Abstract
Central nervous system (CNS) complications resulting from diabetes is a problem that is gaining more acceptance and attention. Recent evidence suggests morphological, electrophysiological and cognitive changes, often observed in the hippocampus, in diabetic individuals. Many of the CNS changes observed in diabetic patients and animal models of diabetes are reminiscent of the changes seen in normal aging. The central commonalities between diabetes-induced and age-related CNS changes have led to the theory of advanced brain aging in diabetic patients. This review summarizes the findings of the literature as they relate to the relationship between diabetes and dementia and discusses some of the potential contributors to diabetes-induced CNS impairments.
Keywords: Hippocampus, Insulin, Glucose, Cognition, Alzheimer’s disease, Obesity
1. Introduction
Diabetes mellitus is an endocrine disorder of carbohydrate metabolism resulting from inadequate insulin release (insulin-dependent diabetes mellitus, or type 1 diabetes; T1D) or insulin insensitivity (non-insulin-dependent diabetes mellitus, or type 2 diabetes; T2D), both of which result in hyperglycemia if uncontrolled. T1D is believed to occur in response to an autoimmune destruction of insulin producing pancreatic β cells, whereas T2D may be triggered or worsened by a number of factors including obesity, hypertension, and other features of the metabolic syndrome. For many years it has been well accepted that diabetes often results in microvascular and macrovascular disease, leading to complications such as retinopathy, peripheral neuropathy, stroke and coronary heart disease [1]. There is now evidence illustrating that both T1D and T2D can also cause complications within the central nervous system (CNS). Manifestations of diabetes-induced CNS complications may include structural alterations or brain atrophy, as well as changes in electrophysiological properties that ultimately result in deficits in cognitive performance [2]. These diabetes-induced CNS complications may be associated with or exacerbated by cardiovascular disease, including hypertension [3,4] and cerebral vascular complications [5–8]. Additional factors that may contribute to diabetes-induced cognitive impairment include disrupted insulin signaling and glucose homeostasis in the CNS [9].
Under normal circumstances glucose is the predominant metabolic fuel source of the adult brain and is transported to the CNS from the periphery via facilitative glucose transporters [10]. Since the brain can neither synthesize nor store glucose for extended periods of time, it is essential that proper glucose regulation be achieved in the periphery to ensure appropriate glucose transport to the CNS [11], processes that may be disrupted in poorly-controlled diabetes. Many brain structures, such as the hippocampus, are extremely sensitive and responsive to changes in glucose homeostasis. For example, dysfunctional glucose regulation and/or insufficient insulin availability elicits neuronal synaptic reorganization [12] and increased proliferation of astrocytes [13] in the hippocampus of streptozotocin (STZ) diabetic rodents, an experimental model of T1D. Additionally, glucose and insulin are both instrumental regulators of cognitive function [14–21], further supporting the hypothesis that inefficient regulation of these two factors may contribute to cognitive deficits in diabetes phenotypes.
The discrete etiologies and pathological manifestations of these subtypes of diabetes provide insight as to the differential deleterious effects of T1D and T2D on cognition. While it could be argued that these impairments may not hinder daily cognitive functioning in diabetic individuals, these alterations in cognition and brain structure may over time lead to an acceleration of CNS aging [22] or an increased risk for age-related diseases such as Alzheimer’s disease (AD) [23]. Accordingly, this review will examine the diabetic brain and its associated changes in both T1D and T2D, with particular emphasis on diabetes-induced deficits in the structural and functional properties of the hippocampus. Additionally, this review will highlight the potential contributors to diabetes-induced brain changes and will provide a general discussion of how several diabetes-associated CNS changes correlate with an increased risk of AD and advanced brain aging in both the clinical and pre-clinical settings. Although animal models of diabetes and aging cannot fully parallel these states in humans, data from non-human studies will be referred to and acknowledged for contributions made in providing insight into mechanisms relevant to the clinical setting.
2. The relationship between cognitive decline and diabetes
There is an increasing appreciation that cognitive decline occurs in both T1D and T2D. However, there are differences concerning the relative degree of cognitive dysfunction and the way in which these cognitive abnormalities are manifested in the two sub-types of diabetes. For example, although there may be commonalities between the underlying mechanisms involved in diabetes-induced cognitive impairments, many studies have shown that T1D is more likely to be associated with psychomotor slowing and reductions in mental efficiency [24], while cognitive deficits in T2D are often in the areas of psychomotor efficiency, attention, learning and memory and executive function [25]. Moreover, some studies suggest that diabetes-induced CNS complications occur more rapidly in T2D than in T1D. For instance, in a comparative study of cognitive function, those with more than 30 years of T1D had comparable cognitive profiles to T2D patients with seven years of disease duration [26]. Also, when compared to T2D patients, T1D patients had better brain magnetic resonance imaging (MRI) ratings [26], which measure changes in brain volume, silent infarcts and white matter lesions (WML). Hyperintense lesions, as measured by T2-weighted and proton density MRI, occur in white matter (referred to as white matter hyperintensities, WMH or WML) as well as in gray matter [27,28]. The clinical significance of WML remains undetermined [28,29]; however, WML have been associated with diabetes, AD, normal aging, impairments in cognition, and accelerated cognitive decline [27,28,30–33]. As such, increases in WML in diabetic individuals may lead to the development of a cognitive phenotype similar to that of aged individuals.
The differential effects of T2D and T1D upon cognition raise the question of the underlying causes for the cognitive differences in these two diseases. One potential clue as to why T1D and T2D may differ in the progression of cognitive impairment is a potential interaction between diabetes and age [34,35]. This theory is consistent with the fact that T2D is more prevalent with increasing age and the observation by Biessels and Kappelle that clinically relevant decreases in cognitive function are more likely to occur in elderly T2D patients [36]. Additionally, T1D is a disease that often affects individuals before the age of 18 [37] which presents the possibility that developmental compensations may contribute to the less severe cognitive deficits observed in T1D patients when compared to T2D patients. Another possible explanation for the differential effects of T1D and T2D upon cognitive function is insulin resistance, a feature more prevalent in T2D than in T1D. In support of this hypothesis, insulin resistance was identified as the best predictor of T2D diagnosis in a study of pre-diabetic individuals [38]. Additionally, insulin resistance is associated with a host of conditions including, hyperglycemia, hyperinsulinemia, hypertension, dyslipidemia, and increased central adiposity collectively termed the metabolic syndrome [39]. Many features of the metabolic syndrome may contribute to cognitive impairment independently or in combination with one another [39]. Although the incidence of cognitive deterioration is more prevalent in older T2D, younger T2D and T1D patients are not immune to the deleterious consequences of diabetes upon brain structure and function.
3. Type 1 diabetes and cognitive impairment
T1D is a life-long disorder usually diagnosed in younger individuals (50–60% younger than 18 years of age) [37] and therefore raises concern regarding how this disease and its treatment may impact cognitive performance. In this regard, it has been reported that children with T1D show impairment on tasks of declarative memory compared to age matched non-diabetic controls [40]. Typically, T1D is treated in either a conventional or intensive manner with exogenous insulin [1]. Insulin treatment increases the risk of hypoglycemic episodes [1,41], and increases the potential for cycling between hypoglycemia and hyperglycemia, both of which can be harmful to the brain, especially the developing brain [42–44]. Additionally, both hyperglycemia and hypoglycemia are known to cause acute cerebral dysfunction [45] and are associated with decline in intellectual performance [39]. While some investigators report no independent correlation between preceding severe hypoglycemic events and cognitive function in adult T1D patients [46], several clinical studies report that hypoglycemic events negatively affect cognition. For example, Hershey et al. reported that children with more frequent and earlier onset of hypoglycemic events had worse performance on tests of short and long delay spatial memory than did other tested groups [47]. A higher frequency of hypoglycemic events during a relatively short period of time, as is more often the case in diabetic children than adults, could account for discrepancies between studies. Nonetheless, these studies emphasize the importance of maintaining good glycemic control for diabetes patients.
