Abstract
Convergent evidence from human and non-human animal studies suggests aerobic exercise and increased aerobic capacity may be beneficial for brain health and cognition. It is thought growth factors may mediate this putative relationship, particularly by augmenting plasticity mechanisms in the hippocampus, a brain region critical for learning and memory. Among these factors, glucocorticoids, brain derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF), hormones that have considerable and diverse physiological importance, are thought to effect normal and exercise-induced hippocampal plasticity. Despite these predictions, relatively few published human studies have tested hypotheses that relate exercise and fitness to the hippocampus, and none have considered the potential links to all of these hormonal components. Here we present cross-sectional data from a study of recognition memory; serum BDNF, cortisol, IGF-1, and VEGF levels; and aerobic capacity in healthy young adults. We measured circulating levels of these hormones together with performance on a recognition memory task, and a standard graded treadmill test of aerobic fitness. Regression analyses demonstrated BDNF and aerobic fitness predict recognition memory in an interactive manner. In addition, IGF-1 was positively associated with aerobic fitness, but not with recognition memory. Our results may suggest an exercise adaptation-related change in the BDNF dose-response curve that relates to hippocampal memory.
Keywords: BDNF, IGF-1, recognition memory, hippocampus, cardiovascular fitness, VO2 max
1. INTRODUCTION
Human and animal studies have converged on the idea aerobic exercise and cardiorespiratory fitness may be beneficial for brain health and cognition. In animal models, rodents that run consistently outperform sedentary controls on memory tests that depend on the hippocampus [e.g.1,2]. It is thought aerobic exercise may enhance hippocampal plasticity and memory through a variety of hormonal and inflammatory factors [see ref. 3 for a review], including glucocorticoids, and neurotrophins—proteins that play special roles in the growth and maintenance of the nervous and cardiovascular systems both developmentally and in adulthood [4–6]. Neurotrophins and neurotrophin related genes (e.g. brain derived neurotrophic factor; BDNF) are upregulated in the hippocampus in response to exercise [7,8] and are thought to be critical for synaptic plasticity [9].
Although rodent studies clearly suggest aerobic exercise may preferentially impact the hippocampal memory system, human studies have mostly focused on executive processes [reviewed in ref. 10]. In humans, links between physical fitness and performance on tasks designed to probe executive functions have been established in meta-analytic studies [11–14]. Recently, exercise-induced changes in aerobic capacity were associated with global intelligence measures in young men [15], positively correlated with delayed free recall in young to middle aged adults [16] and with pattern separation performance in young adults [17]. While a few human studies have also focused on BDNF specifically, results have been mixed. Previous work has suggested changes in serum BDNF levels following chronic exercise are associated with changes in hippocampal volume over a one year period [18], and BDNF may predict enhanced performance on a word-learning task following an acute exercise bout [19].
Although animal models predict circulating growth factors and glucocorticoids may mediate the effects of aerobic exercise on memory, whether the presumed relationship between aerobic capacity and memory enhancement is modulated by these factors in humans is not known. To address this question, our group designed a cross sectional study of aerobic capacity and recognition memory in healthy young adults. We used a paradigm shown in fMRI studies to recruit the hippocampus [20,21] in combination with serum assays for resting levels of BDNF; related neurotrophic factors insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF); and cortisol, the primary glucocorticoid hormone in humans. We predicted an interrelationship between aerobic capacity, memory performance, and hormone levels. Here we report evidence of an interaction between circulating BDNF and aerobic fitness on recognition memory performance in humans and show how other trophic factors may be related to fitness.
2. MATERIALS AND METHODS
2.1. Participants
One hundred and fourteen healthy young participants were recruited from the Boston University student community. Students who indicated their interest in the study were sent a brief explanation of study protocols, and asked to exclude themselves if they met any of the following pre-screening conditions: history of neurological or psychiatric conditions; learning disability; heart, lung, or musculoskeletal conditions or disorders; diabetes mellitus; electrolyte disorder; high cholesterol; eating disorder or obesity; or current use of any prescription or recreational cardioactive or psychoactive drugs, or recreational smoking.
Twenty-one participants were excluded following an initial screening visit, twenty-one withdrew voluntarily, and an additional nine participants were excluded from analyses due to behavioral tasks performance issues (described below; N = 2), or equipment malfunction (N = 7), leaving a final sample size of N = 63 participants. Details of participant characteristics are presented in Table 1. All participants were native English speakers or bilingual, all had normal or corrected to normal vision, and all gave signed, informed consent before participating in the experiment. All protocols were approved by the Boston University Charles River Campus Institutional Review Board and conformed with the Code of Ethics of the World Medical Association [22].
Table 1.
Participant Characteristics
|
N = 63 (39 female) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Meansa |
Correlationsb |
||||||||||
| N | Range | Female | Male | ρaccuracy | ρfitness | ρBDNF | ρcortisol | ρIGF-1 | ρVEGF | ||
| Demographics | |||||||||||
| Age (years) | 63 | 18 – 29 | 20.5 ± 2.1 | 22.3 ± 3.2* | 0.04 | −0.23 | 0.09 | 0.07 | −0.35** | 0.03 | |
| Education (years) | 62 | 12 – 22 | 14.8 ± 1.5 | 16.0 ± 2.5* | −0.06 | −0.24 | 0.06 | 0.11 | −0.29* | −0.10 | |
| Physiology | |||||||||||
| BDNF (ng·mL−1) | 63 | 1.1 – 30.5 | 17.9 ± 6.0 | 17.6 ± 7.3 | −0.23 | −0.22 | -- | 0.01 | −0.12 | 0.15 | |
| Cortisol (ng·mL−1) | 62 | 14 – 182 | 72.8 ± 31.9 | 60.1 ± 35.5 | −0.21 | −0.25 | 0.01 | -- | −0.05 | −0.10 | |
| IGF-1 (ng·mL−1) | 62 | 95 – 271.4 | 165.1 ± 42.6 | 159.0 ± 30.1 | 0.11 | 0.27* | −0.12 | −0.05 | -- | −0.08 | |
| VEGF (ng·mL−1) | 61 | 0.016 – 0.465 | 0.21 ± 0.09 | 0.21 ± 0.11 | −0.05 | −0.08 | 0.13 | −0.06 | −0.10 | -- | |
| Fitness percentile | 63 | 3.3 – 99.9 | 51.4 ± 24.0 | 70.2 ± 26.3** | 0.05 | −0.22 | −0.25 | 0.27* | −0.06 | ||
| VO2 peak (mL·kg−1min−1) | 63 | 24.7 – 66.5 | 38.5 ± 6.3 | 50.5 ± 8.1*** | 0.02 | 0.91*** | −0.17 | −0.30* | 0.18 | −0.03 | |
| RERmax | 63 | 0.98 – 1.56 | 1.19 ± 0.14 | 1.26 ± 0.15* | 0.05 | 0.28* | 0.03 | −0.11 | 0.11 | 0.07 | |
| BMI (kg·m−2) | 63 | 16.8 – 31.2 | 22.9 ± 3.2 | 23.6 ± 3.2 | −0.02 | −0.18 | −0.10 | 0.12 | −0.03 | −0.07 | |
| Body Fat % | 63 | 5.0 – 34.9 | 23.7 ± 4.8 | 11.5 ± 5.6*** | 0.03 | −0.43*** | 0.11 | 0.36** | −0.09 | 0.08 | |
| Hip circumference (cm) | 63 | 73.8 – 114.0 | 93.4 ± 8.7 | 95.2 ± 8.4 | 0.10 | −0.23 | −0.12 | 0.01 | −0.05 | 0.06 | |
| Waist circumference (cm) | 63 | 60.0 – 98.7 | 73.1 ± 7.9 | 80.7 ± 7.6*** | −0.05 | 0.00 | −0.11 | 0.02 | −0.10 | 0.06 | |
| Questionnaires | |||||||||||
| BaeckeWork Index | 62 | 1.25 – 3.625 | 2.1 ± 0.4 | 2.1 ± 0.6 | 0.00 | 0.14 | −0.25* | 0.13 | −0.05 | 0.02 | |
| BaeckeSport Index | 62 | 1.0 – 4.5 | 2.5 ± 0.8 | 3.0 ± 0.8* | 0.06 | 0.48*** | −0.31* | −0.04 | 0.17 | −0.33** | |
| BaeckeLeisure Index | 63 | 1.75 – 4.25 | 3.0 ± 0.5 | 3.1 ± 0.6 | −0.03 | 0.20 | −0.18 | 0.28* | 0.05 | −0.30* | |
| Beck Depression Index | 63 | 0 – 15 | 2.4 ± 3.3 | 2.9 ± 3.3 | −0.17 | 0.17 | −0.06 | 0.09 | 0.07 | 0.05 | |
| Estimated Verbal IQ | 62 | 98 – 123 | 112.4 ± 5.3 | 112.8 ± 4.8 | 0.07 | −0.03 | 0.15 | −0.01 | −0.07 | −0.07 | |
Summarized as mean ± SD; asterisks in the Male column indicate sex differences
We present bivariate Spearman-rank coefficients for our outcome variables to provide a thorough characterization of our study’s sample. We do not draw inference from these simple comparisons. “Accuracy” refers to SMT corrected accuracy (see Methods section 2.2.7. for definition)
P < 0.05
P < 0.01
P < 0.001
2.2. Procedure
2.2.1. Experimental Overview
For each participant, the experiment consisted of three visits: (i) informed consent and screening, (ii) VO2 max aerobic capacity and body composition testing, and (iii) blood draw and cognitive testing. For each subject, visits took place approximately within one month from start to finish, and visit three was performed no later than one week after visit two.
