Abstract
Background. A measles outbreak occurred in Maroua, Cameroon, from January 2008 to April 2009. In accordance with recent World Health Organization guidelines, an outbreak-response immunization (ORI) was conducted in January 2009. The aim of this study was to investigate the causes of the epidemic in order to guide vaccination strategies.
Methods. We performed a stratified household-based survey using cluster sampling to determine measles vaccination coverage in children aged 9 months to 15 years. We defined 3 strata based on measles incidence. Next, we performed a case–control study to measure vaccine effectiveness (VE). Cases were obtained from health center registries. Controls were selected among respondents to the coverage survey.
Results. The vaccination-coverage survey included 2963 children in total. The overall routine vaccination coverage was 74.1% (95% confidence interval [CI]: 70.0%–78.3%). Measles incidence was inversely proportional to routine vaccination coverage, with high incidence associated with coverage of 71% and low incidence associated with coverage of 84%. The overall VE was 94% (95% CI, 86.7%–97.4%). After the ORI in January 2009, the coverage was >90% in all strata and measles incidence declined rapidly.
Discussion. Our results confirm that insufficient vaccination coverage was the main reason for this epidemic. The ORI conducted in January 2009 contributed both to control the epidemic and to increase the vaccination coverage to desirable levels.
Implementation of the comprehensive measles mortality–reduction strategy by high-burden countries supported by the Measles Initiative, a partnership supporting measles mortality reduction in Africa, has resulted in a >90% decline in measles-related mortality in the last decade [1–3]. The 4-pronged strategy focuses on improved routine immunization, providing all children with a second opportunity for measles immunization through either periodic supplemental immunization activities (SIAs) or routine second doses of measles vaccine, improved measles case management, and careful measles surveillance[4].
In the last 10 years, Cameroon has seen great progress in measles control. First-dose coverage has increased from 47% in 2001 to 80% in 2008. Countrywide SIAs were conducted in 2002 and 2006, with >90% administrative coverage in targeted populations (aged 9 mo–14 y in 2002; aged 9 mo–59 mo in 2006) [1, 5]. Measles surveillance and case management has also been reinforced. These interventions have contributed to a reduction in the annual incidence from 41 cases per 100,000 children in 2001–2004 to 2 cases per 100,000 children in 2005–2008 [1].
Despite this progress, an outbreak was declared in the Extreme-Nord region at the beginning of 2008. Most cases were reported in the city of Maroua, with a total of 875 cases and 8 deaths reported between 1 January 2008 and 6 April 2009 (Figure 1a). The most affected area was the center of the city, with an attack rate over 700 cases per 100,000 children (Figure 1b). In response to the epidemic, several interventions were conducted: 1) Routine vaccination activities were reinforced during 2008, 2) a vaccination intervention was performed in certain affected health districts of Maroua in October 2008, and because of the continued reporting of cases, 3) a mass vaccination campaign was implemented in late January 2009 targeting all children aged 9 months to 15 years, irrespective of their vaccination status.
Figure 1.
