Abstract
Background:
Hypertension is more common in adults with type 1 diabetes mellitus (T1DM) than the general population. The aim of this study was to detect the pre-hypertensive stage in children with T1D and to evaluate its correlation with diabetic nephropathy compared to non-diabetic children.
Methods:
This was a prospective cross-sectional study in an out-patient clinic of a university hospital. A total of 62 which consists of 36 males and 26 females patients with stable T1D with a median age of 13 year and 42 age - sex-matched healthy children were entered in the study between September 2008 and February 2011. Three readings of blood pressure were recorded. Fasting blood sample was drawn for hemoglobin A1C (HbA1C), creatinine and a 24 h urine aliquot was collected to measure microalbumin, creatinine and volume to estimate glomerular filtration rate (eGFR).
Results:
From 62 children with T1DM, 25.8% were in pre-hypertensive stage, 4.8% Stage 1, and 1.6% Stage 2. In controls, 1 (2.4%) out of 42 children was in pre-hypertensive stage (P < 0.0001). Abnormal blood pressures were correlated with eGFR and the duration of disease (P < 0.05), but there were not associated with microalbominuria or HbA1C level.
Conclusions:
There was a higher rate of early stage of high normal blood pressure in children with T1DM compared with the healthy controls and this abnormality was only correlated with puberty stage and glomerular filtration rate.
Keywords: Blood pressure, chronic, diabetes mellitus, diabetic nephropathies, glomerular filtration rate, hypertension, kidney failure
INTRODUCTION
Hypertension is a risk factor for development of diabetic nephropathy. Previous case-control study showed hypertension as an independent factor that had a good correlation with microalbominuria in type 1 diabetes (T1D).[1] Because of utilizing old criteria of abnormal hypertension, the prevalence of hypertension reported the same as the prevalence of essential hypertension among normal population.[2] Even nocturnal systolic blood pressure (SBP) elevation precedes the appearance of microalbominuria.[3] The prevalence of masked hypertension in normotensive T1D mellitus (T1DM) is estimated to be around 13%. In this group, nocturnal hypertension is correlated with higher retinopathy.[4] We evaluated the prevalence of abnormal blood pressure in T1DM children with the new classification of blood pressure[5] and assessed its correlation with glomerular filtration rate (GFR) and diabetic control.
METHODS
A case-control study conducted between September 2008 and February 2011 in an out-patient clinic of endocrinology. A total of 62 patients with stable T1DM age 6-20 years old with no history of recent infection were entered in the study. Patients with severe renal insufficiency, diabetic ketoacidosis and patients who received steroid for any reason were excluded from the study. 42 age - sex-matched healthy cases that were referred for height evaluation or the siblings of the index patients who had normal physical examination and normal routine biochemistry lab tests were selected as controls. A pediatric endocrinologist determined the sexual maturity rating (SMR) of subjects. Informed consents were taken from the patients or parents before enrolment. It was performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki revised in Tokyo 2008. The study was approved by the ethical committee of Iran University of medical sciences and followed by the Institution's review board for human subjects guidelines.
Blood pressure was measured in a stable condition on the non-dominant arm while the patient was sitting in a comfortable position. Aneroid sphygmomanometers (Welch Allyn Inc., USA) used for measuring blood pressure. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescent was used to define abnormal blood pressure.[5,6] Abnormal blood pressure was classified to pre-hypertensive (>90 to <95% or ≥120/80 mmHg in adolescence even it is below 90%), Stage 1 hypertension (95-99% +5 mmHg), and Stage 2 hypertension (>99% +5 mmHg).[5,6]
Hemoglobin A1C (HbA1C) less than 7% was considered as good control. Microalbuminuria was measured by the enzyme-linked immunosorbent assay (ELISA) kit (Orgentec, Mainz, Germany) using Awareness STAT FAX 2100 series microplate reader (Awareness Technolgy, USA). Persistent micoalbuminuria was defined by detection of microalbuminuria on two consecutive occasions within 3 months of detection.[7,8] Microalbuminuria was defined by albumin excretion more than 30 mg/g creatinine.
GFR was estimated using Schwatz formula:
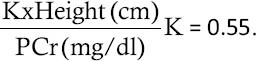
Chronic kidney disease was classified according to GFR: more than 90 ml/min/1.73 m2 (Stage 1), 60-90 (Stage 2), 30-60 (Stage 3), 15-30 (Stage 4) and less than 15 (Stage 5). Glomerular hyperfiltration was defined by GFR more than 140 ml/min/1.73 m.[2,9,10]
Cystatin-c was measured only in diabetic patients and GFRCystC was calculated by using Zappitelli formula: GFR = 75.94/(CysC [1.17]).[11] Serum and urine cystatin-c levels were measured by using a sandwich ELISA method (Biovendor, CZECH Republic) by using Awareness STAT FAX 2100 series microplate reader (USA). The laboratory coefficient variation was 4.8%.
