Abstract
Hypertension after pediatric renal transplant is a common and important risk factor for graft loss and patient survival. The mechanism of post kidney transplant hypertension is complex and multifactorial. Control of blood pressure in renal transplant patients is important but often times blood pressures remain uncontrolled. The management of hypertension and obesity in pediatric kidney transplant patients is based on the pathophysiology. Compared to the general pediatric hypertensive population, special attention needs to be focused on the additional impact of immunosuppressive medications side effects and interactions, recurrent disease, and donor and recipient comorbidities such as obesity on blood pressure control with thoughtful consideration of the risk of graft failure. In general, there is a need for prospective studies in pediatric kidney transplant patients to understand the pathophysiology of hypertension and obesity and the appropriate approach to achieve a balance between the primary need to avoid rejection and the need to lower blood pressure and prevent obesity.
Keywords: Hypertension, obesity, pediatric kidney transplant
INTRODUCTION
Hypertension is a frequent complication following kidney transplantation in children[1,2,3] and has been shown to be a risk factor for increased cardiovascular morbidity and graft failure.[4,5,6] Its presence is a significant and independent risk factor for poor long-term graft survival.[4]
Reported prevalence of hypertension following renal transplantation ranges between 60% and 90% depending on the method used for blood pressure (BP) measurement and diagnostic criteria for hypertension.[7,8,9,10,11,12,13,14,15] In contrast, most recent prevalence rates of post renal transplant hypertension in the UK reported by Sinha and the British Association for Pediatric Nephrology are 66.4% at 6 months and 55.9% at 5 years after transplant if both clinic BP > 95th percentile and use of antihypertensive medications were used to define hypertension.[2]
CAUSES AND RISK FACTORS OF HYPERTENSION
Steroids
Steroids have been an important part of immunosuppression protocols since the early years of transplant and have been highly effective in preventing rejection. However, the side effect profile of steroids, particularly their effects on lipid and glucose metabolism and BPs, has prompted avoidance or early withdrawal during more recent years. Steroids produce hypertension by causing sodium and water retention and this effect is dose dependent. A recent study suggests activation of vascular smooth muscle receptors as well.[16]
Several studies have associated use of steroids with hypertension post-transplant and steroid withdrawal with improvement in BPs. A prospective study done by Höcker et al. showed that steroid withdrawal caused a significant reduction of arterial hypertension which persisted after 2 years from the time steroids were discontinued and that these children required less anti hypertensives.[17] In contrast, the larger European multicenter randomized control study tacrolimus and withdrawal of steroids did not see any significant difference in the incidence of hypertension but approximately 8% of the steroid withdrawal group, compared with 18% of the steroid group were on ≥3 antihypertensive medications 6 months post-transplant.[18] A study done by Maduram et al. demonstrated similar findings and reported that pediatric renal transplant patients who received early steroid withdrawal required significantly less anti hypertensives compared with those on steroids.[19] A meta-analysis by Knight and Morris comparing maintenance steroids with steroid avoidance or withdrawal after renal transplantation in adult patients showed significant reductions in the risk of hypertension in the avoidance or withdrawal group and this relative reduction remained significant even when only the intention-to-treat analysis was included.[20]
Calcineurin inhibitors
The introduction of calcineurin inhibitors as part of the immunosuppressive regimen led to significant reductions in allograft rejection rates but also contributed to an increase in BP in transplant patients. This elevation in BP following treatment with calcineurin inhibitors is dose dependent and is caused by the vasoconstriction of the glomerular afferent arteriole resulting in sodium and water retention. Increased sympathetic nerve discharges and increased vascular resistance have also been suggested.[21] Other mechanisms implicated include endothelial dysfunction, arterial baroreceptor impairment and activation of sympathetic, endothelin and renin-angiotensin systems.[22] More recently, animal studies done by El-mas et al. suggested the role of central nitric oxide and carbon monoxide in the hypertensive response to cyclosporine.[23]
Both cyclosporine and tacrolimus can induce hypertension. In a cross sectional study done by Seeman et al., the authors demonstrated that children with poorly controlled hypertension had significantly higher cyclosporine doses and tacrolimus levels.[11] However, more recent studies suggest that the effects may be greater with cyclosporine use compared with tacrolimus. In a retrospective cohort study done by Denburg et al., both systolic blood pressure (SBP) and diastolic blood pressure (DBP) during later years after transplant were found to be lower, independent of concomitant decrease in steroid use. This was likely due to the transition from cyclosporine to tacrolimus over the last decade.[24] Several other studies have demonstrated a similar effect of cyclosporine on BP compared to tacrolimus for immunosuppression.[2,3,4,5,6,25,26] Vincenti et al. in their study reported that significantly fewer patients treated with tacrolimus required antihypertensive medications to control BP compared with those who were treated with cyclosporine at 5 years post-transplant.[25] Margreiter et al. reported similar results. New onset or worsening hypertension was found to be significantly more common in patients receiving cyclosporine microemulsion, although the duration of follow up in this study was only 6 months.[27] A more recent study by Krämer et al. however did not show a significant difference in the rate of use of antihypertensives between cyclosporine treated and tacrolimus treated patients at 3 years post-transplant.[28]
This difference in findings may potentially be genetic in nature. A recent study has suggested that polymorphisms in the CYP3A5 may be associated with the degree and severity of hypertension in patients receiving calcineurin inhibitors following renal transplant. Ferraresso et al. found that BP was significantly more elevated in patients who were heterozygous for the CYP3A5*1/*3 gene. These patients were also taking significantly more antihypertensive medications and remained hypertensive 6 months after transplant.[29]
Obesity
Children commonly experience weight gain after transplant.[24,30,31,32,33,34,35] The greatest increase in body mass index (BMI) and prevalence of obesity seems to happen during the 1st year post transplant. Furthermore, more children who present for transplant are now obese with a prevalence of 8% of transplanted children before 1995 and 12.4% after 1995 as reported by Hanevold et al.[36] This causes concern since results from a study done by Koulouridis et al. suggested that central obesity and body weight are important contributors to BP control in children and adolescents.[37] In transplant patients, an increase in BMI was found to be a significant contributor to an increase in SBP independent of glucocorticoid exposure.[24] A positive correlation between SBP and BMI that persists years after transplant was also demonstrated by Silverstein et al.[30] Obesity prior to transplant may worsen this association. Mitsnefes et al. reported that children who were obese prior to transplant had significantly higher SBP than children who became obese or overweight after transplant as well as children who were able to maintain a normal BMI.[31]
There are several reasons why patients develop obesity after transplant. Factors identified by Foster et al. as a risk for BMI increase include age 6-12 years at transplant, remote transplant year, female sex, lower BMI at transplant, Hispanic ethnicity and black race.[33] Steroid use was significantly associated with weight gain in several studies.[24,32,33,35] Denburg et al. in their study have reported that the average interval dose of glucocorticoid following transplantation was significantly associated with interval increases in BMI, with greater association in males, younger patients and patients with lower baseline BMI.[24]
The mechanism by which obesity causes hypertension seems to be multifactorial and highly complex. Sodium sensitivity which accompanies obesity, increased renal sodium reabsorption, impaired pressure natriuresis, extracellular volume expansion, increased leptin, activation of the sympathetic nervous system (SNS), increased activity of the renin-angiotensin-aldosterone system (RAAS) and physical compression of the kidneys especially in the presence of visceral obesity may play a contributory role.[37,38,39,40,41]