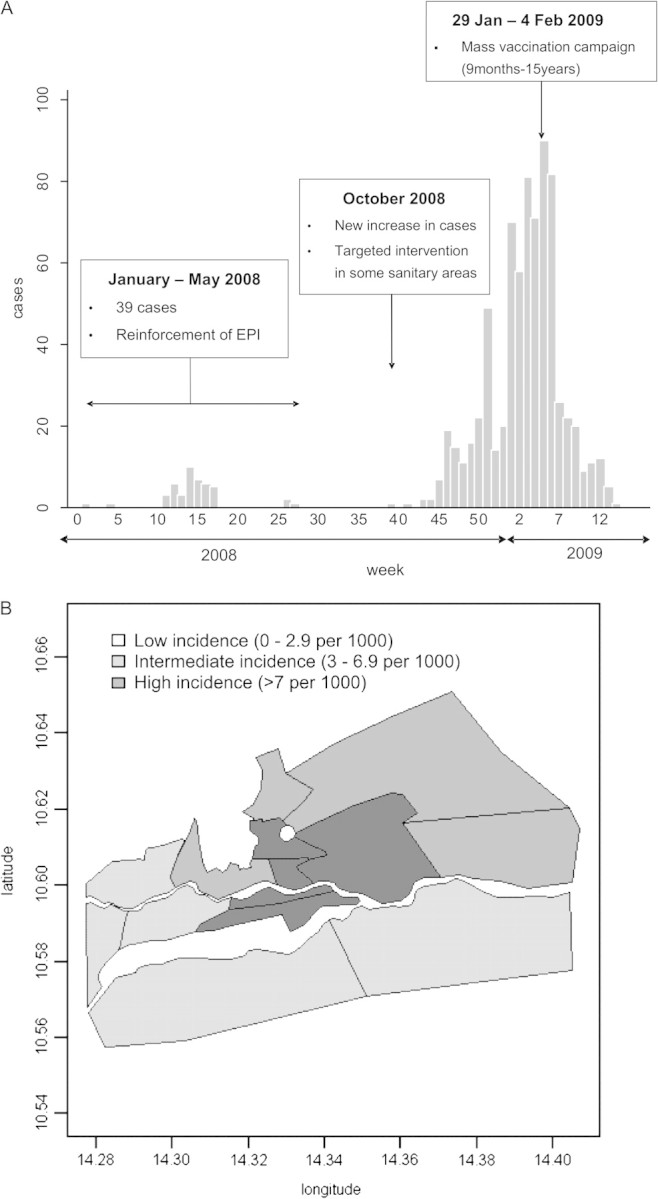
(a) Weekly number of measles cases in Maroua (Cameroon) during weeks 1–52 of 2008 and 1–14 of 2009. (b) Measles attack rates by sanitary area in Maroua at the end of the epidemic, April 2009.
The World Health Organization (WHO) has recently updated guidelines for response to measles outbreaks in mortality-reduction settings including outbreak-response immunization (ORI) [6]. Few evaluations of the impact of reactive vaccination in epidemics have been conducted [7–9]. To investigate the causes of this epidemic, we performed a vaccination-coverage survey and a case-control study to determine vaccine effectiveness. The rationale for this investigation was to document the reactive vaccination activities and provide additional information for the control of measles in northern Cameroon.
METHODS
Vaccine-Coverage Survey
Design Overview
All children living in the city of Maroua at the time of the study aged between 9 months and 15 years were eligible for the survey. The sample was obtained using stratified cluster sampling. We divided Maroua into 3 strata: [1] High-incidence areas (+7‰); [2] intermediate-incidence areas (3‰–6.9‰); and [3] low-incidence areas (0‰–2.9‰). We based the division of strata on attack rates in each health district computed using the number of cases reported to the Ministry of Health of Cameroon and on populations from the most recent census (Figure 1b). In each of these strata, we obtained a representative sample of the population via spatial random sampling. To calculate sample size, we assumed an alpha-error of .05, desired precision of ± 7%, design effect of 3, and expected vaccination coverage of 65%, 75%, and 85% in the high-, intermediate-, and low-incidence strata, respectively. Considering these assumptions, the minimum sample size was estimated to be 535, 441, and 300 in these 3 strata, respectively, corresponding to 24, 20, and 14 clusters of 22 children each [10]. We evaluated children aged 9 months to 15 years from at least 18 households in each cluster.
Survey Teams
Survey teams were recruited locally and underwent a 2-day training prior to the initiation of fieldwork. Training consisted of survey methodology, interview methodology, and a pilot implementation of the questionnaire. Survey teams comprised at least 1 male and 1 female. Survey-team members spoke both French and Fulfulde, the language in which most interviews were conducted. Each team surveyed 5 clusters of 18 households per day.
Cluster Allocation and Household Selection
We allocated clusters within the 3 incidence strata proportionally to the population of each health district. Population data used to allocate clusters was provided by the regional Expanded Program on Immunization (EPI) office. To select households within clusters, we used spatial-based sampling [11] employing a satellite photo from Google Earth. After demarcating each health district, we randomly selected the desired number of starting points for each one from a uniform distribution (Figure 2). When a point was closer than 20 meters to a structure in the satellite image, the point was selected. If not, the point was discarded and another point was drawn at random. This process ensured the same probability of selection for each structure. We defined households as individuals living and eating together under the same roof. We selected households subsequent to the first by proximity. If a household was absent, we asked survey teams to return to the household later in the day. If a household was absent after 2 return attempts, the household was skipped and replaced with another household.
