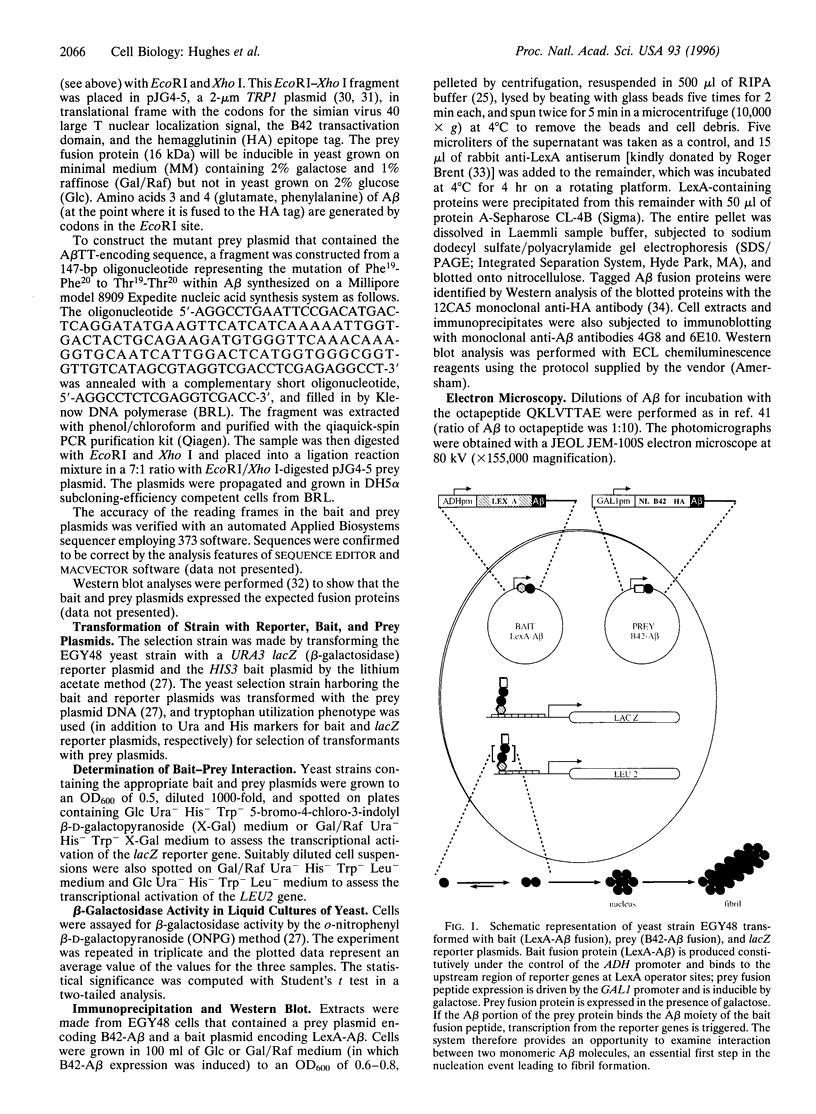
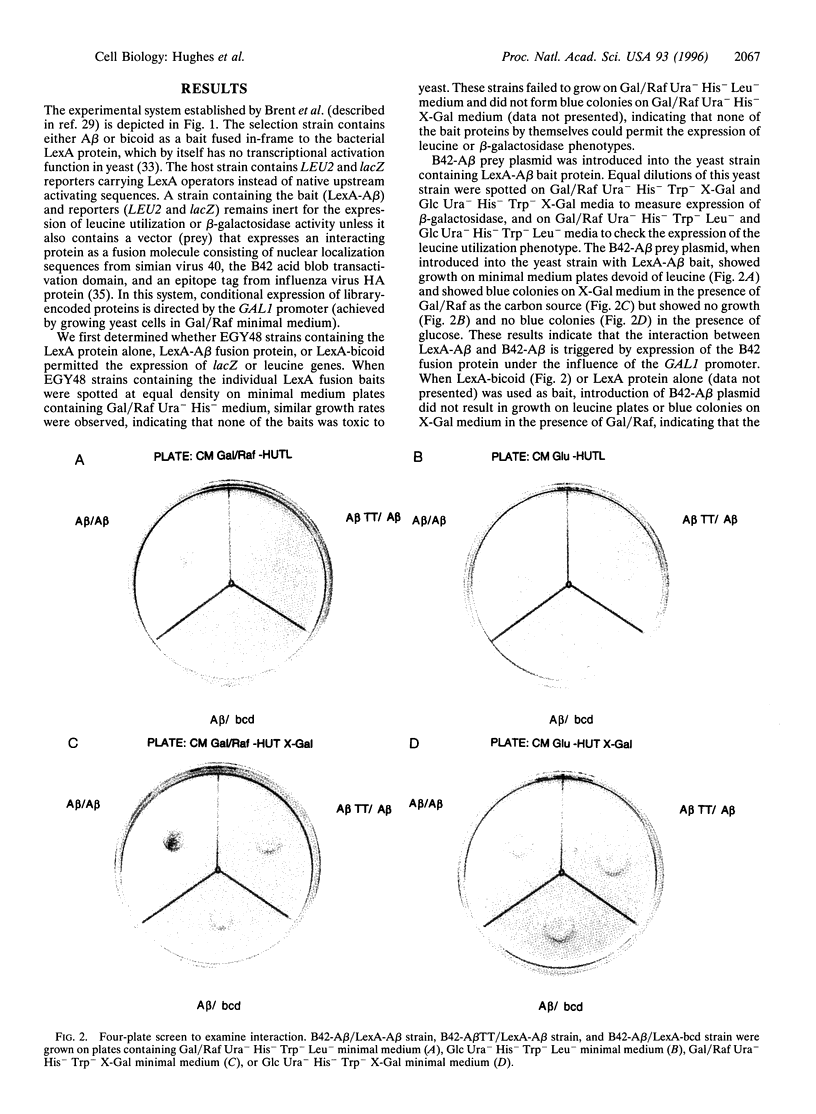
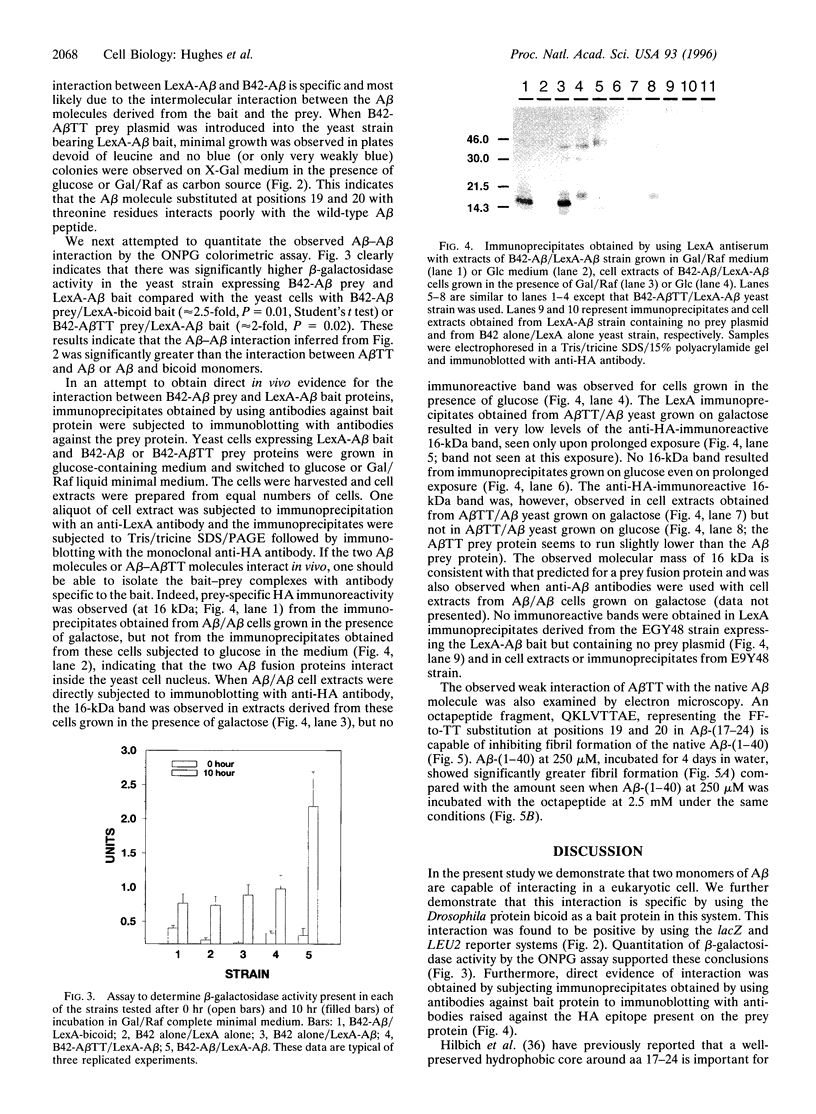
Abstract
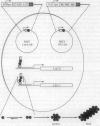
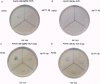
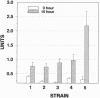
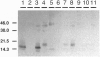
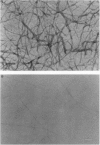
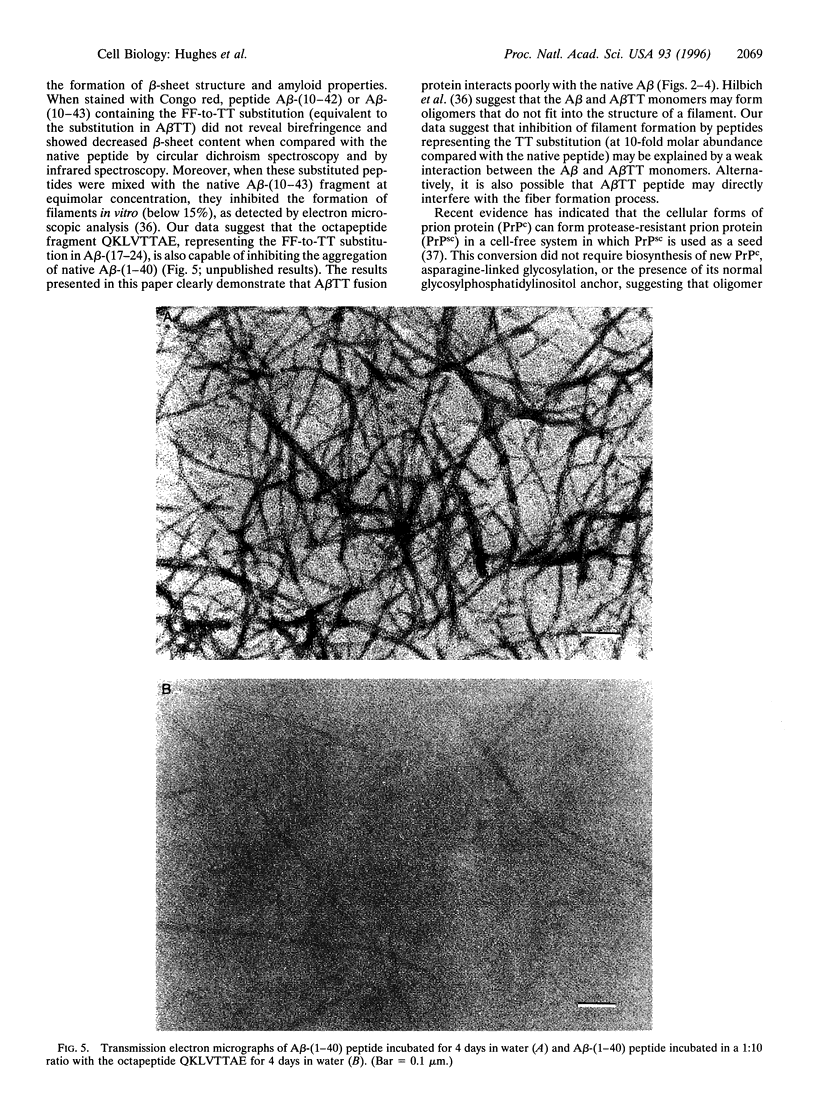
The kinetics of amyloid fibril formation by beta-amyloid peptide (Abeta) are typical of a nucleation-dependent polymerization mechanism. This type of mechanism suggests that the study of the interaction of Abeta with itself can provide some valuable insights into Alzheimer disease amyloidosis. Interaction of Abeta with itself was explored with the yeast two-hybrid system. Fusion proteins were created by linking the Abeta fragment to a LexA DNA-binding domain (bait) and also to a B42 transactivation domain (prey). Protein-protein interactions were measured by expression of these fusion proteins in Saccharomyces cerevisiae harboring lacZ (beta-galactosidase) and LEU2 (leucine utilization) genes under the control of LexA-dependent operators. This approach suggests that the Abeta molecule is capable of interacting with itself in vivo in the yeast cell nucleus. LexA protein fused to the Drosophila protein bicoid (LexA-bicoid) failed to interact with the B42 fragment fused to Abeta, indicating that the observed Abeta-Abeta interaction was specific. Specificity was further shown by the finding that no significant interaction was observed in yeast expressing LexA-Abeta bait when the B42 transactivation domain was fused to an Abeta fragment with Phe-Phe