It is important to note that although T1D patients may perform less well on some cognitive tasks when compared to control subjects, scores for diabetes patients are often within the normal cognitive range [48]. In a study comparing young to middle aged T1D patients with age-matched controls, the diabetic persons performed more poorly on tests of executive function, short term memory, psychomotor efficiency and a measure of mental efficiency; however, all subjects scored within a normal cognitive range [49]. These results imply that although there are deficits in cognition in T1D, these deficits are mild and may not fall below ratings of the general population. However, the potential unfortunate outcome of these ‘mild’ cognitive deficits in T1D patients is a predisposition for more rapid deterioration of cognitive function in later life. Indeed, cumulative CNS complications in the hippocampus may lead to cognitive decline, as well as increased risk for neurological comorbidities [35,50].
3.1. Diabetes and the hippocampus in type 1 diabetes
The hippocampus is an important structure for the integration of learning and memory in the mammalian CNS [51] and is particularly sensitive to changes in glucose homeostasis. For example, improper glucose homeostasis may impact the electrophysiological properties of hippocampal neurons. In particular, studies performed on rodent models of T1D and models of improper glucose regulation show dysfunctional synaptic plasticity with specific deficits in long-term potentiation (LTP) and long-term depression (LTD) [52–54]. Typically these animal models have impairments in LTP and an enhancement of LTD when compared to controls. These electrophysiological changes may be an underlying contributor to some of the impairments exhibited by diabetic animal models on cognitive performance. Indeed, STZ diabetic rats that exhibit these electrophysiological changes in LTP and LTD perform less well on spatial memory tasks when compared with control rats [55].
Clinical imaging studies have provided insight into additional factors that may contribute to cognitive deficits observed in T1D patients, including structural and morphological changes in the hippocampus. In this regard, the odds for better performance on delayed memory tasks were greater in T1D patients with no periventricular WMH [49]. However, this study as well as others identified no overall changes in brain matter composition or volume in T1D patients [43,48,49]. Conversely, some reports have identified changes in brain structures and brain volume in T1D [56–59]. The discrepancies in brain imaging studies of T1D may be related to patient age, age of diabetes onset, hypoglycemic events, or differences in statistical analysis. Some studies have shown that age of diabetes onset may be one of the most critical contributors to CNS structural abnormalities [58], although this may be a secondary effect related to the number of hypoglycemic and/or hyperglycemic events experienced by the patient. Structural analyses in the hippocampus of experimental models of T1D have yielded more consistent findings.
In histological studies, STZ diabetic rats display morphological changes in the hippocampus, including dendritic atrophy in the CA3 pyramidal neurons [12]. In addition, T1D rats exhibit redistribution of the synaptic proteins synaptophysin and post-synaptic density 95 (PSD-95), which could be indicative of ongoing synaptogenesis in the hippocampus of STZ rats [60]. Collectively, these clinical and pre-clinical data illustrate that the extent of cognitive impairment and brain morphology abnormalities in T1D remains to be unequivocally determined. However, the literature to date suggest that structural changes, which may underlie cognitive decline, are likely occurring in T1D patients, effects that may have detrimental consequences over extended periods of time.
4. Type 2 diabetes and cognitive impairment
A number of studies have examined the relationship between T2D and cognition. Some studies suggest that cognitive performance does not differ between T2D patients and non-diabetic controls when the influence of age, premorbid IQ, body mass index (BMI) and depression are taken into account [61]. However, many clinical and pre-clinical studies contradict this finding and support the concept that T2D is associated with cognitive decline. In this regard, T2D patients have been identified as having mini-mental state examination (MMSE, a measure of cognitive ability) scores in the impaired range when compared to age-matched controls [62]. Moreover, lower cognitive scores on performances of general cognition, verbal memory, and category fluency have also been reported in men and women with T2D [63]. These findings are in agreement with other studies suggesting an increased occurrence of cognitive impairment in older T2D individuals [64]. More recently, Convit and coworkers reported that the cognitive deficits in T2D patients may be more selective for hippocampal-related memory performance and that these deficits are correlated with the degree of glycemic control [65]. These clinical data are supported by observations from animal models of T2D. For instance, the db/db mouse exhibits deficits in the water maze, a hippocampal-dependent spatial learning task [66]. Moreover, our previous studies revealed that type 2 Zucker fa/fa diabetic rats perform more poorly compared to their lean littermates in the variable-interval delayed alternation test of learning and memory [67]. It can be concluded from these studies that T2D subjects experience deficits in hippocampal-based cognitive performance, deficits that may be attributed to changes in brain structure and volume.
4.1. Hippocampal structure and function in type 2 diabetes
A number of studies suggest that patients with T2D are more likely to have structural brain abnormalities [64,68], such as cerebral infarcts [69], when compared with T1D patients and non-diabetic individuals. In an MRI study of subjects with vascular disease, patients with T2D were more likely to have a smaller brain parenchymal fraction and more WMH than patients with vascular disease but without diabetes [33]. Alternatively, a review of imaging studies measuring structural brain changes in diabetic patients found no consistent data concerning WML but found a correlation between brain atrophy and T2D [70]. In particular, the hippocampus and other temporal lobe structures appear to have high susceptibility to diabetes-associated atrophy [65,69,71], and most studies of T2D-related structural rearrangement provide evidence for the occurrence of some identifiable CNS damage, especially within the hippocampus [50]. Changes in hippocampal morphology may be accompanied by electrophysiological changes in T2D, which may contribute to cognitive decline. For example, electrophysiological deficits are observed in the hippocampus of experimental models of T2D such as the db/db mouse and the Zucker rat [66,72]. In the clinical setting, a study of middle aged T2D patients identified differences between right, left and overall P300 wave latencies in diabetics compared to controls [62]. The P300 wave is a cortical neurophysiological characteristic reflecting the speed of neuronal events underlying information processing and appears to be strongly associated with attention and short-term memory. Changes in P300 are important to note due to the fact that T2D-induced cognitive changes often include deficits in attention and learning and memory [25]. Interestingly, changes in P300 wave are also a trait observed in normal aging [73], which provides a potential functional deficit common to both diabetes and normal brain aging. Overall, these studies provide data indicating that cognitive decline is a neurological consequence of T2D that may be partly attributable to structural rearrangement and changes in the electrophysiological properties of hippocampal neurons. Importantly, these neurological consequences of diabetes appear to parallel those observed in the aging brain.