2.2.2. Consent and Screening
Screening ensured participants were healthy and able to perform a strenuous treadmill test. Participants gave signed, informed consent during the first visit prior to the start of any other procedures. Participants were then formally screened for the above pre-screening criteria. In addition, height and weight were measured to calculate body mass index (BMI); waist and hip circumference measurements were also taken. Participants were excluded if they met the World Health Organization’s criteria for obesity on both of these measures: BMI greater than or equal to 30 for both men and women, and a waist-to-hip ratio greater than or equal to 1.0 for men, or 0.8 for women (World Health Organization, Geneva, Switzerland). Participants also completed a hand preference questionnaire [23], the North American Adult Reading Test [24,25] to estimate verbal intelligence quotient, and the Baecke physical activity habits questionnaire [26]. At the end of the consent and screening visit, participants were given an exercise and physical activity diary to fill out over the next two weeks.
2.2.3. Assessment of aerobic capacity
To assess participants’ aerobic capacity (VO2 max, the maximal rate of oxygen an individual consumes during exercise measured in milliliters, per minute, per kilogram body-mass) we used a graded maximal exercise test performed on a treadmill [27]. Briefly, the treadmill started out at a speed of 0.8 m/s, and an incline of 10% grade. Throughout the test, the speed and incline of the treadmill increased by an average of 0.35 m/s and 2% grade every three minutes. A physician was on call, and two study staff members with current cardio-pulmonary resuscitation certification were present for each fitness test.
All VO2 max tests were performed between the hours of 8:00 and 10:00 AM at Boston University Sargent College of Health and Rehabilitation Sciences. The ParvoMedics True One 2400 system (ParvoMedics, Sandy, UT, USA) was used during testing to analyze gas exchange in participants’ breath. The True One 2400 system was calibrated against medical grade gasses (ParvoMedics, Sandy, UT, USA), and average VO2 values were computed over 30 second intervals. The system also calculated respiratory exchange ratio (RER; volume expired CO2 divided by volume expired O2), which was used as a reference value for calculated VO2 max; an RER around or above 1.15 is indicative VO2 max has been reached [28].
Participants were asked not to engage in strenuous physical activity for 24 hours before testing, and not to consume food or caffeine 3 hours prior. Immediately before testing, participants’ weight, resting heart rate, and blood pressure were measured, and skin-fold calipers were used to estimate percent body fat using the three site formula [29]. Participants were given a 3 – 5 minute warm-up at 0.5 m/s before testing. Following testing, participants were given a five minute cool-down at 0.5 m/s. Participants’ blood pressure, heart rate, and rating of perceived exertion [30] were monitored continuously during testing and/or cool-down in accordance with American College of Sports Medicine guidelines (ACSM) [29]. All blood pressure measurements were taken manually with an inflatable sphygmomanometer, and pulse rates were measured using an Omron HR-100C (Omron Healthcare Inc., Kyoto, Japan) heart rate monitor or, in cases of monitor failure, were measured by hand. Fitness tests were terminated when participants reached volitional exhaustion. Graded exercise protocol and termination criteria followed ACSM guidelines [29].
2.2.4. Blood draw
All participants had up to 30 mL of blood drawn from the median cubital vein by a trained nurse at the General Clinical Research Unit, Boston University Clinical and Translational Science Institute. Blood was collected to analyze serum levels of brain derived neurotrophic factor (BDNF), cortisol, insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF). Because BDNF [31,32], cortisol [see ref 33 for a review], and VEGF [34] fluctuate with circadian rhythm, all venipunctures were performed between 8:00 – 9:45 AM, prior to cognitive testing. Though not explicitly required, participants were asked to fast for at least 2 hours prior to the blood draw, and were provided a small breakfast directly after.
Whole blood was drawn to a serum separator tube and allowed to clot at room temperature for approximately 30 minutes. The blood samples were then centrifuged for 15 minutes at 4 °C and 1000xg. One mL serum aliquots were prepared and stored at −80 °C. Samples were stored for 2 – 17 months prior to analyses.
2.2.5 Hormone Assays
Serum BDNF, cortisol, IGF-1, and VEGF were determined with Quantikine® quantitative sandwich ELISA kits (R&D Systems Inc., Minneapolis, MN, USA). All assays were performed according to the manufacturer’s instructions. Assays were analyzed in two rounds: (i) BDNF and cortisol levels were determined from the first twenty-eight participants; (ii) IGF-1 and VEGF data were obtained from re-thawed samples from the first twenty-eight participants, and all four hormone levels were determined for the final thirty-five participants. Within each round, all assays were performed in duplicate.
Assay sensitivities, or minimum detectable concentrations, for BDNF, cortisol, IGF-1, and VEGF assays are estimated at 0.020, 0.071, 0.026, and 0.005 ng/mL, respectively. Respective intra- and inter-assay precision coefficients of variation for BDNF, cortisol, IGF-1, and VEGF are estimated at less than 6.2%, 9.2%, 4.3%, and 6.5% within-assay; and less than 11.3%, 21.2%, 8.3%, and 8.5% between assays. No significant cross-reactivities have been observed for BDNF and IGF-1 assays. The cortisol assay may cross-react with the synthetic glucocorticoids Prednisolone (estimated cross-reactivity: 4.4%) and Cortodoxone (3.4%), and/or with endogenous steroid hormones Progesterone (1.7%) and Cortisone (0.2%). The human VEGF assay may cross-react with human VEGF receptors 1 and 2 (genes FLT1 and KDR) if they are present in high concentrations. We did not assay for any of these cross-reactivity factors.
2.2.6. Cognitive Testing
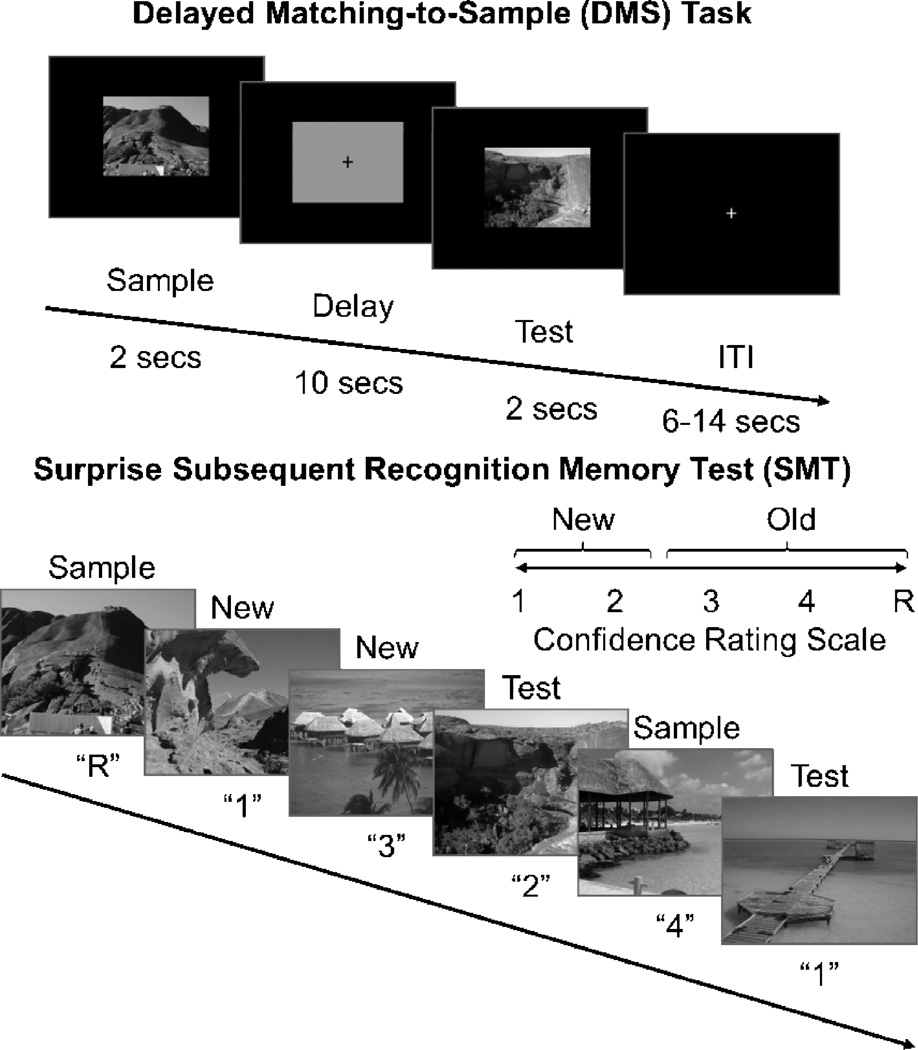
We used an adaptation of a delayed match-to-sample (DMS) paradigm with unfamiliar complex visual scenes in combination with a subsequent memory test (SMT). Thirty-three of the current study’s participants performed the DMS phase of this task in an fMRI scanner. In previous fMRI studies we have demonstrated this paradigm recruits the hippocampus and medial temporal lobe cortex [20,21]. All stimulus presentations and subject response data were displayed and recorded using EPrime 2.0 (Psychology Software Tools, Pittsburgh, PA), and all cognitive testing was performed in the morning, approximately within 60 min after the blood draw. 144 DMS stimuli were drawn from a set of 288 trial unique, but content similar, complex visual outdoor scenes (Fig. 1). Participants completed eight runs of twelve trials each. For each trial, participants saw a sample scene (duration = 2 seconds), followed by a delay period (duration = 10 seconds), a test scene (duration = 2 seconds), and an inter-trial interval fixation (duration = 6 seconds; or 6 – 14 seconds jittered for fMRI participants). Participants were asked to indicate whether sample and test scenes matched or not using keyboard numbers or a button-box in the case of the fMRI participants, and to respond as quickly and as accurately as possible. All trials were evenly split between match and non-match conditions, and pseudo-randomized for each run and participant.