It is also now known that adipose tissue is an active organ that produces all components of the RAAS, including angiotensin, angiotensin converting enzyme (ACE), renin and AngII. Obesity is also a proinflammatory state and several studies have shown that adipose tissue, particularly visceral adipose tissue, secretes adipokines such as leptin, interleukin-6 and tumor necrosis factor-α which have been linked to metabolic syndrome.[42,43]
Recent studies have focused on the involvement of the SNS activation in obesity related hypertension. The SNS activation is thought to be not generalized, but rather differentiated and localized to muscle and kidney SNS, with normal or even reduced cardiac sympathetic activity due to baroreceptor inhibition.[38] Furthermore, studies have shown that the link between presence of obesity and increased SNS activity may be limited to the presence of visceral body fat.[44,45] Alvarez et al. reported an association between SNS activity and abdominal visceral fat that is closer compared with abdominal subcutaneous fat and that is independent of total body fat. In their study, muscle SNS activity was about 55% higher in men with increased abdominal visceral fat compared with age-matched, total fat mass–matched and abdominal subcutaneous fat-matched men with lower abdominal visceral fat level.[44] In a later study, they added that muscle SNS activity was similar in subcutaneously obese and non-obese individuals with similar abdominal visceral fat.[45]
Mechanisms reportedly involved in the increased SNS activity include hyperleptinemia, activation of the central nervous system melanocortin system, hypoadiponectinemia, hypoghrelinemia, hyperinsulinemia, increased angiotensin II levels and baroreceptor dysfunction.[38] However, leptin has been inconsistently connected to increased SNS activity. Alvarez et al. did not see increased SNS activity despite higher levels of leptin in the subcutaneously obese individuals.[45] Another factor which is common in obesity and has been reported to contribute to the increased SNS activity is obstructive sleep apnea (OSA).[40,46,47,48,49] However, this may again be related to the presence of visceral fat. Vgontzas et al. reported in their study that in contrast to those with obesity but no sleep apnea, obese sleep apneics had a significantly greater amount of visceral fat and that visceral but not subcutaneous fat was significantly correlated with indexes of sleep apnea.[48] In contrast, Grassi et al. demonstrated that OSA can contribute to adrenergic activation in both obese and lean subjects.[49]
Obese patients also have activated RAAS and increased circulating levels of renin, angiotensinogen and AngII, which would all contribute to increased BP.[42,43] Aside from synthesis of the components of the RAAS by visceral fat, several other mechanisms have been implicated that include sympathetic stimulation and hemodynamic alterations possibly resulting from compression of the kidneys by visceral fat. Increased RAAS activity combines with increased SNS activity to impair pressure natriuresis and increase renal tubular sodium reabsorption, finally resulting in volume expansion and an increase in BP.[50,51] Free fatty acids have also been reported as contributory to elevations in BP. FFA may increase vascular tone via stimulation of the SNS.[52]
Donor factors
The source of donor kidney could also affect BP after transplant. Sinha et al. reported those with hypertension are more likely to have received kidney from a deceased donor.[2] Similarly, Heidotting et al. demonstrated that a lower donor age and living donors can lead to a significantly lower number of antihypertensive medications up to 36 months after renal transplant.[53] The reason for this is not clear but may be due to longer cold ischemia times resulting to more severe ischemic-reperfusion injury. Other donor factors that may contribute to increased risk of post-transplant hypertension include family history of hypertension, smaller size, female gender, presence of hypertension and overall quality of the allograft and degree of reperfusion injury.[54] “Nephron mass underdosing” from smaller or female donors[54] together with allograft dysfunction from cellular and antibody mediated rejection can lead to chronic kidney disease, which can also be an independent risk factor contributing to post transplant hypertension.
In addition, studies of adult kidney transplant recipients have demonstrated effects of kidney-donor genetic variants on allograft function. Some of the genes implicated include apolipoprotein L1 gene (APOL1), which appear to explain the effects of African donor population ancestry on graft survival and has been associated with increased risk for HIV-associated nephropathy, focal segmental glomerulosclerosis and hypertension-attributed end stage renal disease (ESRD), the caveolin 1 gene (CAV1) linked to renal fibrosis and ascending polyomavirus infection and multi-drug resistance 1 encoding P-glycoprotein gene (ABCB1) which affects drug metabolism.[54,55] Reeves-Daniel et al. reported that graft survival was significantly shorter in donor kidneys with two APOL1 risk variants after renal transplantation than those with zero or one risk variants and that the presence of both variants had more effect compared to human leukocyte antigen mismatch and cold ischemia time. 75% of those with two APOL1 risk variants were also noted to have biopsy-proven APOL1-associated lesions including FSGS and donor-acquired nephron scarring with arteriosclerosis compared to only 11.8% among grafts with 0/1 APOL1 risk alleles.[56] However, there is no difference in allograft survival at 5 years post-transplant for recipients with high-risk APOL1 genotypes as reported by Lee et al.[57] On the other hand, donors who have decreased expression of CAV1, which affects transforming growth factor-β (TGF-β) receptor degradation may have significantly greater renal fibrosis and shorter allograft survival.[55] Moore et al. reported a significantly increased risk for graft loss among those with donor CAV1 risk genotypes compared with donor non-CAV1 risk genotypes with increased incidence of biopsy-proven interstitial fibrosis.[58] Further studies about the effects of genetic variations may provide opportunities to improve long-term graft survival in pediatric kidney transplants. For example, in the future, CAV1 genotyping of the donor may provide opportunities to prevent fibrosis in the transplanted kidney by pre-emptively treating the recipient with ACE1 inhibitor which can inhibit profibrogenic TGF β in the kidney.
Chronic allograft dysfunction
There are numerous mechanisms leading to nephron loss and eventual graft dysfunction and failure post-transplant. The North American Pediatric Renal Trials and Collaborative Studies Annual Report in 2008 reported that chronic rejection accounted for 40.4% of all graft failures and that this continues to be the most common cause of graft failure for children.[59] Other causes include acute rejection, renal artery stenosis, thrombosis, calcineurin inhibitors nephrotoxicity, bacterial and viral infections, chronic arterial hypertension, chronic obstruction, poor adherence to medications and malignancy.[59,60]
Stenosis of transplant renal artery
Renal artery stenosis of the transplanted kidney should be suspected in any kidney transplant patient with severe or worsening hypertension and/or renal function deterioration not attributable to rejection or cyclosporine toxicity. Renal artery stenosis can cause hypertension via activation of the RAAS leading to increase in salt and water retention. However, studies done among adults suggest that renal artery stenosis may not be the actual reason for the clinically significant changes in BP since improvement in BP is not consistently achieved after angioplasty.[61,62] Nonetheless, stenosis of the renal artery may contribute to worsening allograft function.
Hypertension prior to transplant
It is important to note that the pre transplant BP may affect the development of hypertension post-transplant. A recent study done by Sinha et al. showed that half of the non-hypertensive patients post-transplant eventually developed hypertension with incidence rates highest among patients who were pre-hypertensive at baseline.[3] They also estimated that half of the patients who had pre-hypertension (SBP and/or DBP 90-95th percentile) and normal BP (SBP and/or DBP ≥ 50th but < 90th percentile) will become hypertensive in 2 and 3 years respectively. In contrast, <50% of those with optimal BP (BP < 50th percentile) will become hypertensive after a follow-up period of 7 years.
Figure 1 shows the causes of hypertension in pediatric renal transplant patients.
Figure 1.
