatric age group.[30] The gender differences documented in some studies may be explained by various factors as pubertal status, as well as physical activity and dietary patterns.
In the current study, significant differences existed in the frequency distribution of HBP according to the living area. Elevated SBP and elevated SBP and/or DBP were more prevalent in urban than in rural residents. Such difference was not documented for elevated DBP. Some studies in other populations have reported conflicting results in this regard. In a study in India, the prevalence rates of pre HTN and HTN were 2.9% and 2.8% in urban children and 2.8% and 2% in rural children, without statistically significant difference between them.[39] Likewise, in another region of India, 5.9% of children had HTN and 12.3% had pre HTN, with comparable rates of elevated BP in urban and rural areas.[40] Contrary to their findings, in Ghana, rural children showed lower rates of HTN than urban children.[41] In contrast, in a study in children living in the rural area of Alabama, USA, the prevalence rates of pre-HTN and HTN were 6.7% and 14.9%, respectively, which shows much higher rates than our study.[42] Lower rates of HBP in rural residents is probably due to a better life-style including healthier dietary and physical activity habits and lower mental stress and pollutants levels in rural than in urban areas. However as the Alabama study shows, the situation is not always the same.
It must be remembered that the definition of HBP in the pediatric population is based on the age- and gender-specific BP percentiles. Due to regional differences in the BP percentiles, using various definitions for HBP, different distribution of reference BP data and difficulties in the measurement of BP in children including the necessity of using appropriate cuff size, the findings of different studies may show some variations.[8,30]
Overall, the level and pattern of HBP in children and adolescents which we studied needs attention.
CONCLUSIONS
The prevalence rate of HBP including pre-HTN and HTN is noteworthy in the pediatric population of Iran. Screening and tracking BP from childhood is of crucial importance. It is necessary to use national standards for BP percentiles and design and implement targeted interventions based on research findings for various age groups.
ACKNOWLEDGMENTS
The authors wish to thank all participating students and their parents, their schools staff, executive research team and all relevant administrators.
Footnotes
Source of Support: This study was conducted as a national surveillance program funded by the Iranian Ministry of Health and Medical Education
Conflict of Interest: None declared
REFERENCES
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global action plan for the prevention and control of noncommunicable diseases 2013-2020.1. Chronic diseases. 2. Cardiovascular diseases. 3. Neoplasms. 4. Respiratory tract diseases. 5. Diabetes mellitus. 6. Health planning. 7. International cooperation. I. World Health Organization. ISBN 978 92 4 150623 6 (NLM classification: WT 500) [Last accessed on 2014 Jan 3]. Available from: http://www.apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf .
- 3.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 4.Miersch A, Vogel M, Gausche R, Siekmeyer W, Pfäffle R, Dittrich K, et al. Blood pressure tracking in children and adolescents. Pediatr Nephrol. 2013;28:2351–9. doi: 10.1007/s00467-013-2596-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation. 2008;117:3171–80. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juhola J, Magnussen CG, Viikari JS, Kähönen M, Hutri-Kähönen N, Jula A, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–90. doi: 10.1016/j.jpeds.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Virdis A, Ghiadoni L, Masi S, Versari D, Daghini E, Giannarelli C, et al. Obesity in the childhood: A link to adult hypertension. Curr Pharm Des. 2009;15:1063–71. doi: 10.2174/138161209787846900. [DOI] [PubMed] [Google Scholar]