Figure 1.
Recognition memory task. Adapted from Schon et al. [20], participants were first shown a series of 144 pseudo-randomized, trial unique but content similar outdoor scenes in the context of a delayed match to sample (DMS) working memory paradigm. Approximately 15 minutes later, participants were administered a surprise subsequent memory test (SMT) where they were shown all 144 DMS images, plus 144 lure images, and asked to rate their recognition confidence. Participants were blind to the ratio of old to new images.
Approximately 15 minutes after the DMS task, participants performed a surprise self-paced SMT. Participants were shown all 144 old images randomized with an equal number of new images (lures). Participants were unaware of the ratio of old to new images, and were asked to rate their recognition memory strength for each SMT image along a 5-point scale [35]: 1-sure, new; 2-unsure, new; 3-unsure, old; 4-sure, old; R-sure, old, plus accompanied by some subjective association with the scene, e.g. memory for which run the scene came from or a thought prompted by the original viewing.
2.2.7. Behavioral Performance Measures
For the DMS task, performance was assessed using simple percent correct and correct trial reaction times. Responses from incorrect DMS trials were discarded from SMT analysis on a per-subject basis. For the subsequent memory test (SMT), since an image could either be old or new, and a subject could classify it as either old or new, all responses were sorted into one of four bins on a standard two by two truth table: True Positives (hits) and Negatives (correct rejections), and False Positives (false alarms) and Negatives (misses). Because a participant could, in theory, attain a perfect True Positive rate by indiscriminately classifying all stimuli as old, we used the corrected hit rate (accuracy; defined as hits minus false alarms) as a dependent measure of recognition memory in our analyses. Using accuracy instead of the True Positive (hit) rate provides a partial correction for this potential bias.
2.2.8. Statistical Methods
All raw data were analyzed in R 2.15.2 [36]. Demographic variables are summarized as mean ± standard deviation; those grouped by sex were analyzed with two-sample t-tests assuming equal variance except for BMI, which is typically highly skewed [37], and fitness percentile, which has a uniform distribution. These exceptions were analyzed with the Wilcoxon rank sum test.
In all regression analyses, continuous input variables were centered to have a mean of zero, except fitness percentile, which was centered to the 50th percentile for better interpretability. Centered input variables were then standardized by dividing by twice their respective marginal standard deviations [38]. Resultant regression coefficients can be interpreted as the expected change in y per mean ± one standard deviation in x. This practice allows for greater consistency when comparing coefficients on continuous versus binary predictors than common z-score rescaling [38]. Note that this standardization affects the scale, but not the shape of a variable’s distribution. All regression coefficients we report come from models fit to these standardized inputs. Binary and other discrete group variables were left un-centered and un-rescaled. Throughout the modeling process, we assessed the internal validity of our regression models by comparing estimated coefficients and their standard errors, adjusted- or pseudo-R2, and Akaike’s information criterion.
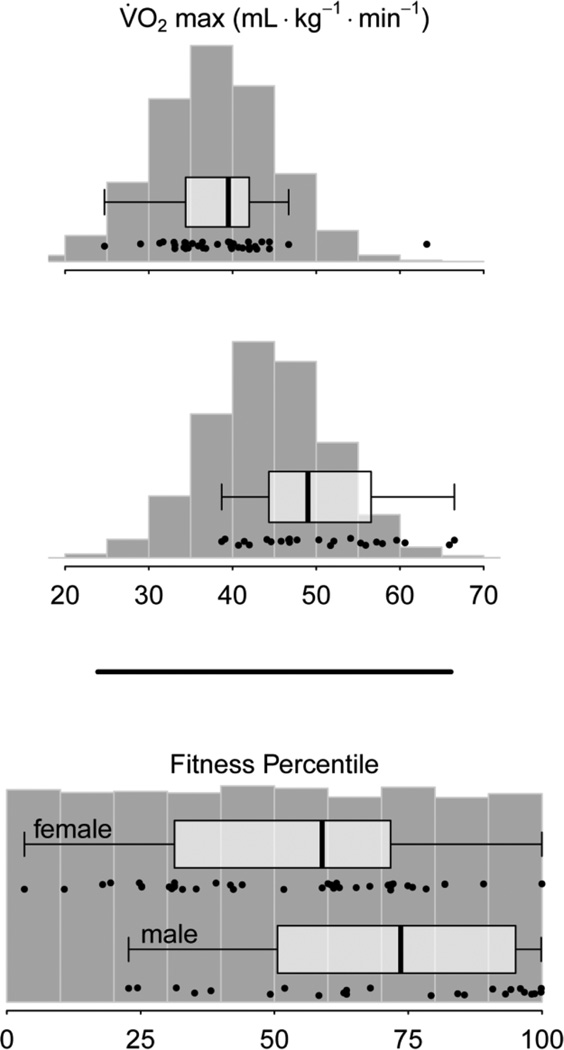
In an effort to control for inherent sex differences in aerobic capacity (men typically have higher VO2 max due to larger lung capacity and lower average body fat percentages), VO2 max data were transformed to fitness percentile scores calculated based on norms compiled by the American College of Sports Medicine (ACSM) [29]. The goal of this transformation was to put observed VO2 peak values on an age and gender independent scale. Note this transformation changes the shape of the population distribution from approximately normal (for VO2 max), to uniform (for fitness percentile; Fig. 2). Despite this effort to control for expected sex differences, we included sex as a covariate in fitness percentile-based regression analyses as we observed a slight gender self-selection bias: in our data, fit individuals with high VO2 max scores tended to be male. In our regression analyses, positive values on the gender coefficient reflect higher outcomes for male participants. Since exactly half of our participants did not reach the 1.15 RER benchmark (see above) and so may not have reached their true VO2 max, we created an indicator variable for participants with RERmax < 1.15 and participants with RERmax ≥ 1.15. This group variable was also used to adjust fitness percentile-based regression analyses, and positive values on its coefficient reflect higher outcomes for individuals whose RER was greater than or equal to 1.15.
Figure 2.
Summary of raw VO2 peak data and fitness percentile transformation. Boxplots and raw data points from our sample are overlaid on histograms of simulated population distributions of VO2 max and fitness percentile based on [29]. The goal of transforming VO2 peak to fitness percentile was to compare individuals on an age and gender independent scale. Boxplots indicate quartiles and medians for VO2 peak data for (top) female and (middle) male participants, and fitness percentile data for both genders (bottom). Individual data points are jittered slightly in the y dimension to appear more visually distinct.
Other variables of interest include measures of memory performance, and hormone concentrations obtained from the blood sample assays. Since all memory variables are expressed as proportions (e.g. SMT corrected accuracy, see above), they make for inappropriate outcome variables for standard ordinary least squares (OLS) regression methods. Proportions are often skewed and heteroskedastic, and exist only on the closed unit interval [0,1]. We have therefore modeled our memory variables using the beta distribution, which is more appropriate for analysis of proportions [39,40]. This was accomplished using the betareg function from the R betareg package [41] specifying the logit link. Resultant regression coefficients can then be exponentiated and interpreted as odds ratios (OR).
