Abstract
A rare case of malignant transformation of uterine leiomyoma is reported. A 54 year old lady, nulliparous and 2 years postmenopausal presented to gynecology clinic with a pelvi – abdominal mass and ultrasound scan suggestive of multiple uterine fibroid. Total abdominal hysterectomy performed. Histopathology report showed leiomyosarcomative changes from benign leiomyoma within the huge mass.
Introduction
The overwhelming data strongly suggest that uterine leiomyosarcoma are isolated lesions and are not routinely found in association with uterine leiomyomas.
Malignant transformations of uterine leiomyomas occur very rarely. 1,2 The hypothesis that uterine leiomyosarcoma arise from or as a result of the malignant transformation of benign leiomyomas has not been proved. 3 A review of the literature suggests however, that only a limited number of case reports have demonstrated a histologic transition of a benign leiomyoma into a leiomyosarcoma. 4
Case Report
In May 2006, a 54-year-old lady, nulliparous, 2 years postmenopausal, married 10 months ago presented herself to gynaecology clinic, complaining of abdominal distention for 2 months' duration. This was associated with frequency & nocturia but there was no dysurea. There was no history of post menopausal bleeding or vaginal discharge. She also had no history of nausea, vomiting or change in bowel habits. The patient did not have any previous major medical or surgical illness and she did not complain about loss of appetite or weight loss. On physical examination, she was found generally well and vital signs are normal.
Abdominal examination revealed distended abdomen. There were no dilated vessels, and no scars. Huge pelvis -abdominal mass was felt extending upto xephisternum with irregular outline, not tender and firm in consistency. The mass was mobile from side to side. No supraclavicular palpable lymph nodes and no other lymphadenopathy were found. Impression is of multiple uterine fibroid.
Investigations
Complete blood count, Hb: 11.4 g/dl, WBC 5.3 × 103, PLT 320 × 103
Liver Function test – Normal
Kidney Function test – Normal
Tumor marker: CA 125 37.7 μ/ml
normal upper limit in our labarotory – 36 μ/ml
CEA – Normal
B – HCG – < 5 IU/ L – Normal
Ultrasonography – showed enlarged uterus with multiple uterine fibroid with one large fibroid mass measuring (25 × 30 cm), Rt. & Lt. ovaries – not seen.
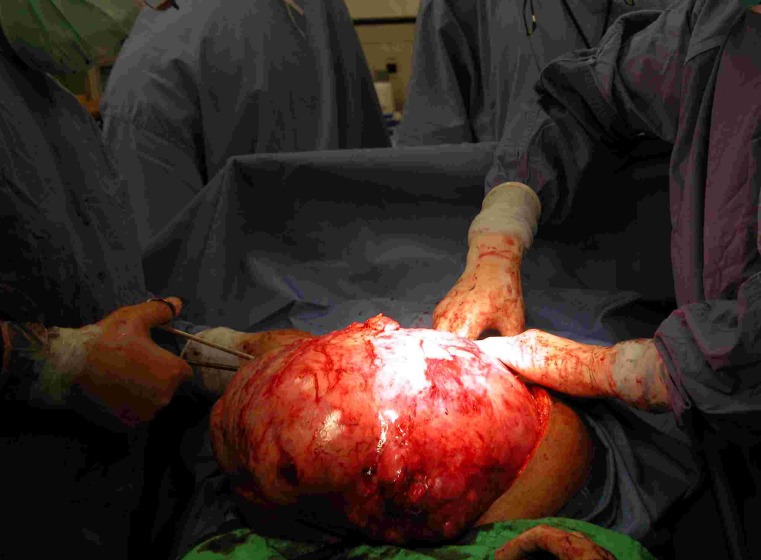
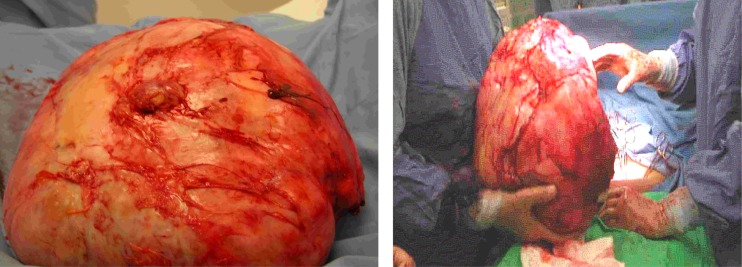
Total abdominal hysterectomy was advised. Intraoperatively an enlarged uterus with multiple uterine fibroids was revealed. There was one huge pedunculated fibroid about 24 × 28 cm. which was attached to the posterior wall of the uterus. The pedicle was about 4 cm. wide. Moreover, there was dense omental adhesions with small bowel attached to this mass. Peritoneal washing taken for cytology. There was no obvious palpable para -aortic lymph nodes and no obvious tumor felt over the liver. The pedunculated fibroid was separated first from the posterior wall of the uterus to facilitate the hysterectomy. So total abdominal hysterectomy was performed with preservation of the ovary. The omentum and small bowel was dissected away from the mass carefully and then we succeeded to separate the mass and delivered it separately. The uterus and fibroid weighed 2.6 kg and the huge fibroid mass weighed 10.6 kg (see Figures 1 and 2).
Figure 1.
Intraoperative dissection of the mass.
Figure 2.