5. Aging, neurodegenerative diseases and diabetes
5.1. The normal aging brain
The process of normal aging is characterized by decreased ability to remember new facts, decline in cognitive performance, structural and morphological brain changes, modifications in electrophysiological properties, and increased risk of diabetes and insulin resistance [73,74]. There is a trend for a slight decrease in brain weight and volume in aged people [74] and a correlation between atrophy rates and decline in cognitive ability [75,76]. In a six year longitudinal study of older individuals, brain atrophy was reported to be most correlated with age, heavy drinking and glycosylated hemoglobin index (HbA1c), an indicator of long term glycemic control [77]. Reorganization of the structural and chemical balance of the hippocampus in aged people and animals can be correlated to age-related memory deficits and changes in synaptic plasticity [73,78–80]. For example, with increasing age, there is a decrease in the number of synapses in the dentate gyrus and specific synapse loss of cortical inputs to the hippocampus in both humans and animals [73]. In aged rats, the hippocampus has approximately one-third fewer synaptic contacts from the entorhinal cortex when compared to younger rats [73]. In addition to decreased synapse number, hippocampal interneuron populations are decreased in the aged brain; specifically, GAD67 immunoreactive interneurons are decreased in the CA1 region of the hippocampus of old rats [81]. These data compliment reports of decreases in specific interneuronal populations in other hippocampal regions such as the hilus [82]. Decline in hippocampal interneuron populations may have functional consequences since interneurons have been shown to be crucial for a variety of cognitive functions including memory [83]. As described above, many of these changes observed in the aging hippocampus are also seen in diabetic patients and animal models of diabetes, changes that may predispose diabetic subjects to neurodegenerative diseases such as AD. The commonalities between the diabetic brain and the aging brain support the idea that diabetes induces advanced brain aging, especially in T2D patients [84]. However, advanced age is a risk factor for both diabetes and cognitive decline. As a result, it is challenging to differentiate between these factors in order to determine whether diabetes induces brain aging or interacts with normal aging to create an exacerbated phenotype. A concept referred to as “the brain reserve hypothesis” may provide insight into this question.
The brain reserve hypothesis was first suggested to explain incongruence between pathological brain damage and cognitive and functional performance in older individuals [85]. This hypothesis is based on the theory that individuals with higher levels of education have an increased capacity to make adjustments so that cognition does not suffer in response to brain atrophy or structural changes [85]. A modified theory of brain reserve in relation to diabetes has been discussed by Ryan and Geckle, which proposes that older individuals have less brain reserve making them more susceptible to diabetes-induced CNS changes [84]. It is possible that this revised hypothesis is not only applicable to older diabetic patients but perhaps to all diabetic patients irrespective of age. If this hypothesis is correct, diabetes patients would be more susceptible to CNS insults arising from diabetes and less capable of adapting in order to reduce or eliminate cognitive deficits. The addition of an increasing number of insults, such as the features of the metabolic syndrome in T2D or hypoglycemic episodes in T1D patients, would further increase CNS vulnerability. As such, poorly controlled diabetes would create a neuronal milieu in which diabetes patients are more vulnerable to the aging process and age-related diseases such as AD.
5.2. Diabetes and age-related comorbidities: Alzheimer’s disease
Alzheimer’s disease (AD) is the most common form of dementia in the United States [86,87]. Risk factors for AD may include age, genetics, a variety of cardiovascular complications and diabetes [88]. The onset of AD is generally subtle, followed by a progressive decrease in cognitive function characterized by memory loss [87,89]. Histologically, AD is identified by an abundance of neuritic plaques and neurofibrillary tangles in areas of the cerebral cortex, hippocampus and other temporal lobe structures of postmortem tissue [87,90]. Neuritic plaques are formed from an accumulation of amyloid-β (Aβ) peptide which is produced by cleavage of amyloid precursor protein (APP) [87]. Neurofibrillary tangles are composed of abnormally hyperphosphorylated tau protein, an axonal protein that promotes microtubule assembly and stability [87]. Hyperphosphorylation disrupts proper tau function and may lead to synapse dysfunction, neuron degeneration and cognitive impairment [91].
Some clinical and epidemiological studies have demonstrated an association between diabetes and the development of comorbid AD. Data from the Religious Orders study, a study of aging in Catholic priests, nuns and brothers across the US, show a 65% increased risk of developing clinical manifestations of AD in diabetic patients compared to non-diabetic controls [23], findings that are consistent with a number of other studies [4,92–94] but not all [95]. In addition, some researchers have identified an association between borderline non-overt diabetes and increased AD risk [96]. One plausible explanation for potential increased risk of AD in diabetes is insulin resistance. Plasma insulin levels are elevated in type 2 patients and in AD patients [88,92,97], which presumably reflects insulin resistance. Additionally, in AD patients, administration of insulin increases plasma levels of Aβ [98], which would potentially increase CNS levels of Aβ that could accumulate into neuritic plaques. In control non-demented subjects, insulin causes a decrease in plasma Aβ levels [98], further supporting differences in the insulin system of AD patients compared to non-AD subjects. Unlike the increases in plasma insulin levels, there is a decrease in cerebrospinal fluid (CSF) levels of insulin in AD patients compared to non-AD controls and a positive correlation between CSF insulin and dementia severity in AD [97]. Such results would support the idea that insulin resistance may be a contributor to cognitive impairment and dementia in AD patients.
Some researchers suggest that insulin aids in the clearance of Aβ, at least in the periphery [98], but others support the concept that insulin leads to the release of intracellular Aβ, thereby increasing secretion into the extracellular space and facilitating Aβ accumulation [88]. Under physiological conditions, insulin signaling cascades have been implicated in the regulation of tau phosphorylation [99]. Briefly, insulin signaling leads to the activation of phosphoinositide 3-kinase (PI3K), which phosphorylates and activates Akt. Activated Akt in turn phosphorylates and inactivates the cytoplasmic enzyme glycogen synthase kinase-3β (GSK3β). In the brain, GSK3β is one of the kinases involved in the phosphorylation of tau [99]. In the normal brain, insulin signaling activation reduces the phosphorylation state of tau, whereas deficits in insulin signaling could cause hyperphosphorylation of tau [99]. Whether these mechanisms contribute to hyperphosphorylation of tau in AD patients or diabetes patients remains to be verified. However, hyperphosphorylation of tau is observed in experimental models of diabetes. In a study of Alzheimer-associated pathologies in animal models of diabetes, both T1D and T2D rats exhibited decreases in phosphorylated Akt and phosphorylated GSK-3β [100], which may have contributed to the development of histological AD features. Additionally, tau is hyperphosphorylated in STZ diabetic mice [101–103]. Moreover, histological studies from our lab identified hyperphosphorylated tau immunoreactivity in the hippocampus of fatty Zucker rats, AD-like pathology that is not observed in lean control rats (Fig. 1).
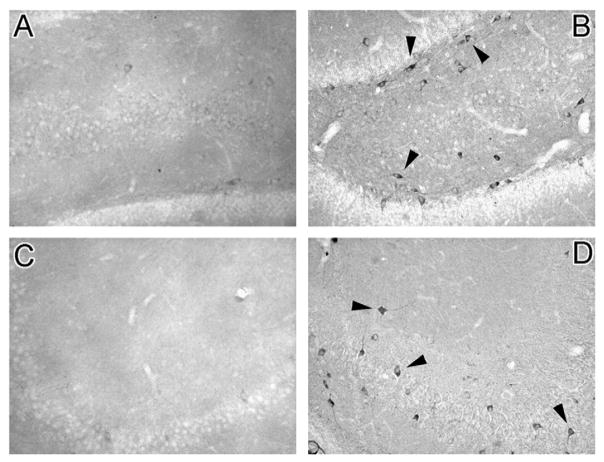
Fig. 1.
Hyperphosphorylation of tau is increased in the hippocampus of Zucker type 2 diabetic rats. Representative photomicrographs demonstrate an absence of hyperphosphorylated tau immunoreactivity in the dentate gyrus (Panel A) and the CA3 region (Panel C) of lean control rats. Conversely, hyperphosphorylated tau immunoreactivity is significantly increased in the dentate gyrus (Panel B, arrow heads) and the CA3 region (Panel D, arrow heads) of fatty Zucker diabetic rats. This AD-like pathology may contribute to the development of behavioral deficits observed in this experimental model of type 2 diabetes.