Prior to analysis, since two participants had perfect scores and the beta distribution assumes data on the open unit interval (0, 1), DMS accuracy was transformed with the formula [ (x (n-1) − ½) / n ] where n is the number of observations in x [40]. SMT scores were left untransformed as these were all comfortably within the (0, 1) range. For the reader’s benefit, we present both OLS and beta regression results side-by-side.
We opted to log-transform (using the natural logarithm) hormone level measurements whenever they were used as outcome variables. Since hormone levels are all-positive continuous variables they are likely to have effects on a multiplicative scale, making the log-transformation an appropriate choice for improved statistical inference [42]. Since serum assays were conducted in two rounds, we adjusted hormone regression analyses for the effect of assay round. Technical reasons for this are twofold: (i) measurements are expected to vary slightly between assays (see above), and (ii) round one samples were thawed one extra time prior to IGF-1/VEGF measurements, which may have partially degraded these samples. Positive values on this coefficient reflect higher outcomes for the second assay round. Furthermore, we considered hormone by fitness interactions. Given the results of our analysis of subsequent memory, we specified a full model, predicting SMT accuracy from BDNF, fitness percentile, and their interaction, controlling for group effects of gender, RER ≥ 1.15, and assay round.
3. RESULTS
3.1. Participants
Overall, N = 63 participants completed all phases of the study. See Table 1 for an overview of participant characteristics, including summaries of demographic and physiological variables, and questionnaire scores. One subject was removed from analyses of serum cortisol, because that subject’s measured cortisol concentration exceeded the highest standard solution concentration, even when the sample was reanalyzed. This subject’s cortisol levels, therefore, could not be reliably determined. Insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) data were also unavailable for one subject. This subject consented to BDNF and cortisol but not IGF-1 and VEGF assays. Moreover, we observed one clear outlier in the VEGF data. This subject’s measurement was more than four standard deviations away from the group mean, and was discarded from all VEGF analyses and summaries.
Raw VO2 peak scores ranged from 24.7 – 63.2 mL·kg−1·min−1 for women, and 38.7 – 66.5 mL·kg−1·min−1 for men in our sample; corresponding fitness percentiles ranged from the 3.3rd – 99.9th percentile for women, and the 22.8th – 99.9th percentile for men (Fig. 2). Resulting normative fitness percentiles were greater for men in our sample (W = 280, z = 2.66, P = 0.008), indicating a possible gender self-selection bias. While we have good coverage of the expected population distributions of VO2 max and fitness percentiles for women, we may be under-sampling lower-fit men and/or oversampling higher-fit men (Fig. 2). Roughly one-third of our participants (N = 22; 18 female) did not attain the threshold RER of 1.15 and so may not have reached their true VO2 max.
3.2. DMS Results
Accuracy on the DMS task, assessed as a simple proportion correct, was very high for most participants (mean = 95% ± 4.6%), and was not predicted by fitness in either beta or OLS regression models (both P > 0.19). When the models were adjusted for sex and RER ≥ 1.15, however, marginal negative relationships between fitness and accuracy emerged (beta: OR = 0.74, P = 0.08, 95% CI = [0.53, 1.03]; OLS: β = −0.02, P = 0.08, 95% CIβ = [−0.05, 0.002]). Median reaction time (in milliseconds) was also analyzed for correct trials. We found a marginal log-level relationship between faster reaction times and fitness (β = −0.11, P = 0.08, 95% CIβ = [−0.23, 0.01]) including when the model was corrected for sex and RER (β = −0.11, P = 0.12, 95% CIβ = [−0.24, 0.02]). We did not expect performance on the DMS task to be associated with fitness. These data seem to reflect a slight speed-accuracy tradeoff which may be incidentally related to fitness, and we do not interpret them further.
3.3. SMT Results
3.3.1. Relationship between hormone levels and subsequent recognition memory
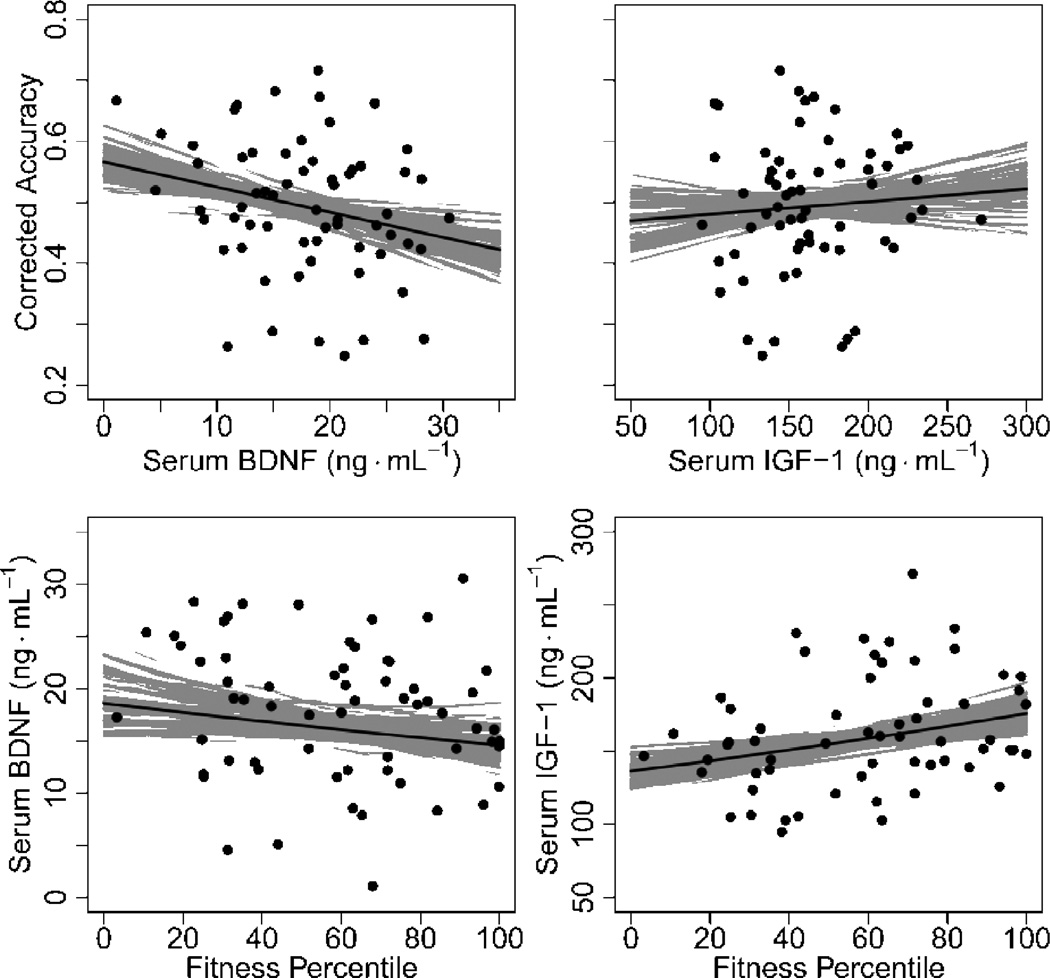
A summary of SMT results and a brief comparison of beta and OLS regression models are presented in Table 2; see Supplementary Fig. 1 for a summary of raw SMT data. Mean SMT corrected accuracy (See section 2.2.7.; mean = 49% ± 11%) was, expectedly, substantially lower than the raw True Positive rate (mean = 67% ± 10%), demonstrating the importance of the accuracy bias correction. The two measures were, however, predictably moderately correlated (Spearman’sp = 0.56, P < 0.001). In unadjusted beta regression models, BDNF was a significant negative predictor of SMT accuracy (Fig. 3; OR = 0.81, P = 0.05, 95% CI = [0.65, 0.999]); cortisol, IGF-1, and VEGF were all non-significant (all P > 0.19). Results were comparable when models were adjusted for assay round, except cortisol became a marginal negative predictor of accuracy (OR = 0.85, P = 0.12, 95% CI = [0.69, 1.04]), and the effect of BDNF on SMT accuracy weakened very slightly (OR = 0.82, P = 0.06, 95% CI = [0.66, 1.01]). Assay round itself was at least marginally significant in all cases (all P < 0.08). Together, these results point to a negative relationship between resting BDNF and subsequent recognition memory.
Table 2.