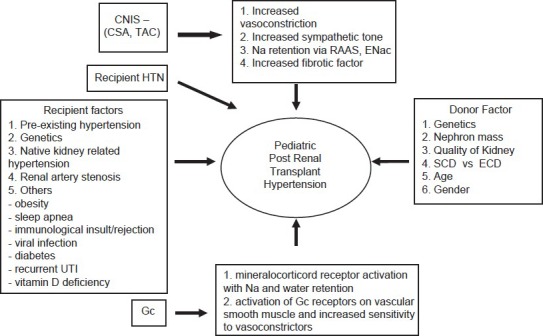
Causes of hypertension in pediatric patients with renal transplant (Modified from hypertension after kidney transplantation: A pathophysiologic approach by Thomas, et al.[54]) *CNIS - Calcinurin inhibitors *CSA - Cyclosporin *TAC - Tacrolimus *HTN-Hypertention *CKD-Chronic Kidney Disease *UTI-urinary tract infection *Gc-Glucocorticoids *Na-Sodium *RAAS-Renin-angiotensin aldosterone system *ENac-Sodium Channel *SCD-Standard Criteria Donor *ECD-Extended Criteria Donor
Vitamin D deficiency
A study done by Shroff suggested vitamin D deficiency as a possible modifiable risk factor for hypertension post kidney transplant.[63] They found an inverse correlation between 25 (OH) D and SBP, but this was no longer significant after multiple regression analysis. Studies of adults reported potential effects of vitamin D on BP and cardiovascular disease (CVD).[64,65,66,67,68] Some of the mechanisms reported in these studies, which may lead to CVD protection include negative endocrine regulation of the renin-angiotensin system, inhibition of vascular smooth muscle proliferation, suppression of vascular calcification, downregulation of pro-inflammatory cytokines and up regulation of anti-inflammatory cytokines. However, the available data is insufficient and more research is needed to determine the amount of vitamin D supplementation necessary to lower BPs.
MONITORING: AMBULATORY BLOOD PRESSURE MONITORING (ABPM) VERSUS CLINIC BP MONITORING
Over the past years, 24 h ambulatory BP monitoring for evaluating hypertension in children and adolescent transplant patients has been more widely used and recommended.[14,69] Several studies have demonstrated its superior ability to detect hypertension.[14,69,70,71,72,73,74] Compared with clinic BP monitoring, ABPM can detect nocturnal hypertension. McGlothan et al. in their study have reported that hypertension during sleep was more common than when awake, with as much as 55% of patients having systolic hypertension when asleep versus 38% when awake and 52% having diastolic hypertension during sleep versus 21% when awake.[71] Ferraris et al. also reported that 30% of pediatric renal transplant patients who were normotensive during clinic visits had uncontrolled BPs when ABPM was used.[72] Moreover, the study done by Paripovic et al. showed that 68% versus 42% of pediatric post renal patients were hypertensive when automatic monitor of blood pressure (AMBP) versus clinic BP measurements were used. What is more concerning from this study is that as much as 21% had hidden uncontrolled hypertension despite antihypertensive medications and an additional 24% had masked hypertension in which the office BP is normal, but daytime systolic and/or diastolic AMBP is elevated in those without antihypertensive medications.[73] Similarly, Basiratnia et al. diagnosed uncontrolled hypertension in 20% and untreated and masked in 27% of patients.[74] These studies should prompt transplant clinics to include ABPM in their practice.
IMPACT OF HYPERTENSION AND OBESITY RENAL TRANSPLANT OUTCOMES
There have been several studies done looking at the effect of hypertension and obesity on graft survival in adult renal transplant which may provide insight on their effects in pediatric renal transplant recipients.
Hypertension after kidney transplantation predicts reduced patient and allograft survival. Mange et al. showed that the level of BP during the 1st year affected the survival of renal allografts from living donors, independent of renal function).[75] Similarly, results from a larger study done by Opelz et al. that included 29, 751 patients followed for up to 7 years demonstrated that uncontrolled hypertension at 1, 3 and 5 years from transplantation is associated with graded increase in subsequent allograft failure and death-censored graft failure irrespective of rejection episodes and antihypertensive medication use.[76] Raiss Jalali et al. compared transplanted kidney function in hypertensive patients with satisfactory BP control (arterial pressure < 160/90 mmHg) and unsatisfactory control who were matched for age, gender, donor–recipient relation, primary disease, early post-transplant course, immunosuppression and antihypertensive medications. After 3 years, they found a slow but significant increase in the mean creatinine levels in those with unsatisfactory BP control with a significant negative correlation between creatinine clearance and 3-year BP.[77] Kasiske et al. reported that each 10 mm Hg of systolic BP was associated with a significantly increased relative risk for graft failure, death-censored graft failure and death even after adjusting for the effects of acute rejection and other variables.[78]
One of the largest studies done on the impact of obesity on renal transplant was done by Meier-Kriesche et al., who retrospectively analyzed 51, 927 primary adult renal transplants registered in the USRDS and reported a very strong association between pre-transplant BMI and outcomes after renal transplantation. The extremes of very high and very low BMI were associated with significantly worse patient and graft survival.[79] Several other retrospective analysis showed poorer patient and/or graft survival in obese or overweight patients.[80,81] Kovesdy et al., however, in a prospective cohort of 993 kidney transplant recipients, found that waist circumference rather than BMI per se was associated with patient mortality. Higher BMI was associated with lower mortality after adjustment for waist circumference while higher waist circumference was more strongly associated with higher mortality after adjustment for BMI. This suggests the important effect of obesity from visceral adiposity which is reflected by waist circumference rather than non-visceral adiposity in kidney transplant recipients.[82]
Obesity and increased weight after transplant also leads to inferior graft outcomes. A retrospective study by el-Agroudy et al. reported on 650 non-diabetic live donor kidney recipients with a BMI at transplant of <25 kg/m2 who were followed for a maximum of 10 years. Obesity developing after renal transplant was associated with significantly higher incidence of chronic allograft nephropathy, post-transplant hypertension, post-transplant hyperlipidemia, diabetes mellitus, ischemic heart disease, increased incidence of patient death from CVD and reduction in graft function based on serum creatinine at 10 years.[83] Similarly, Ducloux et al. studied 292 renal transplant recipients and found that patients with an increase in BMI of more than 5% at 1 year post-transplant had an increased risk of graft loss.[84]
These significant differences associated with obesity or weight gain may be associated to the effects of obesity on the kidney or due to several comorbidities related to the weight gain. Nonetheless, the effects of both obesity and hypertension on renal transplant cannot be ignored and both must be addressed aggressively.
MANAGEMENT
Control of hypertension after transplant in children has been difficult and studies indicate that only about 20-50% of treated children attain normal BP.[11,26] Similarly, the prevalence of uncontrolled hypertension in the UK cohort as reported by Sinha is around 30%.[2]
Excellent control of BPs in patients with kidney disease is of utmost importance. Seeman et al. reported that children who remained hypertensive had significantly decreased graft function after 2 years compared with those who reached normal BP levels.[85] The kidney disease outcomes quality initiative recommends that for children with chronic kidney disease, BP should be maintained lower than the 90th percentile for normal values adjusted for age, gender and height or 130/80 mm Hg, whichever is lower.[86] However, the results of the ESCAPE trial showed that intensified BP control with target BP less than the 50th percentile adjusted for age, gender and height is associated with a significant slowing of progression of renal disease. In this study, 29.9% children whose BP was maintained in the low range of normal had a decline of 50% in the glomerular filtration rate or progression to ESRD as compared to 41.7% in the group with BP maintained between the 50th and 95th percentiles.[87] However, it is still not known if this lower treatment goal and more aggressive hypertension control should be recommended for children with transplanted kidneys. In addition, it might also be worthwhile to consider extending this goal prior to transplant given the findings from the study by Sinha. Their study reported reduced occurrence of hypertension post-transplant in those with lower levels of BP in the normal and optimal range before transplant.[3] Similarly in adults, achieving lower SBP is associated with improved graft and patient survival even several years after transplantation. In a study involving 24,404 primary deceased-donor kidney transplant recipients, patients with uncontrolled hypertension (SBP > 140 mmgHg) at 1 year who were able to achieve adequate BP control (SBP ≤ 140 mmHg) at 3 years had significantly improved 10 year graft survival than those with sustained hypertension at 3 years. Better BP control after year 3 was also associated with improved 10-year graft survival while even a temporary increase in SBP at 3 years was associated with worse graft survival. In addition, the authors also performed a subset analysis in patients whose serum creatinine was < 130 μmol/L at 1 and 3 years to account for renal impairment as a cause of elevated BP. The association of SBP changes with subsequent graft outcome remained in this subgroup of recipients with excellent 1-, 3- and 10 year graft function. Subjects with SBP ≤ 140 mmHg at 1 and 3 years had a significantly better 10-year graft survival rate than those whose SBP increased from ≤ 140 mmHg at 1 year to >140 mmHg at 3 years.[88] However, most of the studies done are observational studies and whether this association between hypertension and poorer graft outcomes is purely dependent on BP control and not affected by other factors such as acute rejection and polyomavirus infection remains to be proven. More prospective trials are needed to determine optimal BP goals for children pre and post renal transplant.