at residues 19 and 20 replaced by Thr-Thr (AbetaTT), a finding that is consistent with in vitro observations made by others. Moreover, when a peptide fragment bearing this substitution was mixed with native Abeta-(1-40), it inhibited formation of fibrils in vitro as examined by electron microscopy. The findings presented in this paper suggest that the two-hybrid system can be used to study the interaction of Abeta monomers and to define the peptide sequences that may be important in nucleation-dependent aggregation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brent R., Ptashne M. A bacterial repressor protein or a yeast transcriptional terminator can block upstream activation of a yeast gene. Nature. 1984 Dec 13;312(5995):612–615. doi: 10.1038/312612a0. [DOI] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Colon W., Kelly J. W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992 Sep 15;31(36):8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- Come J. H., Fraser P. E., Lansbury P. T., Jr A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988 Aug 25;334(6184):721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Golemis E. A., Brent R. Fused protein domains inhibit DNA binding by LexA. Mol Cell Biol. 1992 Jul;12(7):3006–3014. doi: 10.1128/mcb.12.7.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J., Golemis E., Chertkov H., Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993 Nov 19;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Lavender J. P., Pepys M. B. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990 Aug 23;323(8):508–513. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- Hendriks L., van Duijn C. M., Cras P., Cruts M., Van Hul W., van Harskamp F., Warren A., McInnis M. G., Antonarakis S. E., Martin J. J. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the beta-amyloid precursor protein gene. Nat Genet. 1992 Jun;1(3):218–221. doi: 10.1038/ng0692-218. [DOI] [PubMed] [Google Scholar]
- Hilbich C., Kisters-Woike B., Reed J., Masters C. L., Beyreuther K. Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer's disease beta A4 peptides. J Mol Biol. 1992 Nov 20;228(2):460–473. doi: 10.1016/0022-2836(92)90835-8. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994 Jul;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Berger E. P., Lansbury P. T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993 May 11;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Amyloid fibril formation requires a chemically discriminating nucleation event: studies of an amyloidogenic sequence from the bacterial protein OsmB. Biochemistry. 1992 Dec 15;31(49):12345–12352. doi: 10.1021/bi00164a008. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Morris S., Yates C. M., Moffoot A., Sharpe C., Brock D. J., St Clair D. Mutation in codon 713 of the beta amyloid precursor protein gene presenting with schizophrenia. Nat Genet. 1992 Jul;1(4):306–309. doi: 10.1038/ng0792-306. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kocisko D. A., Come J. H., Priola S. A., Chesebro B., Raymond G. J., Lansbury P. T., Caughey B. Cell-free formation of protease-resistant prion protein. Nature. 