The fibroid mass removed completely after careful dissection from the bowel.
Histopathology report revealed that the section of hysterectomy showed benign leiomyoma with more than15 uterine fibroids (0.8–11 cm in diameter) and inactive endometrium. A single leiomyoma showed atypical smooth muscle tumour of undetermined significance. The huge fibroid mass shows extensive coaguloative necrosis along with areas of intersecting bundles of smooth muscle fibres.
However other areas of the tumor shows sheets of ephithelioid smooth muscle cells with abundant clear cytoplasm, numerous mitotic figures, some of which are abnormal as well as moderate pleomorphism suggesting epitheliod leiomyosarcomative changes. Peritoneal cytology was negative for malignancy.
Discussion
We described a 54–year–old woman who was nulliparous and 2 years postmenopausal and underwent total abdominal hysterectomy for multiple uterine fibroid and histopathology showed malignant transformation to leiomyosarcoma. This case report illustrates that malignant transformation of leiomyoma should be considered in certain situations. The age at presentation is an important factor although the study which was carried out in Australia in 1999 by Gard & colleagues showed that the incidence was approximately one leiomyosarcoma of the uterus in one million Australian women each year and the mean age of presentation was 55 years. 5 The uterine size for asymptomatic uteri greater than 12 weeks in size is not associated with an increased risk of malignant transformation to leiomyosarcoma, however, a very rapidly growing uterine tumour with large uteri there appears to be a risk for leiomyosarcoma. 6 This rapid growth of uterine myomas was arbitrarily defined as again at 6 weeks or more in gestational size within an interval of a year or less. Recent data have not supported an association between rapid growth of myoma and an increased risk of malignancy: One out of 371 women operated on for rapidly growing myomas was found to have a leiomyosarcoma. 7
Recent data suggest the use of different techniques for preoperative diagnosis of leiomyosarcoma including serum lactate dehydrogenase determination, dynamic MRI and transcervical needle biopsy before any treatment decision are made. However, this data suggest that more studies are needed before considering these methods as standard guidelines.
It has been debated whether myomas and leiomyosarcoma are part of a disease continuum. Cytogenetic studies have demonstrated that leiomyosarcoma arise de novo and may be unrelated to benign myomas. Recent micro assay data however identified a rare subset of myomas with deletions of chromosome – 1 that have transcriptional profiles that cluster with those of leiomyosarcomas 8 suggesting that some rare leiomyosarcoma may arise from a specific subset of myomas. 3 The histopathological description of our case suggests that the previous statement should be considered in counseling a woman with large rapid growing myoma. The woman reported here had total hysterectomy with preservation of her ovaries. From the limited data on these types of leiomyosarcoma and even in the clinical experience of gynecological oncologists the clinical benefits of oopherectomy, lymphadenectomy and cytotoxic chemotherapy remains controversial. 9
Conclusion
In conclusion malignant transformation of benign leiomyoma to leiomyosarcoma, although a rare occurance but can happen in certain patients with large myomas, post menopausal women, rapid growing myomas and in certain subset of leiomyoma. MRI, serum lactate dehydrogenase might be helpful in preoperative predetection of these changes.
References
- 1.Mayerhofer K, Obermair A, Windbichler G, Petru E, Kaider A, Hefler L, Czerwenka K, Leodolter S, Kainz C. Leiomyosarcoma of the uterus: a clinico pathologic multicenter study of 71 cases. Gynecol Oncol. 1999;74:196–201. doi: 10.1006/gyno.1999.5436. [DOI] [PubMed] [Google Scholar]
- 2.Rotmensch T, Boenyak S, Mantag A. Malignant transition of uterine leiomyomata. Int J Gynecol Obstet. 1993;42:47–49. doi: 10.1016/0020-7292(93)90449-7. [DOI] [PubMed] [Google Scholar]
- 3.Arici Aydin, Rayburn William F. Obstetrics and Gyne clinics. Myomas: Elsevier Saunders; 2006. p. 195. [Google Scholar]
- 4.Clement PB. Pure mesenchymal tumours. In: Clement PB, Young RH, editors. Tumors and tumor – like lesions of the uterine corpus and cervix. New York: Churchill Livingston; 1993. pp. 265–328. [Google Scholar]
- 5.Reiter RC, Wagner PL, Gambone JC. Routine hysterectomy for large asymptomatic uterine leiomyomata: a reappraisal. Obs Gyn. 1992;79(4):481–484. [PubMed] [Google Scholar]
- 6.Gard GB, Mulvany NJ, Quinn MA. Management of uterine leiomyosarcoma in Australia. Aust N Z J Obstet Gynaecol. 1999;39:93–98. doi: 10.1111/j.1479-828x.1999.tb03453.x. [DOI] [PubMed] [Google Scholar]
- 7.Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414–418. [PubMed] [Google Scholar]
- 8.Walker Cl, Stewart EA. Uterine fibroids; the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 9.David L, Beverly V, Gareth W. “Uterine Fibroid” Gynecology. (3rd ed.) 2002;Chapter 33:477–492. [Google Scholar]
- 10.Scurry J, Hack M. Leiomyosarcoma arising in a poleiomyoma. Gynecol Oncol. 1990;39:381–383. doi: 10.1016/0090-8258(90)90271-l. [DOI] [PubMed] [Google Scholar]