In general, the control of BP is based on the cause of hypertension. For renal artery stenosis, angioplasty or surgical repair may be necessary. In children with native kidney induced hypertension poorly controlled by medical treatment, nephrectomy may be beneficial. In addition to the causes of hypertension discussed previously, use of supplements or other medications, which have the potential to increase BP should be elicited and addressed during the clinic visits. Examples include caffeine, licorice, ephedra, non-steroidal anti-inflammatory drugs, herbal supplements, steroids and oral contraceptives. Table 1 gives a summary of the management of hypertension in pediatric transplant patients.
Table 1.
Management of hypertension in pediatric renal transplant patients
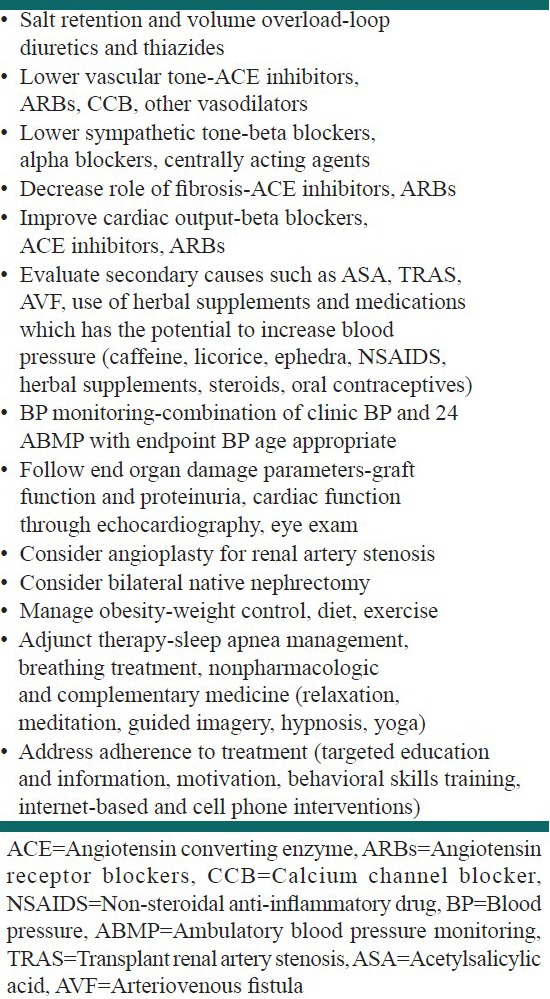
For most patients, more than one class of antihypertensive agent may be necessary to adequately control hypertension. Calcium channel blockers (CCB) are the most commonly prescribed antihypertensives, in combination with diuretics, angiotensin converting enzyme inhibitors (ACEI) or beta-adrenoceptor antagonists but practice differs among practitioners. Results from a meta-analysis on antihypertensives among adult renal transplant patients done by Cross et al. suggested that CCBs are preferred as first-line agents for hypertensive kidney transplant recipients. CCBs compared to placebo or no treatment was found to significantly reduce the risk of graft loss at 1 year post transplant. However, they also noted differences in serum creatinine outcomes among different classes of CCB.[89] This may be explained by inhibition of CYP3A4 and p-glycoprotein by non-dihydropyridine CCB such as verapamil and diltiazem which can increase cyclosporine concentrations by approximately 30%, thus necessitating dose reductions when these drugs are used in combination.[90] Moreover, results of the meta-analysis showed that compared with patients on CCBs, patients receiving ACEI had significantly lower GFR by as much as 12 mL/min and higher creatinine at the end of follow-up.[89] Thus, some physicians are reluctant to use ACEI because of the potential for decrease in renal perfusion especially with a single kidney, cyclosporine use and risk of transplant renal artery stenosis. However, ACEI in majority of pediatric recipients do not cause decrease in renal perfusion and have renoprotective mechanisms, which include inhibition of glomerulosclerosis and antiproteinuric effect in addition to a decrease in systemic and intraglomerular BP. More recent data showed benefits and safety of ACEI in pediatric transplant patients. A retrospective analysis by Arbeiter et al. demonstrated significantly improved BP control during the 1st year of ACEI use in those who failed other medications previously. BP control was achieved in 94% in 6 months and in 100% in 12 months after initiation of ACEI therapy and the number of antihypertensive medications also decreased.[91] Seeman et al. suggested that control of hypertension in children post renal transplant could be improved by increasing antihypertensive drugs such as ACEI and diuretics after an important although non-significant findings of use of fewer antihypertensive medications and less use of ACEI and diuretics in pediatric post renal transplant patients with uncontrolled hypertension. They cited that children with controlled hypertension received twice as much diuretics and five times as much ACEI than children with uncontrolled hypertension.[11] In a later prospective trial, they demonstrated that intensified control of BP by ABPM and addition of more antihypertensive medications, particularly ACEI and diuretics, can improve BP control especially nocturnal BP.[85] Several adult studies also report ACEI as effective, safe and well tolerated among adult renal transplant patients. A recent retrospective study done by Aftab et al. showed that the use of a beta-blocker therapy and ACE1 inhibitor or angiotensin receptor blocker (ARB) was associated with better survival when adjusted for age, sex, diabetes and coronary artery disease. This benefit was seen in all subgroups of patients with different comorbidities including those without diabetes mellitus or left ventricular systolic dysfunction.[92] Another study by Hillebrand et al. showed graft survival was longer in patients who received long-term treatment with ACEI/ARB, CCB, or a combination of ACEI/ARB and CCB. Effects were more pronounced in those with uncontrolled (>130/80 mmHg) BP at 1 year after transplant who were treated with ACE/ARBs.[93] Stigant et al. also reported a significant decrease in arterial BP after initiation of ACEI or AT II antagonist therapy with serious side effects of hyperkalemia, anemia, hypotension and worsening renal function occurring in only 9% of the study population.[94] Other classes of anti-hypertensives that are used are loop diuretics and centrally acting agents. Addition of loop diuretics may be advantageous for patients with volume overload who present with edema or unexplained weight gain. An option for refractory hypertension would be clonidine, which is a centrally acting agent available as oral tablet or transdermal patch, but this commonly causes sedation and increased sympathetic activity if discontinued abruptly.[90]
Non-pharmacologic measures including weight loss, dietary changes and increased physical activity should also be instituted for all hypertensive children to maximize the therapeutic effects of the medications.