The primary objective of this study was to evaluate the frequency of abnormal blood pressure then the correlation between blood pressure and microalbuminuria and estimate GFR (eGFRCr).
Chi-square, ANOVA, and Student's t-test, and general linear model of variance were used to analyze data. P < 0.05 was considered to be significant.
RESULTS
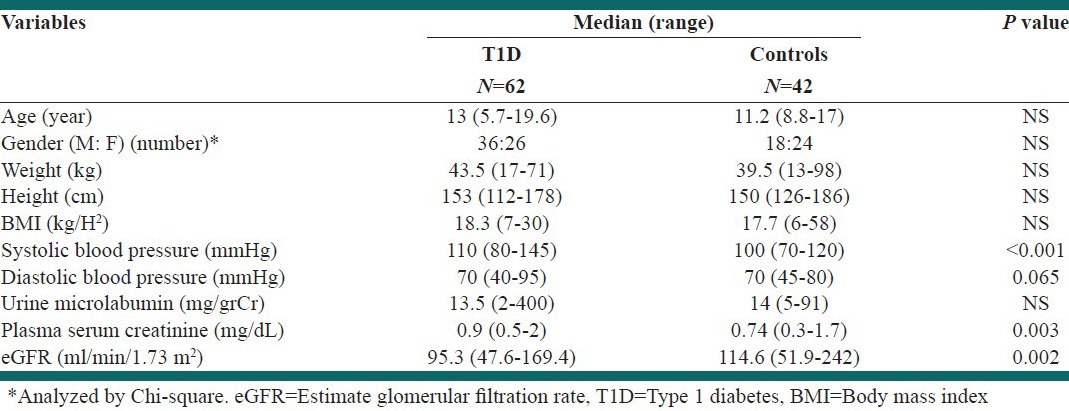
A total of 62 (36 males, 26 females) patients with stable T1DM with the median age 13 year (range: 5.8-19.6) and 42 age - sex-matched healthy cases were entered in the study between September 2008 and February 2011. Table 1 depicts demographic data of groups that show both groups were similar in basic data; however, the diabetic patients had significantly higher SBP and lower eGFR.
Table 1.
Demographic data of diabetic patients and healthy children
In overall, 28 (45.2%) out of 62 diabetic patients had abnormal blood pressure, but only two (4.8%) of controls had abnormal blood pressure (P < 0.001). From 62 children with T1DM, 25.8% were in pre-hypertensive stage, 4.8% Stage 1, and 1.6% Stage 2. In controls, one (2.4%) out of 42 children was in pre-hypertensive stage (P < 0.0001).
As shown in Table 2, the diabetic patients classified to pre-hypertensive, hypertensive and normotensive cases. Patients with normal blood pressure were younger, had lower body mass index (BMI) and lower SMR (P < 0.05). Hyperfiltration was seen in two normotenisve cases (5.4%) and six normotensive controls (15.4%) (P < 0.05). Table 3 depicts the frequency of abnormal blood pressure in each GFR classifications.
Table 2.
Independent variables in diabetic patients with different systolic blood pressure stages
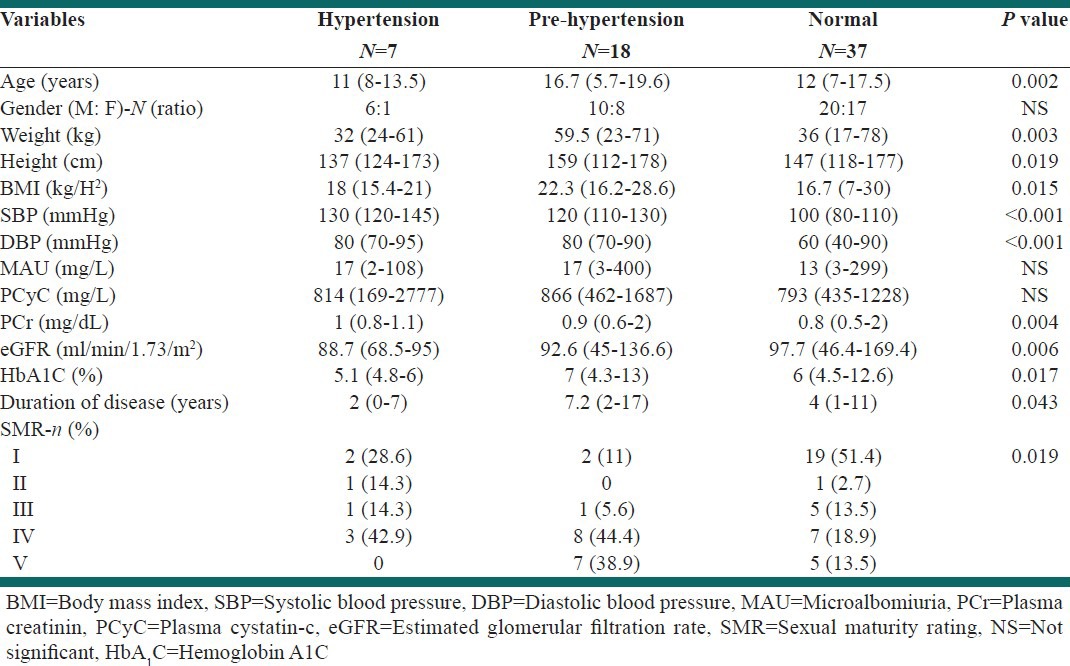
Table 3.