- 8.Falkner B. Hypertension in children and adolescents: Epidemiology and natural history. Pediatr Nephrol. 2010;25:1219–24. doi: 10.1007/s00467-009-1200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luma GB, Spiotta RT. Hypertension in children and adolescents. Am Fam Physician. 2006;73:1558–68. [PubMed] [Google Scholar]
- 10.Kelishadi R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol Rev. 2007;29:62–76. doi: 10.1093/epirev/mxm003. [DOI] [PubMed] [Google Scholar]
- 11.Kelishadi R, Poursafa P, Keramatian K. Overweight, air and noise pollution: Universal risk factors for pediatric pre-hypertension. J Res Med Sci. 2011;16:1234–50. [PMC free article] [PubMed] [Google Scholar]
- 12.Kelishadi R, Ardalan G, Gheiratmand R, Majdzadeh R, Delavari A, Heshmat R, et al. Blood pressure and its influencing factors in a national representative sample of Iranian children and adolescents: The CASPIAN study. Eur J Cardiovasc Prev Rehabil. 2006;13:956–63. doi: 10.1097/01.hjr.0000219109.17791.b6. [DOI] [PubMed] [Google Scholar]
- 13.Khan FS, Lotia-Farrukh I, Khan AJ, Siddiqui ST, Sajun SZ, Malik AA, et al. The burden of non-communicable disease in transition communities in an Asian megacity: Baseline findings from a cohort study in Karachi, Pakistan. PLoS One. 2013;8:e56008. doi: 10.1371/journal.pone.0056008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naghavi M, Abolhassani F, Pourmalek F, Lakeh M, Jafari N, Vaseghi S, et al. The burden of disease and injury in Iran 2003. Popul Health Metr. 2009;7:9. doi: 10.1186/1478-7954-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization; 2012. [Last accessed on 2013 Jan 05]. Noncommunicable diseases country profiles 2011, WHO Global Report. Available from: http://www.who.int . [Google Scholar]
- 16.Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, et al. Third national surveillance of risk factors of non-communicable diseases (SuRFNCD-2007) in Iran: Methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9:167. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelishadi R, Amirkhani A, Ardalan G, Ziaoddini H, Majdzadeh R. An overview of a national surveillance program in Iran for prevention of chronic non-communicable diseases from childhood: CASPIAN study. Iran J Public Health. 2009;38(Suppl 1):102–10. [Google Scholar]
- 18.Khashayar P, Heshmat R, Qorbani M, Motlagh ME, Aminaee T, Ardalan G, et al. Metabolic syndrome and cardiovascular risk factors in a national sample of adolescent population in the Middle East and North Africa: The CASPIAN III study. Int J Endocrinol 2013. 2013:8. doi: 10.1155/2013/702095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelishadi R, Ardalan G, Adeli K, Motaghian M, Majdzadeh R, Mahmood-Arabi MS, et al. Factor analysis of cardiovascular risk clustering in pediatric metabolic syndrome: CASPIAN study. Ann Nutr Metab. 2007;51:208–15. doi: 10.1159/000104139. [DOI] [PubMed] [Google Scholar]
- 20.Farzadfar F, Danaei G, Namdaritabar H, Rajaratnam JK, Marcus JR, Khosravi A, et al. National and subnational mortality effects of metabolic risk factors and smoking in Iran: A comparative risk assessment. Popul Health Metr. 2011;9:55. doi: 10.1186/1478-7954-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Global school-based student health survey (GSHS) [Last accessed on 2013 Dec 28]. Available from: http://www.who.int/chp/gshs/en .