There are a limited number of human postmortem studies that have examined histological features of AD in the diabetic brain and these studies have yielded inconsistent findings. Some studies report no association [104,105], while another study reports a negative correlation between diabetes and the presence of neuritic plaques and neurofibrillary tangles [106]. A number of possibilities could explain the discrepancy between postmortem and clinical data relating AD to diabetes. For example, diabetic individuals with comorbid AD may be less likely to survive to an older age and therefore may not be included in these postmortem studies [106]. Additionally, there is the possibility that diabetes does not increase the risk of developing histological features of AD but instead creates a cognitive phenotype that resembles the clinical symptoms of AD, a phenotype which may be more pronounced in individuals of older age. In support of this concept, data from the Religious Orders study show a correlation between diabetes and clinical symptoms of AD [23] while no correlation was later found between postmortem histopathological AD features and diabetes in this same population [104]. Nonetheless, as detailed above, some features associated with AD are observed in diabetic patients and would support the idea of diabetes increasing the risk of AD or AD-like symptoms. The association between increased risks of developing mild cognitive impairment (MCI) or AD in diabetes patients requires further analysis, including additional post-mortem studies assessing histopathological features such as hyperphosphorylated tau. It is crucial that further investigation be continued in order to elucidate the mechanistic mediators that increase neuronal vulnerability and thereby facilitate cognitive decline and progression of AD symptomatology in diabetic patients.
6. Potential contributors to diabetes-induced brain aging
6.1. Insulin and glucose
The discovery of centrally located insulin receptors [107,108] has lead to a greater appreciation of the multi-faceted functions of insulin within the CNS. The expression of insulin receptors in the hippocampus has driven the hypothesis that insulin is an important contributor to or regulator of cognitive function [109,110] a theory supported by clinical evidence. For example, chronic intranasal insulin administration improves cognitive performance in both AD and non-demented individuals [17,88,111–113], and acute insulin administration has been shown to improve declarative memory in AD patients [114]. Moreover, administration of the insulin sensitizer, rosiglitazone, ameliorates cognitive decline in non-diabetic AD patients [115]. Rosiglitazone-induced cognitive improvement is also observed in animal models of AD [116], which may be mediated in part through increases in dendritic spine density, an effect of rosiglitazone observed in primary cortical cultures [117]. Insulin is also known to exert direct and indirect effects over various neurotransmitter systems. For example, insulin increases N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionate (AMPA) and gamma-aminobutyric acid (GABA) receptor surface expression [118–120], and indirectly regulates particular neurotransmitters, such as epinephrine [18,121–123]. Insulin also modulates basal synaptic transmission [124–126] and restores normal synaptic plasticity in the hippocampus of animal models of diabetes [55]. Additionally, cognitive training strengthens insulin signaling in the hippocampus of non-diabetic rats [127], effects that may further enhance the functional activities of neurotransmitters that are critical for synaptic plasticity and cognitive function. Under pathological conditions, previous studies have revealed that hyperglycemia, endocrine dysfunction, deficits in hippocampal plasticity and behavioral performance observed in experimental models of diabetes are inhibited by insulin replacement [55,128]. Such results suggest that decreases in insulin receptor (IR) activity may be an initiating factor producing deficits in hippocampal plasticity and ultimately cognitive decline.
Another important contributor to brain function, especially as it relates to cognition, is glucose. In this regard, fasting plasma glucose levels were negatively correlated with episodic memory and recall in a study of normal healthy women [129]. Also, glucose administration improves cognitive performance in people and animals irrespective of pre-existing cognitive impairments [19,20,114,130,131]. During hippocampal-dependent cognitive tasks, extracellular levels of glucose are decreased in the hippocampus of rodents, implying increased glucose uptake and utilization by cells [15]. The intimate connection between glucose and cognition [129,132] reinforces the importance of maintaining the delicate balance of glucose levels within the brain. Studies in experimental models of diabetes provide additional support for the importance of tight glycemic control. For example, moderate recurrent hypoglycemia improved performance on a hippocampal dependent cognitive task in the euglycemic state in male rats, as compared to control animals. However, during acute hypoglycemia, recurrent hypoglycemic animals performed no better than chance on a maze task [133]. These data suggest that familiarity with the experience of hyperglycemia or hypoglycemia may cause the brain to become more efficient at utilizing glucose and other means of energy during cognitive tasks, but only when sufficient glucose is available. Although these data suggest that a more constant glycemic index is perhaps better than fluctuations between glycemic states, harmful CNS consequences may nonetheless accompany a non-euglycemic environment, including blood–brain barrier dysfunction [122], deep subcortical white matter lesions and other structural deficits [43,64]. Although the data on the effects of glycemic control show a bit of discrepancy, overall, it appears that bouts of hypoglycemia and hyperglycemia make a significant contribution to cognitive performance impairments via a variety of mechanisms [134].
6.2. Advanced glycation end products and oxidative stress
In addition to altering cognitive performance, poor glycemic control increases the accumulation of advanced glycation end products (AGE) [135], products formed by the non-enzymatic reaction between sugars and amino groups, and oxidative stress in the brain, which may lead to cellular and molecular damage [136]. In this regard, AGE and oxidative stress have both been identified as potential contributors to diabetes-induced brain aging [22,137]. Accumulated AGE are present in normal aging and in certain pathological conditions such as AD and diabetes [138–141]. Among the actions of AGE are effects on extracellular matrix proteins and basement membrane components which can cause or facilitate vascular complications [135,142]. Additionally, AGE generation increases proinflammatory mechanisms in the vessels that enhance oxidative stress. An increase in oxidative stress leads to AGE accumulation and thus creates an unremitting cycle [140] which may contribute to a host of complications and imbalances.
In physiological conditions there is a delicate balance between the synthesis of oxygen free radicals and the activities of anti-oxidant pathways to protect the organism; an imbalance of the oxidant/anti-oxidant equilibrium favoring the former creates a situation of oxidative stress [143]. Studies from animal models have strengthened the relationship between oxidative stress, aging and cognitive performance. For example, increases in oxidative stress in the rat hippocampus are associated with decreases in behavioral performance in hippocampal-dependent learning and memory paradigms, such as the Morris water maze [143–145]. During aging, antioxidant enzyme activity and expression decreases [146–148], suggesting that oxidative status may shift towards accumulation of oxidative stress products. There are similar imbalances in oxidant/anti-oxidant pathways in animal models of diabetes [9]. The free radical hypothesis of aging proposes that aging is the result of random harmful effects on tissue due to oxygen free radicals [149,150], a hypothesis that may be applicable to diabetic individuals as well. As such, deleterious AGE accumulation and imbalances in oxidative stress status may be among the etiological factors that contribute to the pathogenesis of age-related and diabetes-induced cognitive impairments. The potential for disruption of proper AGE metabolism and oxidative stress status in diabetes [137] provide additional explanations as to how diabetes might worsen or accelerate the brain aging process.