Regression models of Subsequent Memory Test (SMT) Accuracy
|
Relationship between hormone levels and subsequent recognition memory | ||||||
| Predictor |
Unadjusted |
Adjusted |
||||
| βa | R2b | βa | βcassay round | R2b | ||
| BDNF | −0.215* (0.109) | 0.059 (0.045) | −0.200 (0.107) | −0.188 (0.106) | 0.103 (0.034) | |
| Cortisol | −0.145 (0.111) | 0.026 (0.009) | −0.166 (0.107) | −0.243* (0.107) | 0.100 (0.001) | |
| IGF-1 | 0.063 (0.113) | 0.005 (−0.011) | 0.065 (0.110) | −0.213 (0.110) | 0.062 (−0.026) | |
| VEGF | −0.003 (0.115) | 0.000 (−0.017) | 0.048 (0.115) | −0.219 (0.115) | 0.056 (0.025) | |
|
Relationship between aerobic fitness and subsequent recognition memory | ||||||
| Predictor |
Unadjusted |
Adjusted |
||||
| βafitness | R2b | βafitness | βcsex | βRER ≥ 1.15 | R2b | |
| Fitness | −0.002 (0.112) | 0.000 (−0.016) | 0.008 (0.118) | −0.086 (0.125) | 0.128 (0.121) | 0.020 (−0.029) |
|
Interaction between BDNF and aerobic fitness on subsequent recognition memory | ||||||
| βBDNF· fitness | βaBDNF | βafitness | βcsex | βRER ≥ 1.15 | βcassay round | R2b |
| 0.609** (0.228) | −0.294** (0.109) | −0.059 (0.105) | -- | -- | -- | 0.157 (0.117) |
| 0.587** (0.222) | −0.252* (0.106) | 0.040 (0.118) | −0.066 (0.116) | 0.181 (0.113) | −0.262* (0.113) | 0.232 (0.158) |
This table presents a series of beta regression models with and without adjusting for confounding covariates; each row corresponds to a different model predicting SMT accuracy. Coefficients are presented as β (SEβ). Exponentiated coefficients can be interpreted as odds-ratios
Continuous input variables were standardized prior to model estimation (see Methods for details)
Beta regression pseudo-R2 (ordinary least squares regression adjusted-R2for comparison)
Positive values on the coefficient for assay round reflect higher outcomes for assay wave two; positive values on the coefficient for sex reflect higher outcomes for male subjects
P < 0.05
P < 0.01
P < 0.001
Figure 3.
Summary of BDNF and IGF-1 findings. Top: maximum likelihood beta regression models of subsequent memory accuracy by resting serum brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1). Corrected accuracy is defined as the proportion of subsequent memory test (SMT) stimuli correctly identified as old less the proportion of SMT lure stimuli incorrectly identified as old. The top-left panel shows a significant negative association between BDNF and SMT accuracy. Bottom: ordinary least squares (OLS) regression models of BDNF and IGF-1 by cardiovascular fitness percentile. Outcome variables were modeled on the log scale. The bottom-right panel shows strong evidence of a positive association between IGF-1 and fitness. Aggregated gray lines represent 95% confidence interval estimates for each regression.
3.3.2. Relationship between aerobic fitness and subsequent recognition memory
A summary of SMT results and a brief comparison of beta and OLS regression models are presented in Table 2. Fitness percentile did not predict SMT accuracy in unadjusted beta regression models. Results did not change appreciably when models were adjusted for sex and RER ≥ the 1.15 benchmark.
3.3.3. Interaction between BDNF and aerobic fitness on subsequent recognition memory
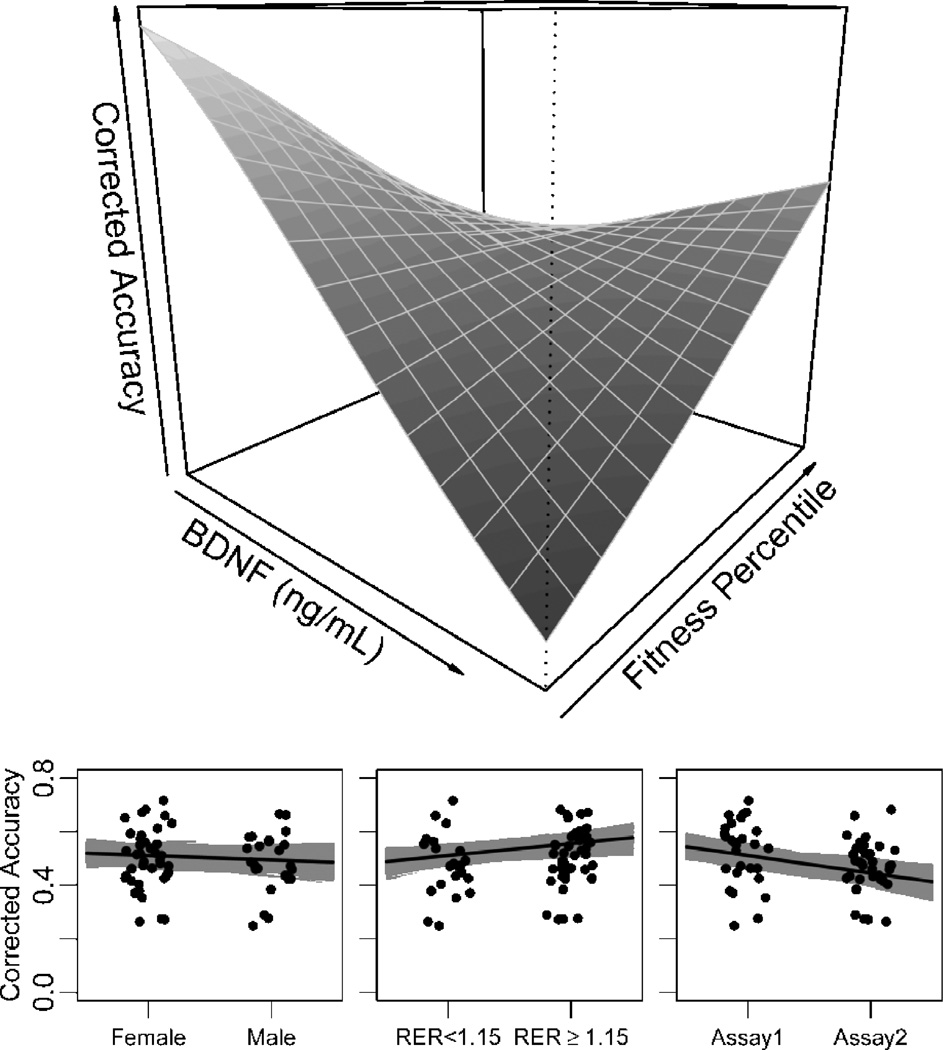
A summary of SMT results and a brief comparison of beta and OLS regression models are presented in Table 2. We also considered possible interactions between hormones and fitness on subsequent recognition memory. Given the results of the above SMT analysis, we modeled accuracy from predictors BDNF, fitness, and a BDNF by fitness interaction, controlling for confounding variables sex, RER ≥ 1.15, and assay round. Results of this analysis (Fig. 4) indicated main effects of BDNF (OR = 0.78, P = 0.02, 95% CI = [0.63, 0.96]) and assay round (OR = 0.77, P = 0.02, 95% CI = [0.62, 0.96]), and a strong, positive interaction between BDNF and fitness predicting SMT accuracy (OR = 1.80, P = 0.01, 95% CI = [1.16, 2.78]; overall model: R2pseudo = 0.23). Note the interaction term coefficient and standard error remained relatively stable when sex was included as a covariate in the model (Table 2). Although the coefficient on resting BDNF suggests a negative relationship with subsequent recognition accuracy, this relationship becomes positive as fitness percentile increases (Fig. 4), with an inflection point estimated around the 75th fitness percentile.
Figure 4.
Interaction between BDNF and fitness on subsequent memory accuracy. SMT corrected accuracy was modeled, using beta regression, as a function of resting serum BDNF, cardiovascular fitness percentile, and their interaction, adjusting for confounding covariates sex, an indicator of aerobic threshold (RER ≥ 1.15), and assay round. The top panel conveys the shape of a strong, positive interaction between continuous variables BDNF and fitness percentile. For example, at low fitness, increasing serum BDNF clearly predicts lower SMT accuracy. As fitness increases, BDNF begins to positively predict subsequent memory accuracy, with an estimated inflection point around the 75th aerobic fitness percentile. Bottom panels show effects of covariates on the regression at mean BDNF and 50th fitness percentile.
Given we also observed a possible self-selection bias of higher-fit male participants we wanted to confirm the BDNF by fitness interaction could not simply be explained by a BDNF by sex interaction. Adding this second interaction term to the model strengthened the BDNF by fitness interaction (beta regression βaccuracy± SEβ increased from 0.59 ± 0.22 to 0.70 ± 0.24), indicating the observed BDNF by fitness term should not be taken as simply a substitute for a BDNF by sex term. The BDNF by sex coefficient was not significant (P = 0.23). Furthermore, adding the BDNF by sex term to the model had a minimal effect on R2pseudo estimates (R2pseudo increased by 0.017) and slightly lessened the model’s expected out of sample predictive power, given by a small increase in Akaike’s information criterion. For these reasons we discarded the two-interaction term model in favor of the simpler model presented above, and detailed in Table 2 and Fig. 4.