Obesity is a modifiable risk factor that may be exacerbated by renal transplantation and contribute significantly to hypertension and cardiovascular mortality risk. Given the role of obesity in hypertension presented in this review, prevention and treatment of pretransplant obesity is a logical and necessary approach to hypertension in renal transplant patients. Recently, among adult renal transplant patients, there has been more focus on dietary protocols to manage obesity. Guida et al. showed that diet with light physical activity during the first post-transplant year improved body weight with decreased body fat, total cholesterol and plasma fasting glucose.[95] Similarly, Bellinghieri et al. reported that a low calorie, low fat diet intervention improved BMI to normal and lipid levels in post-transplant patients after 12 months.[96]
For children, both parents and patients should be counseled regarding diet and exercise with focus on prevention of significant weight gain and obesity. Sodium restriction is particularly important because high salt consumption can blunt the BP lowering effects of antihypertensive drugs and directly increase BP through volume expansion. One of the dietary approaches recommended to control hypertension is the dietary approaches to stop hypertension (DASH) or the DASH diet. This approach recommends intake of fruits, vegetables, whole grains, lean meats and low fat dairy foods to create a diet that is low in fat and sodium and rich in magnesium, potassium, calcium and fiber. Couch et al. studied the effects of a modified DASH diet versus routine dietary changes among adolescents who were pre-hypertensive or hypertensive. They found a significant change in SBP among DASH participants versus the control group despite higher initial BP measures among those in the DASH group.[97] In contrast, a cross-over randomized control trial done by Saneei et al. demonstrated that a modified DASH diet among children with metabolic syndrome prevented an increase in DBP compared with the usual dietary recommendations.[98]
In addition to nutritional changes, careful and vigorous monitoring of weight gain and other risk factors such as OSA is also warranted. Treatment of OSA with nocturnal continuous positive airway pressure, which has been demonstrated to decrease sympathetic activity in patients with OSA,[99] may be considered. Information about community based resources that promote healthy lifestyles and offer patient education programs should also be discussed with parents. These resources include the jump rope for heart and hoops for heart offered by the American Heart Association (AHA),[100] and the Alliance for a Healthier Generation which is a joint project of the AHA and the Clinton Foundation.[101] All these should be done as routine pre-transplant and post-transplant care since weight gain after transplant may potentially persist for years.
Another nondrug option that has been recently utilized to decrease BP is paced breathing using a device called RESPeRATE (InterCure, Inc). Use of this device aims to decrease breathing rate to <10 respirations/min by following musical tones.[102] The proposed mechanism is decreased peripheral sympathetic nerve activity during device-guided slow respiration[103] or reflex control of the cardiovascular system from stimulation of pulmonary stretch receptors or increased baroreceptor sensitivity.[104] However, studies which have been conducted to assess its effects have shown differing results. A meta-analysis done by Mahtani et al. showed an overall improvement in BP control, but the effects became non-significant when trials sponsored by or involving the manufacturers of the device were excluded.[105] Following this meta-analysis, another randomized placebo controlled trial conducted by Landman et al. showed no significant difference in systolic and diastolic BP between the device guided breathing group and the control group with noted respiratory difficulties in the intervention group.[106]
Other therapies include relaxation, meditation, guided imagery, hypnosis and yoga.[102] However, further studies are needed to elucidate the effectiveness of these approaches.
Finally, a key issue that needs to be addressed especially in adolescent patients is the issue of compliance to treatment. In a systematic review by Dobbels, adolescents have a reported weighted mean of prevalence of non-adherence to treatment of 43.2%, which is significantly higher than the weighted mean of 22.4% found in studies of adherence involving younger recipients or a mixed younger and adolescent populations.[107] According to Fredericks and Dore-Stites, medication non-adherence is associated with increased disease frustration, poor regimen adaptation/cognitive issues, difficulty with ingestion (e.g. number of medications, taste) and lack of parental monitoring and involvement. They recommend intervention that includes targeted education and information, motivation and behavioral skills training to increase adherence to treatment, as well as use of technology such as internet-based and cell phone interventions (eHealth).[108] Reviews of eHealth and healthcare interventions using technology revealed that enhancing standard care with reminders, disease monitoring and management and education through the use of cell phone voice and text messaging can help improve health outcomes and that interventions that incorporate behavioral methods (e.g. self-monitoring, goal setting, immediate feedback, contingency management) have better effects.[109,110]
CONCLUSIONS
The mechanism of post kidney transplant hypertension is complex and multifactorial. Control of BP in renal transplant patients is imperative to improve patient and graft survival. The management of hypertension and obesity in pediatric kidney transplant patients is based on the pathophysiology. Compared with the general pediatric hypertensive population, special attention needs to be focused on the additional impact of the side-effects and interactions of immunosuppressive medications, recurrent disease and donor and recipient comorbidities on BP control with thoughtful consideration of the competing risk of graft failure. All these taken into account make control of BP in pediatric kidney transplant patients more challenging. There is a need for prospective studies in pediatric kidney transplant patients to understand the pathophysiology of hypertension and obesity and the appropriate approach to achieve a balance between the primary need to avoid rejection and the need to lower BP and prevent obesity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Nagasako SS, Koch Nogueira PC, Machado PG, Medina Pestana JO. Arterial hypertension following renal transplantation in children - A short-term study. Pediatr Nephrol. 2003;18:1270–4. doi: 10.1007/s00467-003-1297-8. [DOI] [PubMed] [Google Scholar]
- 2.Sinha MD, Kerecuk L, Gilg J, Reid CJ British Association for Paediatric Nephrology. Systemic arterial hypertension in children following renal transplantation: Prevalence and risk factors. Nephrol Dial Transplant. 2012;27:3359–68. doi: 10.1093/ndt/gfr804. [DOI] [PubMed] [Google Scholar]
- 3.Sinha MD, Gilg JA, Kerecuk L, Reid CJ British Association for Paediatric Nephrology. Progression to hypertension in non-hypertensive children following renal transplantation. Nephrol Dial Transplant. 2012;27:2990–6. doi: 10.1093/ndt/gfr784. [DOI] [PubMed] [Google Scholar]
- 4.Mitsnefes MM, Khoury PR, McEnery PT. Early posttransplantation hypertension and poor long-term renal allograft survival in pediatric patients. J Pediatr. 2003;143:98–103. doi: 10.1016/S0022-3476(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 5.Koshy SM, Guttmann A, Hebert D, Parkes RK, Logan AG. Incidence and risk factors for cardiovascular events and death in pediatric renal transplant patients: A single center long-term outcome study. Pediatr Transplant. 2009;13:1027–33. doi: 10.1111/j.1399-3046.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 6.Mitsnefes MM. Hypertension and end-organ damage in pediatric renal transplantation. Pediatr Transplant. 2004;8:394–9. doi: 10.1111/j.1399-3046.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 7.Baluarte HJ, Gruskin AB, Ingelfinger JR, Stablein D, Tejani A. Analysis of hypertension in children post renal transplantation – A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Pediatr Nephrol. 1994;8:570–3. doi: 10.1007/BF00858130. [DOI] [PubMed] [Google Scholar]