6.3. HPA axis dysfunction
Many pathological conditions are associated with hypothalamic–pituitary–adrenal (HPA) axis dysregulation, including diabetes (For review see [9]). The relationship between glucocorticoids (GCs) and obesity, a common feature in T2D patients, is relevant in terms of induction of insulin resistance. In this sense, lipolysis induced by GCs increases free fatty acid levels in blood, which in turn causes hyperglycemia through the stimulation of hepatic gluconeogenesis and a reduction in glucose uptake by competing with glucose for oxidation [151]. Consequently, a compensatory increment in insulin secretion leads to hyperinsulinemia, downregulation of insulin receptors and insulin resistance. Short-term corticosterone (CORT) administration to rats produces peripheral insulin resistance and also decreases insulin sensitivity in the hippocampus, including decreases in insulin-stimulated translocation of the insulin sensitive glucose transporter GLUT4 [152]. Interestingly, adrenalectomized STZ rats and db/db mice provided low dose of CORT replacement exhibit improved spatial memory and novel object recognition compared to sham operated animals and adrenalectomized animals given high levels of CORT replacement [153]. Such results support the notion that HPA axis dysfunction and/or exposure to elevated glucocorticoid levels contributes to the cognitive deficits observed in diabetic animals. It appears that most, if not all, of the comorbidities associated with diabetes or insulin resistance are characterized by HPA axis dysfunction [154,155]. Although cause and effect have not been determined in many of these situations, these findings may place impairments in HPA axis function at the center of diabetes-induced advanced brain aging.
6.4. Obesity
Ongoing epidemiological studies by the Centers for Disease Control estimates that more than 60% of the adult US population may be categorized as either overweight or obese [156]. Since there is a positive correlation between risk of T2D development and obesity [157], understanding the relationship between obesity and cognitive function is an important health care issue. In this regard, obesity has been both positively and negatively correlated with dementia in older individuals [158–160]. However, among middle-aged people there is a common trend for obesity to be negatively correlated with cognitive performance [3,161]. Obesity-related CNS abnormalities may be due to obesity-induced insulin resistance and/or impaired glucose tolerance [157,162], as described in previous sections. In addition, obesity may contribute to dementia and other cognitive impairments by increasing the incidence of vascular pathologies [163,164]. An additional consideration in obesity phenotypes is the potential for increases in plasma levels of adipocyte-derived hormones, such as leptin, that may have deleterious consequences on the CNS.
Leptin is synthesized and secreted by adipocytes and is transported across the blood–brain barrier (BBB) via a saturable transport system [165]. The actions of leptin in the hypothalamus are well described, especially in relation to normal metabolism and in pathophysiological settings such as diabetes and obesity phenotypes [166]. There is now a growing literature to support a role for leptin in the facilitation of hippocampal synaptic plasticity [167]. For example, studies by Harvey and co-workers determined that leptin enhances hippocampal excitability via NMDA receptor mediated mechanisms and regulates hippocampal plasticity by converting short-term potentiation into LTP in young rats [168]. Confocal immunofluorescence analyses also determined that leptin regulates the morphological features of primary hippocampal cultures, including the motility of dendritic filopodia [169]. These structural and functional enhancements of hippocampal plasticity may contribute to leptin’s ability to improve hippocampal-dependent behavioral performance [170–172]. Alternatively, genetic mutations that result in disrupted leptin signaling such as in the db/db mouse and the Zucker fa/fa rat are associated with reductions in LTP [66,72] and impaired performance of hippocampal-dependent tasks [66,67]. Such results indicate that decreases in leptin signaling contribute to deficits in hippocampal synaptic plasticity and suggest that leptin resistance, a well described phenomenon in the hypothalamus, may also be observed in the hippocampus in diabetes/obesity phenotypes.
7. Conclusions
Through findings from both human and animal studies, the underlying mechanisms involved in diabetes-induced CNS complications are coming under greater scrutiny and becoming better appreciated. It is extremely difficult to decipher a causal relationship between many of the endocrine and metabolic changes observed in diabetes and to appoint specific contributions of each factor. It is unlikely that any single factor is solely responsible for the CNS complications in diabetic subjects. Rather, a variety of factors likely act in additive or synergistic ways to impair neuronal homeostasis, increase neuronal vulnerability and eventually contribute to cognitive decline (see Fig. 2). Since some studies suggest that diabetic patients exhibit cognitive performance that is within the normal range, the magnitude or importance of memory decline in diabetic patients is often debated. However, the combination of the structural and functional changes within the hippocampus that elicit subtle decreases in cognitive function may be progressive and silently lead to increased risk of age-related neurological disorders. Indeed, the deleterious consequences of diabetes undoubtedly accumulate over time and ultimately produce detrimental effects in the CNS. Such realities emphasize the importance of patient education as it relates to maintaining a healthy life style through diet, exercise and medication. Additionally, there is a continued need for clinicians and pre-clinical scientists to identify the factors that are involved in the etiology and progression of the neurological complications of diabetes, as well as identify the fundamental basis that may cause diabetes patients to be more susceptible to develop comorbid MCI or AD. Therefore, one critical mission of ongoing research is the identification of these mechanistic mediators, which may lead to the development of novel therapeutic strategies to prevent or reverse the neurological consequences of diabetes in the CNS.
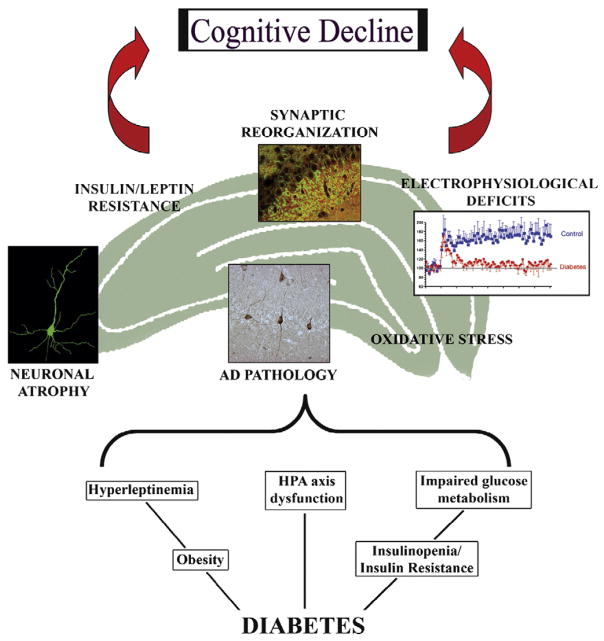
Fig. 2.
Potential mechanistic mediators of diabetes-induced brain aging. The complex pathophysiological features of diabetes may include decreases in insulin activity, impaired glucose homeostasis, dysregulation of hypothalamic–pituitary–adrenal (HPA) axis function, and hyperleptinemia, among others. The consequences of these pathophysiologies in the hippocampus include dendritic atrophy, changes in synapse formation, electrophysiological deficits, as well as the appearance of Alzheimer’s disease-like histopathology, such as hyperphosphorylated tau. Additional pathological consequences that contribute to cognitive decline in diabetic subjects may include changes in oxidative stress status and the development of insulin and/or leptin resistance in the hippocampus. These factors, in combination with other pathological features such as microvascular complications and mediators such as the pro-inflammatory cytokines, act in an additive or synergistic fashion to accelerate brain aging in diabetic subjects, thereby increasing patient vulnerability for the development of age-related disorders such as mild cognitive impairment and AD. See text for details.
Acknowledgments
The authors’ work is supported in part by Juvenile Diabetes Research Foundation grant number 2-03-675 (LPR), NIH grant number NS047728 (LPR) and the Alfred P. Sloan Foundation (SAW). The authors would like to acknowledge the efforts of their collaborators, including: Dr. Bruce McEwen (The Rockefeller University); Dr. Maureen Charron (Albert Einstein College of Medicine); Dr. Carol Greenwood and Dr. Gordon Winocur (University of Toronto); Dr. Randall Sakai (University of Cincinnati); and Dr. Steven Wilson, (University of South Carolina School of Medicine).