3.4. Hormone Measures
3.4.1. Relationship between hormone levels and aerobic capacity
Unadjusted ordinary least squares (OLS) regression models showed a marginal negative log-level association between cortisol and fitness (β = −0.20, P = 0.09, 95% CI, = [−0.43, 0.03]), and a significant positive log-level relationship between IGF-1 and fitness (Fig. 3; β = 0.12, P = 0.02, 95% CIβ, = [0.02, 0.22]). Associations between BDNF and VEGF and fitness were nonsignificant (both P > 0.34). When the models were adjusted for sex, RER ≥ 1.15, and assay round, IGF-1 remained positively associated with fitness (β = 0.16, P = 0.008, 95% CIβ, = [0.05, 0.28]). Fitness did not significantly predict log-hormone levels in the other models (BDNF: β = −0.17, P = 0.22, 95% CIβ, = [−0.45, 0.10]; Cortisol: β = −0.13, P = 0.35, 95% CIβ, = [−0.39, 0.14]; VEGF: β = −0.20, P = 0.16, 95% CIβ, = [−0.47, 0.08]).
3.4.2. Hormone-gender, hormone-hormone, and hormone-assay round comparisons
A summary of hormone concentration ranges and means by sex is presented in Table 1. Of BDNF, cortisol, IGF-1, and VEGF, only cortisol showed marginal evidence of sex differences. We found evidence of a log-log relationship between BDNF and VEGF (β = 0.48, p < 0.001, 95% CIβ, = [0.26, 0.71]), including with the correction for assay round (βBDNF = 0.46, P < 0.001, 95% CIβ, = [0.24, 0.68]); all other hormone correlation pairs were non-significant (all P ≥ 0.20). Only VEGF showed evidence of group differences between assay rounds. Mean VEGF measurement was lower for assay round one, where samples were thawed one extra time for VEGF/IGF-1 analysis (mean = 0.186 ± 0.094, round 1; 0.230 ± 0.092 ng/mL, round 2; t(59) = 1.99, P = 0.05; all other P > 0.38). This suggests the additional thaw cycle may have degraded VEGF levels to some degree, although we should note our observed VEGF range was comparable to ranges reported in previous studies [e.g.43,44].
4. DISCUSSION
4.1. Summary of results in the context of animal models and human studies
The current study was designed to probe relationships between cardio-respiratory fitness, brain-derived neurotrophic factor (BDNF), cortisol, insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and hippocampal memory in healthy young adults. We measured aerobic capacity (VO2 peak); resting levels of serum BDNF, cortisol, IGF-1, and VEGF; and assessed recognition memory. Cotman et al. [3] introduced a framework where aerobic exercise induces upregulated expression of growth factors throughout the body, importantly IGF-1 and VEGF. Circulating IGF-1 and VEGF can then either cross or act on the blood-brain barrier and modulate expression of brain IGF-1, VEGF, and BDNF which in turn effect increased brain plasticity mechanisms that may benefit cognitive function [3]. In support of this framework, on a basic level, we have shown resting serum IGF-1 is positively associated with aerobic fitness, and BDNF and VEGF levels are also correlated with one another. At a more complex level, our data suggest BDNF and fitness interact to predict recognition memory accuracy.
At present human and non-human animal studies of the effects of exercise and aerobic capacity on cognitive function are somewhat disconnected. It is well established aerobic exercise and environmental enrichment induce beneficial effects on the hippocampus and hippocampal memory system in rodents [1,2,45–49]. In contrast, human studies have mostly investigated the effects of exercise on broad cognitive domains, such as executive functions, and until recently have not targeted hypotheses towards specific brain regions [reviewed in ref. 10]. In terms of putative hippocampal memory tasks, a study by Pereira et al. [16] showed delayed free recall performance was positively correlated with fitness in a sample of young to middle-aged adults. In addition, relational memory performance, has been associated with aerobic capacity in pre-adolescent children [50–52]. Finally, change in VO2 peak has recently been shown to modulate brain activity, measured with fMRI, associated with task performance on a virtual navigation task [53], a paradigm shown to recruit the hippocampus [54,55]. None of these studies have reported relationships between BDNF and memory. Complementary to these studies, we have assessed the relationship between BDNF, memory, and physical fitness in healthy young adults using a memory paradigm shown to recruit the hippocampus. With this study, we are among the first few groups to address this gap in the literature. We selected our recognition memory task because it has been shown to engage the hippocampus in fMRI studies [20,21], and depend on hippocampal integrity in patient studies [56], and so should provide a human parallel to the memory tasks used in rodent studies.
4.2 Aerobic fitness as a modifier of the relationship between BDNF and recognition memory
We have observed a large interaction effect suggesting the relationship between BDNF and memory may be modulated by aerobic fitness. Since BDNF mRNA and expression is increased in the hippocampus in response to aerobic exercise [7,8,57], the protein has long been considered a candidate component of the physiological mechanisms underlying the effects of aerobic exercise on the hippocampal memory system. Animal models have suggested exercise benefits memory function specifically by modulating BDNF related mechanisms [58–63]. In support of these hypotheses, our results suggest BDNF and aerobic fitness may interact to predict recognition memory in humans. Although aerobic capacity per se is not typically measured in rodent models, it is well established that exercise training increases aerobic capacity dependent on frequency, duration, and intensity of training [64–66]. This suggests aerobic fitness may underlie the benefits of exercise on brain health and cognition observed in animal models. Consistent with this hypothesis, previous work in older adults has shown aerobic capacity may correlate with overall hippocampal volume, both cross-sectionally and following a yearlong aerobic exercise intervention [18,67].
In our study an interaction between serum BDNF and aerobic fitness, but not fitness alone, predicted recognition memory. It is always challenging to interpret interactions between continuous variables. It can be even more difficult in this case since we did not observe a main effect of fitness to accompany the BDNF by fitness interaction—however, in our view this is a positive outcome: we would not want a single variable as general as “fitness” to explain a considerable amount of variance in memory performance in healthy university students. Aerobic fitness is only one of many indices of healthy physiological function. We would expect fitness to interact with many other physiological parameters, and our data imply fitness should be interpreted in the context of these variables. Our results indicate a strong, positive interaction between BDNF and fitness such that resting serum BDNF is negatively predictive of mean recognition memory accuracy at low fitness, and positively predictive at high fitness. Although we observed a potential gender self-selection bias which likely adds some noise to our estimated effects, we argue the BDNF-fitness interaction cannot be wholly attributable to gender differences. Indeed, our analysis suggests sex plays a relatively small role in the other effects we report on. The coefficients BDNF and the BDNF-fitness interaction remained stable when sex was included in our full model of SMT performance, and the coefficient on sex added very little information to the models overall (Table 2; also see section 3.3.3.). While there are likely alternative interpretations for the interaction effect, one possible explanation that fits well with the extant literature is that there may be an exercise adaptation related change in the BDNF dose-response function.
Exercise is thought to act on BDNF and its signaling pathways through a variety of mechanisms. It is well established exercise increases hippocampal mRNA and protein levels of BDNF [7,8,57] and its “high” affinity tyrosine kinase receptor, tropomyosin receptor kinase B [68,69]. Exercise may also act to increase the density of BDNF in dendrites [70], and the activity of tissue-type plasminogen activator [62]—a protein related to the proteolytic conversion of BDNF into its mature form. BDNF is synthesized as a pro- form, which can trigger apoptotic pathways via the low affinity p75 neurotrophin receptor. Classically “beneficial” effects of BDNF signaling are thought to be greatest when the balance of neurotrophin/receptor binding shifts toward mature BDNF and tropomyosin receptor kinase B [see ref. 71 for a review]. BDNF, IGF-1, and other neurotrophic factors, moreover, are thought to be important agents of healthy cellular function and mechanisms of hormesis, including in neurons [72–74]. It is hypothesized cells adapt to free radical induced accumulation of toxins and oxidative stress through a collection of hormetic mechanisms whereby low doses of these stressors or toxins induce a positive effect on cells and cell systems [see ref. 75 for a review]. Exercise adaptation and conditioning is thought to mirror hormesis by generating oxidative damage the body must then work to repair, improving systemic capacity to deal with reactive oxygen species in the future [see ref. 76 for a review]. This literature suggests BDNF and IGF-1 may be differentially regulated in individuals whose physiology has adapted to the metabolic demands of chronic exercise, and our results may be taken as a compelling behavioral indicator of this putative effect.