- 8.Sorof JM, Sullivan EK, Tejani A, Portman RJ. Antihypertensive medication and renal allograft failure: A North American Pediatric Renal Transplant Cooperative Study report. J Am Soc Nephrol. 1999;10:1324–30. doi: 10.1681/ASN.V1061324. [DOI] [PubMed] [Google Scholar]
- 9.Sorof JM, Poffenbarger T, Portman R. Abnormal 24-hour blood pressure patterns in children after renal transplantation. Am J Kidney Dis. 2000;35:681–6. doi: 10.1016/s0272-6386(00)70016-3. [DOI] [PubMed] [Google Scholar]
- 10.Morgan H, Khan I, Hashmi A, Hebert D, McCrindle BW, Balfe JW. Ambulatory blood pressure monitoring after renal transplantation in children. Pediatr Nephrol. 2001;16:843–7. doi: 10.1007/s004670100668. [DOI] [PubMed] [Google Scholar]
- 11.Seeman T, Simková E, Kreisinger J, Vondrák K, Dusek J, Gilík J, et al. Control of hypertension in children after renal transplantation. Pediatr Transplant. 2006;10:316–22. doi: 10.1111/j.1399-3046.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 12.Lingens N, Dobos E, Witte K, Busch C, Lemmer B, Klaus G, et al. Twenty-four-hour ambulatory blood pressure profiles in pediatric patients after renal transplantation. Pediatr Nephrol. 1997;11:23–6. doi: 10.1007/s004670050226. [DOI] [PubMed] [Google Scholar]
- 13.Kitzmueller E, Vécsei A, Pichler J, Böhm M, Müller T, Vargha R, et al. Changes of blood pressure and left ventricular mass in pediatric renal transplantation. Pediatr Nephrol. 2004;19:1385–9. doi: 10.1007/s00467-004-1672-0. [DOI] [PubMed] [Google Scholar]
- 14.Seeman T. Ambulatory blood pressure monitoring in pediatric renal transplantation. Curr Hypertens Rep. 2012;14:608–18. doi: 10.1007/s11906-012-0301-8. [DOI] [PubMed] [Google Scholar]
- 15.Büscher R, Vester U, Wingen AM, Hoyer PF. Pathomechanisms and the diagnosis of arterial hypertension in pediatric renal allograft recipients. Pediatr Nephrol. 2004;19:1202–11. doi: 10.1007/s00467-004-1601-2. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin JE, Zhang J, Geller DS. A critical role for vascular smooth muscle in acute glucocorticoid-induced hypertension. J Am Soc Nephrol. 2008;19:1291–9. doi: 10.1681/ASN.2007080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Improved growth and cardiovascular risk after late steroid withdrawal: 2-year results of a prospective, randomised trial in paediatric renal transplantation. Nephrol Dial Transplant. 2010;25:617–24. doi: 10.1093/ndt/gfp506. [DOI] [PubMed] [Google Scholar]
- 18.Grenda R, Watson A, Trompeter R, Tönshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: The TWIST study. Am J Transplant. 2010;10:828–36. doi: 10.1111/j.1600-6143.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 19.Maduram A, John E, Hidalgo G, Bottke R, Fornell L, Oberholzer J, et al. Metabolic syndrome in pediatric renal transplant recipients: Comparing early discontinuation of steroids vs. steroid group. Pediatr Transplant. 2010;14:351–7. doi: 10.1111/j.1399-3046.2009.01243.x. [DOI] [PubMed] [Google Scholar]
- 20.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1–14. doi: 10.1097/TP.0b013e3181c518cc. [DOI] [PubMed] [Google Scholar]
- 21.Scherrer U, Vissing SF, Morgan BJ, Rollins JA, Tindall RS, Ring S, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323:693–9. doi: 10.1056/NEJM199009133231101. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JJ. Hypertensinogenic mechanism of the calcineurin inhibitors. Curr Hypertens Rep. 2002;4:377–80. doi: 10.1007/s11906-002-0067-5. [DOI] [PubMed] [Google Scholar]
- 23.El-Mas MM, Omar AG, Helmy MM, Mohy El-Din MM. Crosstalk between central pathways of nitric oxide and carbon monoxide in the hypertensive action of cyclosporine. Neuropharmacology. 2012;62:1890–6. doi: 10.1016/j.neuropharm.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Denburg MR, Pradhan M, Shults J, Jones A, Palmer JA, Baluarte HJ, et al. Longitudinal relations between obesity and hypertension following pediatric renal transplantation. Pediatr Nephrol. 2010;25:2129–39. doi: 10.1007/s00467-010-1572-4. [DOI] [PubMed] [Google Scholar]
- 25.Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: Evidence for improved allograft survival at five years. Transplantation. 2002;73:775–82. doi: 10.1097/00007890-200203150-00021. [DOI] [PubMed] [Google Scholar]
- 26.Seeman T. Hypertension after renal transplantation. Pediatr Nephrol. 2009;24:959–72. doi: 10.1007/s00467-007-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margreiter R European Tacrolimus vs Ciclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: A randomised multicentre study. Lancet. 2002;359:741–6. doi: 10.1016/S0140-6736(02)07875-3. [DOI] [PubMed] [Google Scholar]
- 28.Krämer BK, Del Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, Ortuño J, et al. Efficacy and safety of tacrolimus compared with ciclosporin a in renal transplantation: Three-year observational results. Nephrol Dial Transplant. 2008;23:2386–92. doi: 10.1093/ndt/gfn004. [DOI] [PubMed] [Google Scholar]
- 29.Ferraresso M, Turolo S, Ghio L, Tirelli AS, Belingheri M, Villa R, et al. Association between CYP3A5 polymorphisms and blood pressure in kidney transplant recipients receiving calcineurin inhibitors. Clin Exp Hypertens. 2011;33:359–65. doi: 10.3109/10641963.2011.561896. [DOI] [PubMed] [Google Scholar]
- 30.Silverstein DM, Leblanc P, Hempe JM, Ramcharan T, Boudreaux JP. Tracking of blood pressure and its impact on graft function in pediatric renal transplant patients. Pediatr Transplant. 2007;11:860–7. doi: 10.1111/j.1399-3046.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 31.Mitsnefes MM, Khoury P, McEnery PT. Body mass index and allograft function in pediatric renal transplantation. Pediatr Nephrol. 2002;17:535–9. doi: 10.1007/s00467-002-0863-9. [DOI] [PubMed] [Google Scholar]
- 32.Boschetti SB, Nogueira PC, Pereira AM, Fisberg M, Pestana JO. Prevalence, risk factors, and consequences of overweight in children and adolescents who underwent renal transplantation – Short- and medium-term analysis. Pediatr Transplant. 2013;17:41–7. doi: 10.1111/petr.12020. [DOI] [PubMed] [Google Scholar]
- 33.Foster BJ, Martz K, Gowrishankar M, Stablein D, Al-Uzri A. Weight and height changes and factors associated with greater weight and height gains after pediatric renal transplantation: A NAPRTCS study. Transplantation. 2010;89:1103–12. doi: 10.1097/TP.0b013e3181d3c9be. [DOI] [PubMed] [Google Scholar]
- 34.Omoloja A, Stolfi A, Mitsnefes M. Pediatric obesity at renal transplantation: A single center experience. Pediatr Transplant. 2005;9:770–2. doi: 10.1111/j.1399-3046.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 35.Vester U, Schaefer A, Kranz B, Wingen AM, Nadalin S, Paul A, et al. Development of growth and body mass index after pediatric renal transplantation. Pediatr Transplant. 2005;9:445–9. doi: 10.1111/j.1399-3046.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 36.Hanevold CD, Ho PL, Talley L, Mitsnefes MM. Obesity and renal transplant outcome: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics. 2005;115:352–6. doi: 10.1542/peds.2004-0289. [DOI] [PubMed] [Google Scholar]
- 37.Koulouridis E, Georgalidis K, Kostimpa I, Kalantzi M, Ntouto P, Koulouridis I, et al. Factors influencing blood pressure control in children and adolescents. Int Urol Nephrol. 2008;40:741–8. doi: 10.1007/s11255-008-9340-0. [DOI] [PubMed] [Google Scholar]
- 38.da Silva AA, do Carmo J, Dubinion J, Hall JE. The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep. 2009;11:206–11. doi: 10.1007/s11906-009-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalaitzidis RG, Siamopoulos KC. The role of obesity in kidney disease: Recent findings and potential mechanisms. Int Urol Nephrol. 2011;43:771–84. doi: 10.1007/s11255-011-9974-1. [DOI] [PubMed] [Google Scholar]
- 40.Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2009;33:116–24. doi: 10.1016/j.neubiorev.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry SL, Barzel B, Wood-Bradley RJ, Burke SL, Head GA, Armitage JA. Developmental origins of obesity-related hypertension. Clin Exp Pharmacol Physiol. 2012;39:799–806. doi: 10.1111/j.1440-1681.2011.05579.x. [DOI] [PubMed] [Google Scholar]