References
- 1.The diabetes control and complications trial research group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes-mellitus. N Eng J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- 3.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol Aging. 2005;26:S11–S16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Brown CM, Marthol H, Zikeli U, Ziegler D, Hilz MJ. A simple deep breathing test reveals altered cerebral autoregulation in type 2 diabetic patients. Diabetologia. 2008;51:756–761. doi: 10.1007/s00125-008-0958-3. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Bonilla JF, Quirce R, Hernandez A, Vallina NK, Guede C, Banzo I, Amado JA, Carril JM. Assessment of cerebral perfusion and cerebrovascular reserve in insulin-dependent diabetic patients without central neurological symptoms by means of Tc-99m-HMPAO SPET with acetazolamide. Eur J Nucl Med. 2001;28:1647–1655. doi: 10.1007/s002590100595. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB. Diabetes and cardiovascular-disease — Framingham Study. Am J Cardiol. 1975;35:147. [Google Scholar]
- 8.Tuttolomondo A, Pinto A, Salemi G, Di Raimondo D, Di Sciacca R, Fernandez P, Ragonese P, Savettieri G, Licata G. Diabetic and non-diabetic subjects with ischemic stroke: differences, subtype distribution and outcome. Nutr Metab Cardiovasc Dis. 2008;18:152–157. doi: 10.1016/j.numecd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol. 2008;585:64–75. doi: 10.1016/j.ejphar.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcewen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Boyle PJ, Nagy RJ, Oconnor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose-uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A. 1994;91:9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magarinos AM, Mcewen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci U S A. 2000;97:11056–11061. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saravia FE, Revsin Y, Gonzalez Deniselle MC, Gonzalez SL, Roig P, Lima A, Homo-Delarche F, De Nicola AF. Increased astrocyte reactivity in the hippocampus of murine models of type 1 diabetes: the nonobese diabetic (NOD) and streptozotocin-treated mice. Brain Res. 2002;957:345–353. doi: 10.1016/s0006-8993(02)03675-2. [DOI] [PubMed] [Google Scholar]
- 14.Hall JL, Gonder-Frederick LA, Chewning WW, Silveira J, Gold PE. Glucose enhancement of performance on memory tests in young and aged humans. Neuropsychologia. 1989;27:1129–1138. doi: 10.1016/0028-3932(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 15.Mcnay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mcnay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem. 2001;75:325–337. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- 17.Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86:136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- 18.Kopf SR, Baratti CM. Effects of posttraining administration of insulin on retention of a habituation response in mice: participation of a central cholinergic mechanism. Neurobiol Learn Mem. 1999;71:50–61. doi: 10.1006/nlme.1998.3831. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood CE, Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiol Learn Mem. 2001;75:179–189. doi: 10.1006/nlme.2000.3964. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr. 2000;72:825–836. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- 21.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 22.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441:1–14. doi: 10.1016/s0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- 23.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 24.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 25.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Brands AM, Biessels GJ, Kappelle LJ, de Haan EH, de Valk HW, Algra A, Kessels RP. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dement Geriatr Cogn Disord. 2007;23:343–350. doi: 10.1159/000100980. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien JT, Wiseman R, Burton EJ, Barber B, Wesnes K, Saxby B, Ford GA. Cognitive associations of subcortical white matter lesions in older people. Alzheimer’s Disease: Vasc Etiology Pathol. 2002;977:436–444. doi: 10.1111/j.1749-6632.2002.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 28.Pantoni L, Garcia JH. The significance of cerebral white-matter abnormalities 100 years after Binswangers report — a review. Stroke. 1995;26:1293–1301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt R, Schmidt H, Kapeller P, Enzinger C, Ropele S, Saurugg R, Fazekas F. The natural course of MRI white matter hyperintensities. J Neurol Sci. 2002;203:253–257. doi: 10.1016/s0022-510x(02)00300-3. [DOI] [PubMed] [Google Scholar]
- 30.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O’Brien JT, Ince PG. White matter lesions in an unselected cohort of the elderly — molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 31.Jongen C, Biessels GJ. Structural brain imaging in diabetes: a methodological perspective. Eur J Pharmacol. 2008;585:208–218. doi: 10.1016/j.ejphar.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, OLeary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people — the cardiovascular health study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 33.Tiehuis AM, Van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman APA, Kappelle LJ, Mali WPTM. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke. 2008;39:1600–1603. doi: 10.1161/STROKEAHA.107.506089. [DOI] [PubMed] [Google Scholar]
- 34.Kamal A, Biessels GJ, Duis SE, Gispen WH. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing. Diabetologia. 2000;43:500–506. doi: 10.1007/s001250051335. [DOI] [PubMed] [Google Scholar]
- 35.Reagan LP. Insulin signaling effects on memory and mood. Curr Opin Pharmacol. 2007;7:633–637. doi: 10.1016/j.coph.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biessels GJ, Kappelle LJ. Increased risk of Alzheimer’s disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans. 2005;33:1041–1044. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- 37.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 38.Abdul-Ghani MA, Williams K, Defronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–1548. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 39.Kumari M, Brunner E, Fuhrer R. Minireview: mechanisms by which the metabolic syndrome and diabetes impair memory. J Gerontol, A, Biol Sci Med Sci. 2000;55:B228–B232. doi: 10.1093/gerona/55.5.b228. [DOI] [PubMed] [Google Scholar]
- 40.Hershey T, Lillie R, Sadler M, White NH. A prospective study of severe hypoglycemia and long-term spatial memory in children with type 1 diabetes. Pediatr Diabetes. 2004;5:63–71. doi: 10.1111/j.1399-543X.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- 41.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Impact of improved glycaemic control on rates of hypoglycaemia in insulin dependent diabetes mellitus. Arch Dis Child. 1998;78:111–115. doi: 10.1136/adc.78.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guleria RS, Pan J, Dipette D, Singh US. Hyperglycemia inhibits retinoic acid-induced activation of Rac1, prevents differentiation of cortical neurons, and causes oxidative stress in a rat model of diabetic pregnancy. Diabetes. 2006;55:3326–3334. doi: 10.2337/db06-0169. [DOI] [PubMed] [Google Scholar]
- 43.Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. 2007;30:2331–2337. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- 44.Yamada KA, Rensing N, Izumi Y, De Erausquin GA, Gazit V, Dorsey DA, Herrera DG. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatr Res. 2004;55:372–379. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]
- 45.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics. 1985;75:921–927. [PubMed] [Google Scholar]
- 46.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- 47.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- 48.Brands AM, Kessels RP, Hoogma RP, Henselmans JM, van der Beek Boter JW, Kappelle LJ, de Haan EH, Biessels GJ. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes. 2006;55:1800–1806. doi: 10.2337/db05-1226. [DOI] [PubMed] [Google Scholar]
- 49.Weinger K, Jacobson AM, Musen G, Lyoo IK, Ryan CM, Jimerson DC, Renshaw PF. The effects of type 1 diabetes on cerebral white matter. Diabetologia. 2008;51:417–425. doi: 10.1007/s00125-007-0904-9. [DOI] [PubMed] [Google Scholar]