The correlation between GFR classification and blood pressure in diabetic patients

Univariate analysis revealed that abnormal blood pressure was correlated with eGFR (P: 0.004), duration of disease (P: 0.037) and high SBP was correlated with glomerular hyperfiltration (eGFR >140 ml/min/1.73 m2) (P = 0.015).
Stepwise regression analysis revealed the correlation between abnormal blood pressure and GFR (P = 0.017) and SMR (P = 0.001) in diabetic patients.
DISCUSSION
This study revealed that rate of pre-hypertension is nine-times higher than the normal population.
The abnormal blood pressure was related to SMR and GFR in diabetic patients.
Nørgaard et al. studies in a large population of Danish people and reported the prevalence of hypertension was at least 3.5 times higher in adults with T1DM and the clinical nephropathy was seen in 4% of this group.[2] However, only 11% of our diabetic patients had hypertension and one-third was in pre-hypertensive stage. Okada et al. assessed glomerular filtration in pre-diabetic and pre-hypertensive in a large number of people who went under check-up. The result was the relation between the hypefiltration and pre-diabetic and prehypertensive stage.[12] This reflected the importance of early diagnosis of abnormal blood pressure and blood glucose level to protect the kidney from hyperfiltration. Similarly, abnormal blood pressure in diabetic patients in our study group was associated with eGFR; moreover, systolic hypertension was correlated with hyperfiltration. Wilkinson et al. studied 35 T1DM and 35 controls who had matched blood pressure evaluated for endothelial dysfunction. They observed that T1D patients had increased systemic stiffness and augmentation index.[13] Another study shows that the presence of hypertension and white coat hypertension in children with T1D are associated more with arterial stiffness when they are compared with normotensive T1D.[14] The impaired baroreceptor reflex might have a role in the night time hypertension or non-dipper status in diabetic children.[15] The duration of diabetes was surprisingly longer in pre-hypertensive patients. We had no justification for this finding. Although, pre-hypertensive patients had higher HA1C level and BMI than hypertensive and normotensive patients. Hypertension in diabetic patients might be a coincident finding during our evaluation. However, we could not rule out the possibility of white-coat hypertension in this group. In addition, the diabetic adolescent in puberty stage had a higher rate of abnormal blood pressure. This finding emphasis the influence of hormonal changes in diabetic cases. This association was absent in healthy adolescent.
Lurbe et al. monitored 75 adolescents with T1DM longitudinally to assess the time correlation between night time hypertension and occurrence of microalbuminuria. The patients’ blood pressures were recorded by ambulatory blood pressure monitoring at the time of enrolment to study and 2 year later. They found elevated night time blood pressure preceded microalbuminuria.[3] In our study, we did not find any correlation between stage of blood pressure and microalbuminuria. It signifies that microalbuminoria is either insensitive or late marker of diabetic nephropathy. Correct blood pressure measurement and interpreting according the new classification of blood pressure are more important than considering microlbuminuria as a sole an alarm for incipient nephropathy. Pre-hypertensive diabetic patients observed more in those who reached to pubertal stage or had higher BMI. Thorn studied the incidence of metabolic syndrome in more than 2000 T1D and found that the odd ratio of diabetic nephropathy increased more than three folds in diabetic patients with metabolic syndrome.[16]
In our study, there was no correlation between HbA1C and abnormal blood pressure. Chen et al. observed that poor controlled type 2 diabetes had a higher rate of hypertension, arterial stiffness.[17] This association was not found in normotensive T1DM patients. A nation-wide study was done by Raile et al.; hypertension, HbA1C, duration of disease and hyperlipidemia increased risk of diabetic nephropathy, in contrast childhood diabetic onset was protective.[18]
It was interesting that GFR based creatinin was associate with abnormal blood pressure compared to cystatin-c. However, cytstatin-c is recommended as sensitive marker to creatingn for estimating GFR. Our previous study had shown that GFR based cystatin-c formulas in diabetic patients were not accurate at least in higher level of GFR.[19]
Small sample size, the absence of 24 h blood pressure measurements and absence of lipid profile measurements were limitation of this study.