A possible alternative is that BDNF may moderate an aerobic fitness dose-response curve. We are not able to distinguish these interpretations with the present study design. Given the ubiquity of multiple feedback mechanism in biological systems, it is likely the signaling mechanisms are bidirectional to some degree. We do, however, give slight preference to the BDNF dose-response interpretation because we observed a main effect of BDNF but not fitness in our fully specified model of SMT accuracy (Table 2). It will be particularly insightful to probe causal and directional components of this interaction in future studies.
4.3. Peripheral versus central BDNF and hippocampal memory function
Researchers studying the effects of BDNF on brain plasticity in animal and cellular models are able to measure the protein directly from nervous tissue. Although BDNF cannot be measured directly in the living human brain, various reports indicate peripheral BDNF may be a viable surrogate for BDNF measured centrally. For example, serum BDNF concentrations are correlated with overall brain BDNF concentrations in multiple species [77], and, in a mouse model, hippocampal BDNF was increased after peripheral BDNF infusion [78]. BDNF, cortisol, and IGF-1 can all cross the blood-brain barrier [79–82], and although VEGF does not cross the blood-brain barrier, peripheral VEGF may induce brain plasticity effects through a variety of other mechanisms [see ref. 83 for a review]. Together, these studies suggest our serum measurements may be a reasonable approximation for levels of these hormones in brain and hippocampus. While other human studies have considered serum BDNF in an aerobic exercise and learning/memory paradigm [18,19,84,85], none have reported relationships between BDNF and memory. Our results extend previous work by suggesting (i) a negative association between resting serum BDNF and recognition memory accuracy and (ii) that this relationship may be moderated by aerobic fitness. These findings are particularly exciting for the field because they are the first demonstration of a relationship between BDNF and memory in a sample of healthy adults.
Related work has found serum and hippocampal BDNF are decreased in various pathological states, for example in Alzheimer’s disease and major depression [86–92]. Another study found plasma BDNF was positively correlated with performance on various neuropsychological tests in older women [93]. Given this background, and our results, it seems clear different dose-response mechanisms may be at play between healthy and patient populations. We speculate that in patients and the elderly, peripheral BDNF might co-vary most with overall health, while it might be more tightly coupled to complex physiological factors in healthy young adults as in our sample. Peripheral BDNF has been linked to a variety of homeostatic mechanisms, including blood glucose control and energy metabolism [94,95], angiogenesis and vascular stability [96], inflammation and pain transduction [reviewed in ref.97], etc. As far as is known, serum BDNF, which we measured directly, is derived mainly from immune system related peripheral blood mononuclear cells, platelets, and vascular endothelial cells [87,98–101]. This highlights the need for studies like ours to characterize these physiological and behavioral relationships in healthy individuals, where latent age-related confounds do not complicate interpretation of results. A thorough understanding of healthy physiology is a necessary prerequisite for an understanding of pathophysiology.
Since peripheral BDNF is correlated with brain and hippocampal BDNF as suggested by animal work [77], it may be physiologically relevant to episodic memory function. Consequently, evidence that relates aerobic exercise to BDNF and hippocampal memory in rodents might be taken to suggest our serum BDNF measurements should be positively associated with recognition memory. We discuss this point from two angles. First, evidence suggests levels of BDNF protein in rodent hippocampus is significantly increased from baseline only after about two to three weeks of consistent wheel running [63]. This implies extant rodent models may be most directly comparable to our higher- fit participants. Consistent with this hypothesis, taking our point-estimates for the coefficients on serum BDNF and the BDNF by fitness interaction at face value, our data suggest BDNF positively predicts mean recognition memory accuracy only in individuals above the 75th aerobic fitness percentile, although the error of this estimate is quite large. In this age range, the 75th percentile corresponds to VO2 max scores of about 43 mL·kg−1·min−1 for women, and 49 mL·kg−1·min−1 for men. Despite these observations in humans it is unknown if endogenous peripheral BDNF levels are associated with hippocampal memory in animal models.
Second, inference drawn from comparisons of hormone levels needs to be placed in its specific context. For example, the timing between blood sample collection for BDNF assays and exercise is critical, because circulating BDNF levels exhibit regular diurnal fluctuation patterns [31,32], and peak in response to acute exercise [reviewed in ref. 102]. We have shown resting serum BDNF, measured from blood drawn in the morning, shortly before cognitive testing, is negatively associated with recognition memory performance in healthy young adults. Since BDNF is a dynamic variable, there may be additional experimental manipulations that might reverse this outcome. Future animal research is needed that more closely resembles the human work by (i) measuring or estimating aerobic fitness (which is possible in rodents [103]), and (ii) by including BDNF measurements from peripheral blood samples.
4.4. Relationship between resting BDNF and IGF-1 and aerobic fitness
Here we have given evidence resting serum IGF-1 is positively associated with aerobic fitness in healthy young adults. This is in accord with extant literature in older adults that suggests a positive relationship between aerobic capacity and IGF-1 [104,105]. We have also given an estimate for the relationship between BDNF and fitness. Currie et al. [106] described a negative relationship between resting serum BDNF and estimated VO2 max in young to middle aged adults that is consistent with our estimated negative relationship. When we replicated Currie and colleagues’ analysis of the relationship between serum BDNF and VO2 peak with our data we found the 95% confidence intervals for each data set’s Pearson-r statistics include the other’s estimate (unpublished observation; we refer interested readers to our similar comparison between BDNF and aerobic fitness percentile, reported here). Although the correlation is not statistically significant in our data, this observation suggests the underlying population parameter may be well approximated in both studies. Together with other studies that report an inverse relationship between serum BDNF and fitness measures [107,108], these findings suggest resting levels of circulating BDNF are on average slightly lower in higher-fit individuals.
4.5. Other interpretive considerations
Although we have made an effort to address potential sources of confounding influence in our data collection and analysis, we acknowledge other potential limitations to our results. For example, we have observed a possible gender self-selection bias whereby we may have undersampled lower-fit males and/or oversampled higher-fit males. We have endeavored to control for this by converting raw VO2 peak scores to ACSM fitness percentiles and adjusting for effects of sex in our regression analyses. Despite these precautions, however, we may not have completely eliminated this bias in our results. Another potential limitation to the present report is its dropout rate. It is unclear how our low study completion rate (55%) may have influenced our results. Perhaps most importantly, we note the magnitudes, causal directions, and replicability of the effects we report here will need to be assessed in future studies.
4.6. Conclusions
Results of this study provide evidence for a novel negative association between resting serum BDNF and recognition memory accuracy in healthy young adults. Consistent with previous experimental work, we have shown positive relationships between IGF-1 and fitness, and BDNF and VEGF. Although aerobic fitness itself was not related to memory directly, which is not surprising since our study sample consisted of healthy young adults in their cognitive prime, we have put forward significant evidence of a large interaction between BDNF and fitness predicting subsequent recognition. In our study, increasing resting serum BDNF is predictive of decreasing memory performance at low fitness, but increasing memory performance at high fitness. This is a striking behavioral indication that could be interpreted to suggest an exercise adaptation-related change in the BDNF dose-response curve that in turn may affect hippocampal memory.
Supplementary Material
Highlights.
We assessed recognition memory, peripheral neurotrophin levels and aerobic fitness.
On its own, resting serum BDNF is negatively associated with recognition memory.
BDNF and aerobic fitness strongly interact to positively predict recognition memory.
This interaction may support an exercise-related change in BDNF dose-response.
Resting serum IGF-1 is positively associated with aerobic fitness.
ACKNOWLEDGMENTS
We would like to dedicate this paper to our co-author Dr. Robert C. Wagenaar, Ph.D., (May 23, 1957 – February 13, 2013) who unexpectedly passed away while this manuscript was in preparation. This work was supported by a Pathway to Independence Award to K.S. (NIH K99AG036845), the Boston University Clinical and Translational Science Institute (CTSI; UL1-TR000157), and a Student Research Award from the Boston University Undergraduate Research Opportunities Program to A.S.W. We would like to thank Ethan Pravetz and Jessica Lam for their assistance with data collection, entry, scoring, and analysis, and the staff of the CTSI General Clinical Research Unit (GCRU) for their support. In addition, we would like to thank Maria Burgess, Kellene Isom, and Chia-Ling Wu for metabolic cart training and support, and Drs. Andrew Budson and Neil Kowall for serving as the study physicians for fitness testing and for the GCRU, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Abbreviations. The following non-standard abbreviations are used throughout the text. ACSM, American College of Sports Medicine; BDNF, Brain-Derived Neurotrophic Factor; BMI, Body Mass Index; DMS, Delayed Matching-to-Sample; ELISA, nzyme-Linked Immunosorbent Assay; IGF-1, Insulin-like Growth Factor-1; MTL, Medial emporal Lobes; OLS, Ordinary Least Squares; RER, Respiratory Exchange Ratio; RERmax, maximum observed Respiratory Exchange Ratio; SMT, Subsequent Memory est; VEGF, Vascular Endothelial Growth Factor; VO2 max, rate of maximal xygen consumption in mL per kg of body weight per min; VO2 peak, peak rate of oxygen consumption in mL per kg of body weight per min, measured during test.