1994 Aug 11;370(6489):471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- Levy E., Carman M. D., Fernandez-Madrid I. J., Power M. D., Lieberburg I., van Duinen S. G., Bots G. T., Luyendijk W., Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. A new class of yeast transcriptional activators. Cell. 1987 Oct 9;51(1):113–119. doi: 10.1016/0092-8674(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Ma J., Yee A., Brewer H. B., Jr, Das S., Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994 Nov 3;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Papayannopoulos I. A., Styles J., Bobin S. A., Lin Y. Y., Biemann K., Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993 Feb 15;301(1):41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Mori H., Takio K., Ogawara M., Selkoe D. J. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J Biol Chem. 1992 Aug 25;267(24):17082–17086. [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Murrell J., Farlow M., Ghetti B., Benson M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz-Wasserman A. J., Kosmoski J., Cribbs D. H., Glabe C. G., Cotman C. W. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995 Jan;64(1):253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M. L., Jackson-Grusby L., Brent R. Gene activation and DNA binding by Drosophila Ubx and abd-A proteins. Cell. 1989 Jun 16;57(6):1045–1052. doi: 10.1016/0092-8674(89)90342-5. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- van Gool W. A., Schenk D. B., Bolhuis P. A. Concentrations of amyloid-beta protein in cerebrospinal fluid increase with age in patients free from neurodegenerative disease. Neurosci Lett. 1994 May 19;172(1-2):122–124. doi: 10.1016/0304-3940(94)90677-7. [DOI] [PubMed] [Google Scholar]