CONCLUSIONS
There was a higher rate of early stage of blood pressure changes in children with T1DM compared with the healthy controls and this abnormality was only correlated with GFR. The odd ratio of abnormal blood pressure was higher in pubertal stage in T1DM.
ACKNOWLEDGMENT
This study has been supported by Endocrine Research Center (Firouzgar), Institute of Endocrinology and Metabolism (Hemmat Campus), Iran University of Medical Sciences, grant number 653 dated 2009.
Footnotes
Source of Support: Endocrine Research Centre (Firouzgar), Institute of Endocrinology and Metabolism (Hemmat Campus), Iran University of Medical Sciences, grant number 653 dated 2009
Conflict of Interest: None declared
REFERENCES
- 1.Wiseman M, Viberti G, Mackintosh D, Jarrett RJ, Keen H. Glycaemia, arterial pressure and micro-albuminuria in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1984;26:401–5. doi: 10.1007/BF00262209. [DOI] [PubMed] [Google Scholar]
- 2.Nørgaard K, Feldt-Rasmussen B, Borch-Johnsen K, Saelan H, Deckert T. Prevalence of hypertension in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:407–10. doi: 10.1007/BF00404089. [DOI] [PubMed] [Google Scholar]
- 3.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues TC, Canani LH, Viatroski RS, Hoffmann LH, Esteves JF, Gross JL. Masked hypertension, nocturnal blood pressure and retinopathy in normotensive patients with type 1 diabetes. Diabetes Res Clin Pract. 2010;87:240–5. doi: 10.1016/j.diabres.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Lurbe E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invitti C, et al. Management of high blood pressure in children and adolescents: Recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–42. doi: 10.1097/HJH.0b013e32832f4f6b. [DOI] [PubMed] [Google Scholar]
- 6.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 7.Karagiannis A, Mikhailidis DP, Tziomalos K, Kakafika AI, Athyros VG. Has the time come for a new definition of microalbuminuria? Curr Vasc Pharmacol. 2008;6:81–3. doi: 10.2174/157016108783955329. [DOI] [PubMed] [Google Scholar]
- 8.Toto RD. Microalbuminuria: Definition, detection, and clinical significance. J Clin Hypertens (Greenwich) 2004;6:2–7. doi: 10.1111/j.1524-6175.2004.4064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarelli F, Verrotti A, Morgese G. Glomerular hyperfiltration increases the risk of developing microalbuminuria in diabetic children. Pediatr Nephrol. 1995;9:154–8. doi: 10.1007/BF00860729. [DOI] [PubMed] [Google Scholar]
- 10.Helal I, Reed B, McFann K, Yan XD, Fick-Brosnahan GM, Cadnapaphornchai M, et al. Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:2439–43. doi: 10.2215/CJN.01010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–30. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 12.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant. 2012;27:1821–5. doi: 10.1093/ndt/gfr651. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson IB, MacCallum H, Rooijmans DF, Murray GD, Cockcroft JR, McKnight JA, et al. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM. 2000;93:441–8. doi: 10.1093/qjmed/93.7.441. [DOI] [PubMed] [Google Scholar]
- 14.Suláková T, Janda J, Cerná J, Janštová V, Feber J. Assessment of arterial stiffness from ambulatory blood pressure monitoring in children with diabetes mellitus type-1 (DMT1) J Hum Hypertens. 2012;26:357–64. doi: 10.1038/jhh.2011.38. [DOI] [PubMed] [Google Scholar]
- 15.Krause M, Rüdiger H, Bald M, Näke A, Paditz E. Autonomic blood pressure control in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:255–63. doi: 10.1111/j.1399-5448.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 16.Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Ferholm K, Saraheimo M, et al. Metabolic syndrome in type 1 diabetes. Diabetes Care. 2005;28:2019–202. doi: 10.2337/diacare.28.8.2019. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Huang Y, Li X, Xu M, Bi Y, Zhang Y, et al. Association of arterial stiffness with HbA1c in 1,000 type 2 diabetic patients with or without hypertension. Endocrine. 2009;36:262–7. doi: 10.1007/s12020-009-9221-z. [DOI] [PubMed] [Google Scholar]
- 18.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, et al. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: Effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30:2523–8. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- 19.Hooman N, Roohani F, Moradi S, Mobarra M, Najafizadeh M. Cystatin C as an early marker of diabetic nephropathy in children with Type 1 diabetes mellitus. Pediatr Nephrol. 2013;28:1533–689. [Google Scholar]