- 42.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–71. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 43.Hutley L, Prins JB. Fat as an endocrine organ: Relationship to the metabolic syndrome. Am J Med Sci. 2005;330:280–9. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–6. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–8. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- 46.Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome – Causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–72. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–90. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: The importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 49.Grassi G, Facchini A, Trevano FQ, Dell’Oro R, Arenare F, Tana F, et al. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46:321–5. doi: 10.1161/01.HYP.0000174243.39897.6c. [DOI] [PubMed] [Google Scholar]
- 50.Rüster C, Wolf G. The role of the renin-angiotensin- aldosterone system in obesity-related renal diseases. Semin Nephrol. 2013;33:44–53. doi: 10.1016/j.semnephrol.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Thethi T, Kamiyama M, Kobori H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep. 2012;14:160–9. doi: 10.1007/s11906-012-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–62. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 53.Heidotting NA, Ahlenstiel T, Kreuzer M, Franke D, Pape L. The influence of low donor age, living related donation and pre-emptive transplantation on end-organ damage based on arterial hypertension after paediatric kidney transplantation. Nephrol Dial Transplant. 2012;27:1672–6. doi: 10.1093/ndt/gfr549. [DOI] [PubMed] [Google Scholar]
- 54.Thomas B, Taber DJ, Srinivas TR. Hypertension after kidney transplantation: A pathophysiologic approach. Curr Hypertens Rep. 2013;15:458–69. doi: 10.1007/s11906-013-0381-0. [DOI] [PubMed] [Google Scholar]
- 55.Palanisamy A, Reeves-Daniel AM, Freedman BI. The impact of APOL1, CAV1, and ABCB1 gene variants on outcomes in kidney transplantation: Donor and recipient effects. Pediatr Nephrol. 2013 doi: 10.1007/s00467-013-2531-7. DOI 10.1007/s00467-013-2531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11:1025–30. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, et al. The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am J Transplant. 2012;12:1924–8. doi: 10.1111/j.1600-6143.2012.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore J, McKnight AJ, Simmonds MJ, Courtney AE, Hanvesakul R, Brand OJ, et al. Association of caveolin-1 gene polymorphism with kidney transplant fibrosis and allograft failure. JAMA. 2010;303:1282–7. doi: 10.1001/jama.2010.356. [DOI] [PubMed] [Google Scholar]
- 59.North American Pediatric Renal Trials and Collaborative Studies (2008). NAPRTCS 2008 Annual Report. [Last retrieved 2013 Sep 07]. Available from: http://www.emmes.com/study/ped/annlrept/Report2008 .
- 60.Höcker B, Tönshoff B. Treatment strategies to minimize or prevent chronic allograft dysfunction in pediatric renal transplant recipients: An overview. Paediatr Drugs. 2009;11:381–96. doi: 10.2165/11316100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Henning BF, Kuchlbauer S, Böger CA, Obed A, Farkas S, Zülke C, et al. Percutaneous transluminal angioplasty as first-line treatment of transplant renal artery stenosis. Clin Nephrol. 2009;71:543–9. [PubMed] [Google Scholar]
- 62.Hagen G, Wadström J, Magnusson M, Magnusson A. Outcome after percutaneous transluminal angioplasty of arterial stenosis in renal transplant patients. Acta Radiol. 2009;50:270–5. doi: 10.1080/02841850902718763. [DOI] [PubMed] [Google Scholar]
- 63.Shroff R, Knott C, Gullett A, Wells D, Marks SD, Rees L. Vitamin D deficiency is associated with short stature and may influence blood pressure control in paediatric renal transplant recipients. Pediatr Nephrol. 2011;26:2227–33. doi: 10.1007/s00467-011-1920-z. [DOI] [PubMed] [Google Scholar]
- 64.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–92. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 65.Feneis JF, Arora RR. Role of vitamin D in blood pressure homeostasis. Am J Ther. 2010;17:e221–9. doi: 10.1097/MJT.0b013e3181d16999. [DOI] [PubMed] [Google Scholar]
- 66.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: Meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–21. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 69.Flynn JT. Ambulatory blood pressure monitoring should be routinely performed after pediatric renal transplantation. Pediatr Transplant. 2012;16:533–6. doi: 10.1111/j.1399-3046.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 70.Giordano U, Matteucci MC, Calzolari A, Turchetta A, Rizzoni G, Alpert BS. Ambulatory blood pressure monitoring in children with aortic coarctation and kidney transplantation. J Pediatr. 2000;136:520–3. doi: 10.1016/s0022-3476(00)90016-7. [DOI] [PubMed] [Google Scholar]
- 71.McGlothan KR, Wyatt RJ, Ault BH, Hastings MC, Rogers T, DiSessa T, et al. Predominance of nocturnal hypertension in pediatric renal allograft recipients. Pediatr Transplant. 2006;10:558–64. doi: 10.1111/j.1399-3046.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 72.Ferraris JR, Ghezzi L, Waisman G, Krmar RT. ABPM vs office blood pressure to define blood pressure control in treated hypertensive paediatric renal transplant recipients. Pediatr Transplant. 2007;11:24–30. doi: 10.1111/j.1399-3046.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 73.Paripovic D, Kostic M, Spasojevic B, Kruscic D, Peco-Antic A. Masked hypertension and hidden uncontrolled hypertension after renal transplantation. Pediatr Nephrol. 2010;25:1719–24. doi: 10.1007/s00467-010-1552-8. [DOI] [PubMed] [Google Scholar]
- 74.Basiratnia M, Esteghamati M, Ajami GH, Amoozgar H, Cheriki C, Soltani M, et al. Blood pressure profile in renal transplant recipients and its relation to diastolic function: Tissue Doppler echocardiographic study. Pediatr Nephrol. 2011;26:449–57. doi: 10.1007/s00467-010-1724-6. [DOI] [PubMed] [Google Scholar]
- 75.Mange KC, Feldman HI, Joffe MM, Fa K, Bloom RD. Blood pressure and the survival of renal allografts from living donors. J Am Soc Nephrol. 2004;15:187–93. doi: 10.1097/01.asn.0000104574.04006.08. [DOI] [PubMed] [Google Scholar]
- 76.Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney Int. 1998;53:217–22. doi: 10.1046/j.1523-1755.1998.00744.x. [DOI] [PubMed] [Google Scholar]
- 77.Raiss Jalali GA, Fazelzadeh A, Mehdizadeh AR. Effect of hypertension on transplant kidney function: Three year of follow-up. Transplant Proc. 2007;39:941–2. doi: 10.1016/j.transproceed.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 78.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–81. doi: 10.1053/j.ajkd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: A significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70–4. doi: 10.1097/00007890-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 80.Papalia T, Greco R, Lofaro D, Maestripieri S, Mancuso D, Bonofiglio R. Impact of body mass index on graft loss in normal and overweight patients: Retrospective analysis of 206 renal transplants. Clin Transplant. 2010;24:E241–6. doi: 10.1111/j.1399-0012.2010.01258.x. [DOI] [PubMed] [Google Scholar]
- 81.Sancho A, Avila A, Gavela E, Beltrán S, Fernández-Nájera JE, Molina P, et al. Effect of overweight on kidney transplantation outcome. Transplant Proc. 2007;39:2202–4. doi: 10.1016/j.transproceed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 82.Kovesdy CP, Czira ME, Rudas A, Ujszaszi A, Rosivall L, Novak M, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant. 2010;10:2644–51. doi: 10.1111/j.1600-6143.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 83.el-Agroudy AE, Wafa EW, Gheith OE, Shehab el-Dein AB, Ghoneim MA. Weight gain after renal transplantation is a risk factor for patient and graft outcome. Transplantation. 2004;77:1381–5. doi: 10.1097/01.tp.0000120949.86038.62. [DOI] [PubMed] [Google Scholar]
- 84.Ducloux D, Kazory A, Simula-Faivre D, Chalopin JM. One-year post-transplant weight gain is a risk factor for graft loss. Am J Transplant. 2005;5:2922–8. doi: 10.1111/j.1600-6143.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- 85.Seeman T, Simková E, Kreisinger J, Vondrák K, Dusek J, Gilík J, et al. Improved control of hypertension in children after renal transplantation: Results of a two-year interventional trial. Pediatr Transplant. 2007;11:491–7. doi: 10.1111/j.1399-3046.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 86.National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. [Last retrieved 2013 Sep 07]. Available from: http://www.kidney.org/professionals/KDOQI/guidelines_bp .