- 22.Motlagh ME, Kelishadi R, Ardalan G, Gheiratmand R, Majdzadeh R, Heidarzadeh A, et al. Rationale, methods and first results of the Iranian National Programme for prevention of chronic diseases from childhood: CASPIAN study. East Mediterr Health J. 2009;15:302–14. [PubMed] [Google Scholar]
- 23.Amirkhani A, Motlagh ME, Sedaghat M, Ardalan G, editors. Tehran, Iran: Publications of the Ministry of Health and Medical Education; 2009. Health Status of Iranian Students According to the Health-Risk Behaviors Survey 2006-2007. [Google Scholar]
- 24.Kelishadi R, Heshmat R, Motlagh ME, Majdzadeh R, Keramatian K, Qorbani M, et al. Methodology and early findings of the third survey of CASPIAN study: A national school-based surveillance of students’ high risk behaviors. Int J Prev Med. 2012;3:394–401. [PMC free article] [PubMed] [Google Scholar]
- 25.Kelishadi R, Ardalan G, Qorbani M, Ataie-Jafari A, Bahreynian M, Taslimi M, et al. Methodology and early findings of the fourth survey of childhood and adolescence surveillance and prevention of adult non-communicable disease in Iran: The CASPIANIV study. Int J Prev Med. 2013;12:1451–60. [PMC free article] [PubMed] [Google Scholar]
- 26.Kelishadi R, Majdzadeh R, Motlagh ME, Heshmat R, Aminaee T, Ardalan G, et al. Development and evaluation of a questionnaire for assessment of determinants of weight disorders among children and adolescents: The Caspian-IV study. Int J Prev Med. 2012;3:699–705. [PMC free article] [PubMed] [Google Scholar]
- 27.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 28.Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, et al. Third National Surveillance of Risk Factors of Non-communicable Diseases (SuRFNCD-2007) in Iran: Methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9:167. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrabi I, Belarbia A, Gaha R, Essoussi AS, Ghannem H. Epidemiology of hypertension among a population of school children in Sousse, Tunisia. Can J Cardiol. 2006;22:212–6. doi: 10.1016/s0828-282x(06)70898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwandt P, Bertsch T, Liepold E, Haas GM. Age- and gender-specific components of the metabolic syndrome in 2228 first graders: The PEP Family Heart Study. Scientifica (Cairo) 2013. 2013 doi: 10.1155/2013/394807. 394807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade H, Antonio N, Rodrigues D, Da Silva M, Pêgo M, Providência LA. High blood pressure in the pediatric age group. Rev Port Cardiol. 2010;29:413–32. [PubMed] [Google Scholar]
- 32.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640–4. doi: 10.1016/j.jpeds.2007.01.052. 644.e1. [DOI] [PubMed] [Google Scholar]
- 33.Koebnick C, Black MH, Wu J, Martinez MP, Smith N, Kuizon BD, et al. The prevalence of primary pediatric prehypertension and hypertension in a real-world managed care system. J Clin Hypertens (Greenwich) 2013;15:784–92. doi: 10.1111/jch.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo JC, Sinaiko A, Chandra M, Daley MF, Greenspan LC, Parker ED, et al. Prehypertension and hypertension in community-based pediatric practice. Pediatrics. 2013;131:e415–24. doi: 10.1542/peds.2012-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–9. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 36.Messiah SE, Arheart KL, Lopez-Mitnik G, Lipshultz SE, Miller TL. Ethnic group differences in cardiometabolic disease risk factors independent of body mass index among American youth. Obesity (Silver Spring) 2013;21:424–8. doi: 10.1002/oby.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stabouli S, Papakatsika S, Kotsis V. The role of obesity, salt and exercise on blood pressure in children and adolescents. Expert Rev Cardiovasc Ther. 2011;9:753–61. doi: 10.1586/erc.11.63. [DOI] [PubMed] [Google Scholar]
- 38.Lurbe E, Thijs L, Torro MI, Alvarez J, Staessen JA, Redon J. Sexual dimorphism in the transition from masked to sustained hypertension in healthy youths. Hypertension. 2013;62:410–4. doi: 10.1161/HYPERTENSIONAHA.113.01549. [DOI] [PubMed] [Google Scholar]
- 39.Narayanappa D, Rajani HS, Mahendrappa KB, Ravikumar VG. Prevalence of prehypertension and hypertension among urban and rural school going children. Indian Pediatr. 2012;49:755–6. doi: 10.1007/s13312-012-0159-5. [DOI] [PubMed] [Google Scholar]
- 40.Sharma A, Grover N, Kaushik S, Bhardwaj R, Sankhyan N. Prevalence of hypertension among schoolchildren in Shimla. Indian Pediatr. 2010;47:873–6. doi: 10.1007/s13312-010-0148-5. [DOI] [PubMed] [Google Scholar]
- 41.Agyemang C, Redekop WK, Owusu-Dabo E, Bruijnzeels MA. Blood pressure patterns in rural, semi-urban and urban children in the Ashanti region of Ghana, West Africa. BMC Public Health. 2005;5:114. doi: 10.1186/1471-2458-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson LE, Daly CM, Danielle D, Wadsworth DD. Body mass index and blood pressure in rural, low socioeconomic children. Health. 2013;5:91–5. [Google Scholar]