- 50.Starr VL, Convit A. Diabetes, sugar-coated but harmful to the brain. Curr Opin Pharmacol. 2007;7:638–642. doi: 10.1016/j.coph.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 52.Artola A, Kamal A, Ramakers GM, Biessels GJ, Gispen WH. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Eur J Neurosci. 2005;22:169–178. doi: 10.1111/j.1460-9568.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 53.Kamal A, Biessels GJ, Urban IJ, Gispen WH. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: impairment of long-term potentiation and facilitation of long-term depression. Neuroscience. 1999;90:737–745. doi: 10.1016/s0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- 54.Kamal A, Biessels GJ, Gispen WH, Ramakers GM. Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res. 2006;1073–1074:276–280. doi: 10.1016/j.brainres.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 55.Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 56.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–333. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- 57.Lobnig BM, Kromeke O, Optenhostert-Porst C, Wolf OT. Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabet Med. 2006;23:32–39. doi: 10.1111/j.1464-5491.2005.01716.x. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, Deary IJ. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care. 2005;28:1431–1437. doi: 10.2337/diacare.28.6.1431. [DOI] [PubMed] [Google Scholar]
- 59.Perros P, Deary IJ, Sellar RJ, Best JJ, Frier BM. Brain abnormalities demonstrated by magnetic resonance imaging in adult IDDM patients with and without a history of recurrent severe hypoglycemia. Diabetes Care. 1997;20:1013–1018. doi: 10.2337/diacare.20.6.1013. [DOI] [PubMed] [Google Scholar]
- 60.Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 61.Asimakopoulou KG, Hampson SE, Morrish NJ. Neuropsychological functioning in older people with Type 2 diabetes: the effect of controlling for confounding factors. Diabet Med. 2002;19:311–316. doi: 10.1046/j.1464-5491.2002.00680.x. [DOI] [PubMed] [Google Scholar]
- 62.Dey J, Misra A, Desai NG, Mahapatra AK, Padma MV. Cognitive function in younger type II diabetes. Diabetes Care. 1997;20:32–35. doi: 10.2337/diacare.20.1.32. [DOI] [PubMed] [Google Scholar]
- 63.Okereke OI, Kang JH, Cook NR, Gaziano JM, Manson JE, Buring JE, Grodstein F. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008;56:1028–1036. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 64.Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GEHM, van der Grond J, Kappelle LJ. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 66.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 67.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, Mcewen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 68.Korf ESC, White LR, Scheltens P, Launer LJ. Brain aging in very old men with type 2 diabetes — the Honolulu-Asia Aging study. Diabetes Care. 2006;29:2268–2274. doi: 10.2337/dc06-0243. [DOI] [PubMed] [Google Scholar]
- 69.den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 70.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes — a systematic review. Diabetes Care. 2006;29:2539–2548. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 71.Manschot SM, Brands AM, van der GJ, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 72.Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese Zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi: 10.1016/s0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 73.Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer’s disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- 75.Jack CR, Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, Schmidt R. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 78.Golomb J, Kluger A, de Leon MJ, Ferris SH, Convit A, Mittelman MS, Cohen J, Rusinek H, de Santi S, George AE. Hippocampal formation size in normal human aging: a correlate of delayed secondary memory performance. Learn Mem. 1994;1:45–54. [PubMed] [Google Scholar]
- 79.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rusinek H, de Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- 81.Shi L, Argenta AE, Winseck AK, Brunso-Bechtold JK. Stereological quantification of GAD-67-immunoreactive neurons and boutons in the hippocampus of middle-aged and old Fischer 344×Brown Norway rats. J Comp Neurol. 2004;478:282–291. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- 82.Cadacio CL, Milner TA, Gallagher M, Pierce JP. Hilar neuropeptide Y interneuron loss in the aged rat hippocampal formation. Exp Neurol. 2003;183:147–158. doi: 10.1016/s0014-4886(03)00126-2. [DOI] [PubMed] [Google Scholar]
- 83.Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 84.Ryan CM, Geckle M. Why is learning and memory dysfunction in Type 2 diabetes limited to older adults? Diabetes Metab Res Rev. 2000;16:308–315. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr141>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 85.Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging — implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- 86.Meyer JS, Xu G, Thornby J, Chowdhury MH, Quach M. Is mild cognitive impairment prodromal for vascular dementia like Alzheimer’s disease? Stroke. 2002;33:1981–1985. doi: 10.1161/01.str.0000024432.34557.10. [DOI] [PubMed] [Google Scholar]
- 87.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 88.Sabayan B, Foroughinia F, Mowla A, Borhanihaghighi A. Role of insulin metabolism disturbances in the development of alzheimer disease: mini review. Am J Alzheimer’s Dis Other Dement. 2008;23:192–199. doi: 10.1177/1533317507312623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yaari R, Corey-Bloom J. Alzheimer’s disease. Semin Neurol. 2007;27:32–41. doi: 10.1055/s-2006-956753. [DOI] [PubMed] [Google Scholar]
- 90.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Luchsinger JA, Mayeux R. Adiposity and Alzheimer’s disease. Curr Alzheimer Res. 2007;4:127–134. doi: 10.2174/156720507780362100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 94.Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. Br Med J. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Snyder AZ, Morris JC, Buckner RL. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005;62:1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- 96.Xu WL, Qiu CX, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56:211–216. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- 97.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 98.Kulstad JJ, Green PS, Cook DG, Watson GS, Reger MA, Baker LD, Plymate SR, Asthana S, Rhoads K, Mehta PD, Craft S. Differential modulation of plasma beta-amyloid by insulin in patients with Alzheimer disease. Neurology. 2006;66:1506–1510. doi: 10.1212/01.wnl.0000216274.58185.09. [DOI] [PubMed] [Google Scholar]
- 99.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li ZG, Mang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:2650. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- 101.Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao YM, Pei JJ, Ji ZJ, Zhao ZW, Qian YY, Sheng SL. Effect of amyloid precursor protein 17mer peptide on microtubule structure and tau protein hyperphosphorylation in hippocampal neurons of experimental diabetic mice. NeuroReport. 2003;14:61–66. doi: 10.1097/00001756-200301200-00012. [DOI] [PubMed] [Google Scholar]
- 104.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 105.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- 106.Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Purohit DP, Perl DP, Davidson M, Mohs RC, Haroutunian V. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol, A, Biol Sci Med Sci. 2005;60:471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dore S, Kar S, Rowe W, Quirion R. Distribution and levels of [125I]IGF-I, [125I] IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience. 1997;80:1033–1040. doi: 10.1016/s0306-4522(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 108.Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- 109.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 110.van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 111.Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32:239–243. doi: 10.1038/sj.npp.1301193. [DOI] [PubMed] [Google Scholar]
- 112.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