REFERENCES
- 1.Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46:123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 2.Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Bradford HF. Nerve growth factors and the control of neurotransmitter phenotype selection in the mammalian central nervous system. Progress Neurobiol. 1997;53:27–43. doi: 10.1016/s0301-0082(97)00030-0. [DOI] [PubMed] [Google Scholar]
- 5.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 6.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89:279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on geneexpression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- 8.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 9.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 10.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 11.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a metaanalytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 12.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Smith PJ, Blumenthal JA, Hoffman BM, Strauman TA, Welsh-bohmer K, Jeffrey N, et al. Aerobic Exercise and Neurocognitive Performance: A Meta-Analytic Review of Randomized Controlled Trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberg MAI, Pedersen NL, Torén K, Svartengren M, Bäckstrand B, Johnsson T, et al. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci USA. 2009;106:20906–20911. doi: 10.1073/pnas.0905307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Déry N, Pilgrim M, Gibala M, Gillen J, Wojtowicz JM, Macqueen G, et al. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7:66. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Human Experimentation: Code of Ethics of the World Medical Association. Brit Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldfield RC. The Assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Blair JR, Spreen OA. Predicting premorbid IQ: A revision of the national adult reading test. Clinical Neuropsychol. 1989;3:129–136. [Google Scholar]
- 25.Strauss E, Sherman EMS, Spreen OA. Compendium of Neuropsychological Tests. 3rd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 26.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 27.Bruce R, Blackmon J, Jones J, Strait G. Exercise Testing in Adult Normal Subjects and Cardiac Patients. Pediatrics. 1963;32:742–756. [PubMed] [Google Scholar]
- 28.Issekutz B, Birkhead NC, Rodahl K. Use of respiratory quotients in assessment of aerobic work capacity. J Appl Physiol. 1962;17:47–50. [Google Scholar]
- 29.Thompson WR, Gordon NF, Pescatello LS, editors. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 30.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 31.Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, Pluchino N, et al. Plasma brainderived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J Endocrinol. 2008;197:429–435. doi: 10.1677/JOE-07-0376. [DOI] [PubMed] [Google Scholar]
- 32.Pluchino N, Cubeddu A, Begliuomini S, Merlini S, Giannini A, Bucci F, et al. Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Hum Reprod. 2009;24:2303–2309. doi: 10.1093/humrep/dep119. [DOI] [PubMed] [Google Scholar]
- 33.Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Jensen LD, Cao Y. Clock controls angiogenesis. Cell Cycle. 2013;12:405–408. doi: 10.4161/cc.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Development Core Team. R A Language and Environment for Statistical Computing. 2012 http://www.R-project.org/
- 37.Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. Int J Obes Relat Metab Disord. 2000;24:807–818. doi: 10.1038/sj.ijo.0801232. [DOI] [PubMed] [Google Scholar]
- 38.Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med. 2008;27:2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari SLP, Cribari-Neto F. Beta Regression for Modelling Rates and Proportions. J Appl Stat. 2004;31:799–815. [Google Scholar]
- 40.Smithson M, Verkuilen J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods. 2006;11:54–71. doi: 10.1037/1082-989X.11.1.54. [DOI] [PubMed] [Google Scholar]
- 41.Cribari-Neto F, Ferrari SLP. Beta Regression in R. J Stat Softw. 2004;34:1–24. [Google Scholar]
- 42.Keene ON. The log transformation is special. Stat Med. 1995;14:811–819. doi: 10.1002/sim.4780140810. [DOI] [PubMed] [Google Scholar]
- 43.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 44.Kraus RM, Stallings HW, Yeager RC, Gavin TP. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J Appl Physiol. 2004;96:1445–1450. doi: 10.1152/japplphysiol.01031.2003. [DOI] [PubMed] [Google Scholar]
- 45.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav Brain Res. 2010;207:321–331. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 50.Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Med Sci Sports Exerc. 2011;43:344–349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- 51.Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monti JM, Hillman CH, Cohen NJ. Aerobic fitness enhances relational memory in preadolescent children: The FITKids randomized control trial. Hippocampus. 2012;22:1876–1882. doi: 10.1002/hipo.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holzschneider K, Wolbers T, Röder B, Hötting K. Cardiovascular fitness modulates brain activation associated with spatial learning. Neuroimage. 2012;59:3003–3014. doi: 10.1016/j.neuroimage.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 55.Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci. 2010;30:7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nichols EA, Kao Y, Verfaellie M, Gabrieli JDE. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:749–56. [PubMed] [Google Scholar]
- 58.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 59.Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 60.Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exerciseinduced improvement in object recognition memory. Neurobiol Learn Mem. 2010;94:278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampaldependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- 62.Ding Q, Ying Z, Gómez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 64.Pedersen PK, Jørgensen K. Maximal oxygen uptake in young women with training, inactivity, and retraining. Med Sci Sports. 1978;10:233–237. [PubMed] [Google Scholar]
- 65.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3:346–356. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 66.Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. J Appl Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- 67.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y-F, Chen H, Yu L, Kuo Y-M, Wu F-S, Chuang J-I, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90:81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Macias M, Dwornik A, Skup M, Czarkowska-Bauch J. Confocal visualization of the effect of short-term locomotor exercise on BDNF and TrkB distribution in the lumbar spinal cord of the rat: the enhancement of BDNF in dendrites? Acta Neurobiol Exp (Wars) 2005;65:177–182. doi: 10.55782/ane-2005-1552. [DOI] [PubMed] [Google Scholar]
- 71.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 72.Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neuroch. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- 73.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Wolkow CA. Life span: getting the signal from the nervous system. Trends Neurosci. 2002;25:212–216. doi: 10.1016/s0166-2236(02)02133-1. [DOI] [PubMed] [Google Scholar]
- 75.Arumugam TV, Gleichmann M, Tang S-C, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 77.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 80.Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- 81.Pan W, Kastin AJ. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology. 2000;1262:171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- 82.Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier: Primary role of albumin-bound hormone. J Clin Invest. 1979;64:145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 84.Flöel A, Ruscheweyh R, Krüger K, Willemer C, Winter B, Völker K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 85.Griffin ÉW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 86.Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, et al. Stagedependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- 87.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry J-M, Bertschy G. Low brainderived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiat. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 88.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry J-M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiat Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 89.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 90.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiat. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 91.Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 92.Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Mol Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 93.Komulainen P, Pedersen M, Hänninen T, Bruunsgaard H, Lakka TA, Kivipelto M, et al. BDNF is a novel marker of cognitive function in ageing women: the DR’s EXTRA Study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 94.Tonra JR, Ono M, Liu X, Garcia K, Jackson C, Yancopoulos GD, et al. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes. 1999;48:588–594. doi: 10.2337/diabetes.48.3.588. [DOI] [PubMed] [Google Scholar]
- 95.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann NY Acad Sci. 2012;1264:49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, et al. Neurotrophins promote revascularization by local recruitment of TrkB + endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115:653–663. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, et al. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia: Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gielen A, Khademi M, Muhallab S, Olsson T, Piehl F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scand J Immunol. 2003;57:493–497. doi: 10.1046/j.1365-3083.2003.01260.x. [DOI] [PubMed] [Google Scholar]
- 100.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 102.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40:765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 103.Pica AJ, Brooks GA. Effects of training and age on VO2max in laboratory rats. Med Sci Sports Exerc. 1982;14:249–252. [PubMed] [Google Scholar]
- 104.Vale RGDS, de Oliveira RD, Pernambuco CS, de Meneses YPDSF, Novaes JDS, de Andrade ADFD. Effects of muscle strength and aerobic training on basal serum levels of IGF-1 and cortisol in elderly women. Arch Gerontol Geriatr. 2009;49:343–347. doi: 10.1016/j.archger.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Tissandier O, Péres G, Fiet J, Piette F. Testosterone, dehydroepiandrosterone, insulin-like growth factor 1, and insulin in sedentary and physically trained aged men. Eur J Appl Physiol. 2001;85:177–184. doi: 10.1007/s004210100420. [DOI] [PubMed] [Google Scholar]
- 106.Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci Lett. 2009;451:152–155. doi: 10.1016/j.neulet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 107.Nofuji Y, Suwa M, Moriyama Y, Nakano H, Ichimiya A, Nishichi R, et al. Decreased serum brain-derived neurotrophic factor in trained men. Neurosci Lett. 2008;437:29–32. doi: 10.1016/j.neulet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 108.Jung SH, Kim J, Davis JM, Blair SN, Cho H. Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. Eur J Appl Physiol. 2011;111:303–311. doi: 10.1007/s00421-010-1658-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.