- 87.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, et al. ESCAPE Trial Group. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–50. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 88.Opelz G, Döhler B Collaborative Transplant Study. Improved long-term outcomes after renal transplantation associated with blood pressure control. Am J Transplant. 2005;5:2725–31. doi: 10.1111/j.1600-6143.2005.01093.x. [DOI] [PubMed] [Google Scholar]
- 89.Cross NB, Webster AC, Masson P, O’connell PJ, Craig JC. Antihypertensives for kidney transplant recipients: Systematic review and meta-analysis of randomized controlled trials. Transplantation. 2009;88:7–18. doi: 10.1097/TP.0b013e3181a9e960. [DOI] [PubMed] [Google Scholar]
- 90.Dunn BL, Teusink AC, Taber DJ, Hemstreet BA, Uber LA, Weimert NA. Management of hypertension in renal transplant patients: A comprehensive review of nonpharmacologic and pharmacologic treatment strategies. Ann Pharmacother. 2010;44:1259–70. doi: 10.1345/aph.1P004. [DOI] [PubMed] [Google Scholar]
- 91.Arbeiter K, Pichler A, Stemberger R, Mueller T, Ruffingshofer D, Vargha R, et al. ACE inhibition in the treatment of children after renal transplantation. Pediatr Nephrol. 2004;19:222–6. doi: 10.1007/s00467-003-1317-8. [DOI] [PubMed] [Google Scholar]
- 92.Aftab W, Varadarajan P, Rasool S, Kore A, Pai RG. Beta and angiotensin blockades are associated with improved 10-year survival in renal transplant recipients. J Am Heart Assoc. 2013;2:e000091. doi: 10.1161/JAHA.112.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hillebrand U, Suwelack BM, Loley K, Lang D, Reuter S, Amler S, et al. Blood pressure, antihypertensive treatment, and graft survival in kidney transplant patients. Transpl Int. 2009;22:1073–80. doi: 10.1111/j.1432-2277.2009.00922.x. [DOI] [PubMed] [Google Scholar]
- 94.Stigant CE, Cohen J, Vivera M, Zaltzman JS. ACE inhibitors and angiotensin II antagonists in renal transplantation: An analysis of safety and efficacy. Am J Kidney Dis. 2000;35:58–63. doi: 10.1016/S0272-6386(00)70302-7. [DOI] [PubMed] [Google Scholar]
- 95.Guida B, Trio R, Laccetti R, Nastasi A, Salvi E, Perrino NR, et al. Role of dietary intervention on metabolic abnormalities and nutritional status after renal transplantation. Nephrol Dial Transplant. 2007;22:3304–10. doi: 10.1093/ndt/gfm345. [DOI] [PubMed] [Google Scholar]
- 96.Bellinghieri G, Bernardi A, Piva M, Pati T, Stoppa F, Scaramuzzo P, et al. Metabolic syndrome after kidney transplantation. J Ren Nutr. 2009;19:105–10. doi: 10.1053/j.jrn.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Couch SC, Saelens BE, Levin L, Dart K, Falciglia G, Daniels SR. The efficacy of a clinic-based behavioral nutrition intervention emphasizing a DASH-type diet for adolescents with elevated blood pressure. J Pediatr. 2008;152:494–501. doi: 10.1016/j.jpeds.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 98.Saneei P, Hashemipour M, Kelishadi R, Rajaei S, Esmaillzadeh A. Effects of recommendations to follow the Dietary Approaches to Stop Hypertension (DASH) diet v. usual dietary advice on childhood metabolic syndrome: A randomised cross-over clinical trial. Br J Nutr. 2013;110:2250–9. doi: 10.1017/S0007114513001724. [DOI] [PubMed] [Google Scholar]
- 99.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–5. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 100. [Last accessed on 2013 Sep 07]. Available from: http://www.heart.org .
- 101. [Last accessed on 2013 Sep 07]. Available from: http://www.healthiergeneration.org .
- 102.DeSimone ME, Crowe A. Nonpharmacological approaches in the management of hypertension. J Am Acad Nurse Pract. 2009;21:189–96. doi: 10.1111/j.1745-7599.2009.00395.x. [DOI] [PubMed] [Google Scholar]
- 103.Oneda B, Ortega KC, Gusmão JL, Araújo TG, Mion D., Jr Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens Res. 2010;33:708–12. doi: 10.1038/hr.2010.74. [DOI] [PubMed] [Google Scholar]
- 104.Grossman E, Grossman A, Schein MH, Zimlichman R, Gavish B. Breathing-control lowers blood pressure. J Hum Hypertens. 2001;15:263–9. doi: 10.1038/sj.jhh.1001147. [DOI] [PubMed] [Google Scholar]
- 105.Mahtani KR, Nunan D, Heneghan CJ. Device-guided breathing exercises in the control of human blood pressure: Systematic review and meta-analysis. J Hypertens. 2012;30:852–60. doi: 10.1097/HJH.0b013e3283520077. [DOI] [PubMed] [Google Scholar]
- 106.Landman GW, Drion I, van Hateren KJ, van Dijk PR, Logtenberg SJ, Lambert J, et al. Device-guided breathing as treatment for hypertension in type 2 diabetes mellitus: A randomized, double-blind, sham-controlled trial. JAMA Intern Med. 2013;173:1346–50. doi: 10.1001/jamainternmed.2013.6883. [DOI] [PubMed] [Google Scholar]
- 107.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: A systematic review. Pediatr Transplant. 2010;14:603–13. doi: 10.1111/j.1399-3046.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 108.Fredericks EM, Dore-Stites D. Adherence to immunosuppressants: How can it be improved in adolescent organ transplant recipients? Curr Opin Organ Transplant. 2010;15:614–20. doi: 10.1097/MOT.0b013e32833d3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: A systematic review. Telemed J E Health. 2009;15:231–40. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 110.Cushing CC, Steele RG. A meta-analytic review of eHealth interventions for pediatric health promoting and maintaining behaviors. J Pediatr Psychol. 2010;35:937–49. doi: 10.1093/jpepsy/jsq023. [DOI] [PubMed] [Google Scholar]