- 113.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JCS, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 114.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 115.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 116.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 117.Brodbeck J, Balestra ME, Saunders AM, Roses AD, Mahley RW, Huang Y. Rosiglitazone increases dendritic spine density and rescues spine loss caused by apolipoprotein E4 in primary cortical neurons. Proc Natl Acad Sci U S A. 2008;105:1343–1346. doi: 10.1073/pnas.0709906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D-aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 120.Vetiska SM, Ahmadian G, Ju W, Liu L, Wymann MP, Wang YT. GABAA receptor-associated phosphoinositide 3-kinase is required for insulin-induced recruitment of postsynaptic GABAA receptors. Neuropharmacology. 2007;52:146–155. doi: 10.1016/j.neuropharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 121.Berk MA, Clutter WE, Skor D, Shah SD, Gingerich RP, Parvin CA, Cryer PE. Enhanced glycemic responsiveness to epinephrine in insulin-dependent diabetes-mellitus is the result of the inability to secrete insulin — augmented insulin-secretion normally limits the glycemic, but not the lipolytic or ketogenic, response to epinephrine in humans. J Clin Invest. 1985;75:1842–1851. doi: 10.1172/JCI111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mooradian AD. Central nervous system complications of diabetes mellitus — a perspective from the blood–brain barrier. Brain Res Rev. 1997;23:210–218. doi: 10.1016/s0165-0173(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 123.Guy DA, Sandoval D, Richardson MA, Tate D, Davis SN. Effects of glycemic control on target organ responses to epinephrine in type 1 diabetes. Am J Physiol: Endocrinol Metab. 2005;289:E258–E265. doi: 10.1152/ajpendo.00311.2004. [DOI] [PubMed] [Google Scholar]
- 124.Huang CC, You JL, Lee CC, Hsu KS. Insulin induces a novel form of postsynaptic mossy fiber long-term depression in the hippocampus. Mol Cell Neurosci. 2003;24:831–841. doi: 10.1016/s1044-7431(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 125.Huang CC, Lee CC, Hsu KS. An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J Neurochem. 2004;89:217–231. doi: 10.1111/j.1471-4159.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- 126.van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-D-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- 127.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 128.Magarinos AM, Jain K, Blount ED, Reagan L, Smith BH, Mcewen BS. Peritoneal implantation of macroencapsulated porcine pancreatic islets in diabetic rats ameliorates severe hyperglycemia and prevents retraction and simplification of hippocampal dendrites. Brain Res. 2001;902:282–287. doi: 10.1016/s0006-8993(01)02400-3. [DOI] [PubMed] [Google Scholar]
- 129.Rolandsson O, Backestrdm A, Eriksson S, Hallmans G, Nilsson LG. Increased glucose levels are associated with episodic memory in nondiabetic women. Diabetes. 2008;57:440–443. doi: 10.2337/db07-1215. [DOI] [PubMed] [Google Scholar]
- 130.Gold PE. Glucose and age-related changes in memory. Neurobiol Aging. 2005;26:S60–S64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 131.Sunram-Lea SI, Foster JK, Durlach P, Perez C. The effect of retrograde and anterograde glucose administration on memory performance in healthy young adults. Behav Brain Res. 2002;134:505–516. doi: 10.1016/s0166-4328(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 132.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28:71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 133.Mcnay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55:1088–1095. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- 134.Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 135.Goh SY, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 136.Dominguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care. 1998;21:1736–1742. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- 137.Reagan LP. Glucose, stress, and hippocampal neuronal vulnerability. Int Rev Neurobiol. 2002;51:289–324. doi: 10.1016/s0074-7742(02)51009-6. [DOI] [PubMed] [Google Scholar]
- 138.Takeuchi M, Sato T, Takino JI, Kobayashi Y, Furuno S, Kikuchi S, Yamagishi SI. Diagnostic utility of serum or cerebrospinal fluid levels of toxic advanced glycation end-products (TAGE) in early detection of Alzheimer’s disease. Med Hypotheses. 2007;69:1358–1366. doi: 10.1016/j.mehy.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 139.Tan KCB, Chow WS, Tam S, Bucala R, Betteridge J. Association between acute-phase reactants and advanced glycation end products in type 2 diabetes. Diabetes Care. 2004;27:223–228. doi: 10.2337/diacare.27.1.223. [DOI] [PubMed] [Google Scholar]
- 140.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE — a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 141.Li YM, Dickson DW. Enhanced binding of advanced glycation endproducts (AGE) by the ApoE4 isoform links the mechanism of plaque deposition in Alzheimer’s disease. Neurosci Lett. 1997;226:155–158. doi: 10.1016/s0304-3940(97)00266-8. [DOI] [PubMed] [Google Scholar]
- 142.Koyama H, Shoji T, Fukumoto S, Shinohara K, Shoji T, Emoto M, Mori K, Tahara H, Ishimura E, Kakiya R, Tabata T, Yamamoto H, Nishizawa Y. Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 2007;27:147–153. doi: 10.1161/01.ATV.0000251502.88818.4b. [DOI] [PubMed] [Google Scholar]
- 143.Kelly SJ, Bernard K, Munoz C, Lawrence RC, Thacker J, Grillo CA, Piroli GG, Reagan LP. Effects of the AMPA receptor modulator S 18986 on measures of cognition and oxidative stress in aged rats. Q3 Psychopharmacology. doi: 10.1007/s00213-008-1301-x. in press. [DOI] [PubMed] [Google Scholar]
- 144.Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- 145.Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann NY Acad Sci. 2001;928:168–175. doi: 10.1111/j.1749-6632.2001.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 146.Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging. 2004;25:501–508. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 147.Colombrita C, Calabrese V, Stella AM, Mattei F, Alkon DL, Scapagnini G. Regional rat brain distribution of heme oxygenase-1 and manganese superoxide dismutase mRNA: relevance of redox homeostasis in the aging processes. Exp Biol Med (Maywood ) 2003;228:517–524. doi: 10.1177/15353702-0322805-16. [DOI] [PubMed] [Google Scholar]
- 148.Sugaya K, Chouinard M, Greene R, Robbins M, Personett D, Kent C, Gallagher M, McKinney M. Molecular indices of neuronal and glial plasticity in the hippocampal formation in a rodent model of age-induced spatial learning impairment. J Neurosci. 1996;16:3427–3443. doi: 10.1523/JNEUROSCI.16-10-03427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berr C. Cognitive impairment and oxidative stress in the elderly: results of epidemiological studies. Biofactors. 2000;13:205–209. doi: 10.1002/biof.5520130132. [DOI] [PubMed] [Google Scholar]
- 150.Harman D. Aging: overview. Ann NY Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 151.Ferrannini E, Barrett EJ, Bevilacqua S, Defronzo RA. Effect of fatty-acids on glucose-production and utilization in man. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Piroli GG, Grillo CA, Reznikov LR, Adams S, Mcewen BS, Charron MJ, Reagan LP. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- 153.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Andrews RC, Herlihy O, Livingstone DEW, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab. 2002;87:5587–5593. doi: 10.1210/jc.2002-020048. [DOI] [PubMed] [Google Scholar]
- 155.Vogelzangs N, Suthers K, Ferrucci L, Simonsick EM, Ble A, Schrager M, Bandinelli S, Lauretani F, Giannelli SV, Penninx BW. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology. 2007;32:151–159. doi: 10.1016/j.psyneuen.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 157.Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10:97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 158.Ng TP, Feng L, Niti M, Yap KB. Albumin, haemoglobin, BMI and cognitive performance in older adults. Age Ageing. 2008;37:423–429. doi: 10.1093/ageing/afn102. [DOI] [PubMed] [Google Scholar]
- 159.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 160.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cournot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, Ruidavets JB. Relation between Body Mass Index and cognitive function in healthy middle-aged men and women. Eur Heart J. 2006;27:937. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 162.Ronnemaa T, Koskenvuo M, Marniemi J, Koivunen T, Sajantila A, Rissanen A, Kaitsaari M, Bouchard C, Kaprio J. Glucose metabolism in identical twins discordant for obesity. The critical role of visceral fat. J Clin Endocrinol Metab. 1997;82:383–387. doi: 10.1210/jcem.82.2.3763. [DOI] [PubMed] [Google Scholar]
- 163.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 164.De La Torre JC. Vascular basis of Alzheimer’s pathogenesis. Alzheimer’s Disease: Vasc Etiology Pathol. 2002;977:196–215. doi: 10.1111/j.1749-6632.2002.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 165.Banks WA. The many lives of leptin. Peptides. 2004;25:331–338. doi: 10.1016/j.peptides.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 166.Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 167.Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC 186, 1–6. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35:559–572. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 171.Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]