Figure 2.
Distribution of the randomly selected starting points (black dots) in the different sanitary areas.
Data Collection
All information was elicited by interviews. Interviews were conducted preferentially with the mother or female caregiver. A standardized questionnaire was used to collect demographic data, vaccination status, vaccination history (place, date of vaccination, and injection site on the body), reasons for nonvaccination, previous measles episodes, and stays outside Cameroon in the preceding 6 months for each child included in the survey. We verified vaccination status retrospectively by vaccination cards provided either in the routine vaccination or in the mass vaccination interventions. When card verification was not possible, history was relied on. We also asked respondents their degree of literacy, place of usual residence, and the number of children residing in the household.
Definitions
We employed the following definitions for the vaccine-coverage survey.
▪ Vaccinated: an individual who had received ≥1 dose of measles-containing vaccine. Vaccination was verified by vaccination card or history.
▪ Unvaccinated: an individual who had no vaccination card and whose parent or guardian confirmed on interview that she or he has received no measles vaccination.
▪ Routine vaccination: an individual who had received a dose of measles-containing vaccine on the routine schedule. Routine vaccination was verified either by its registration on a routine vaccination card or by report during interview that the child had received the “9-months vaccine.”
▪ October 2008 intervention: excluding persons vaccinated through the routine system, an individual with measles vaccination registered on a vaccination card with date during the intervention or for whom vaccination in October 2008 was confirmed by report during interview.
▪ January 2009 mass vaccination campaign: excluding persons vaccinated through the routine system, an individual with vaccination registered on a vaccination card or for whom the interviewed person reported that the child had received a dose of measles vaccine between 29 January 2009 and 5 February 2009.
Data Entry and Analysis
Our main outcomes were measures of measles vaccine coverage in each incidence stratum and an overall estimate of vaccination coverage for Maroua. Our secondary outcomes included vaccine coverage by age group and reasons for nonvaccination. We obtained crude vaccination-coverage estimates, considering the survey design. We calculated the design effect to estimate the loss of precision due to the cluster-based sampling strategy [12]. We obtained adjusted vaccination-coverage estimates using generalized linear and latent mixed models to account for household, cluster, and area-level correlation [13]. We calculated sampling weights at each level to account for the different cluster size. We used EpiData 3.1 software for data entry and Stata 10.0 software for data analysis.
Vaccine Effectiveness Assessment: Case-Control Study
Definition and Selection of Case and Control Participants
A case was defined as any patient between age 9 months and 15 years who sought care at a governmental or private health facility and who was registered in the surveillance logbook as meeting the WHO measles case definition: fever, maculopapular (ie, nonvesicular) rash, and cough, coryza (ie, runny nose), or conjunctivitis (ie, red eyes). Case participants also needed to be residents of Maroua with known vaccination status.
Children included in the vaccination-coverage survey were included in the case-control study as controls. Eligibility as a control required: oral informed consent; residency in Maroua; age between 9 months and 15 years; known vaccination status and no previous measles episode.
For each case, we enrolled 4 controls that were individually matched to the case date of birth (± 3 mo) and health district of residence. To control for potential differences in vaccine effectiveness throughout the period of investigation, we selected 3 different dates of analysis (1 January–30 September 2008, 15 November 2008–28 January 2009, and 19 February–17 April 2009); we defined cases and controls for each period to calculate vaccine effectiveness (Table 1).
Table 1.
Definition of Cases and Controls, Vaccine Effectiveness Study, Maroua, Cameroon, April 2009
| Measurement: Vaccine Effectiveness | Casesa | Controlsa | Exposure |
| Routine immunization until October 2008 | Registered cases from 1 January to 30 September 2008 | 4 controls that were individually matched to each case by date of birth (±3 mo) and sanitary area of residence | Vaccination through the routine activities |
| Routine immunization + October 2008 intervention | Registered cases from 15 November 2008 to 28 January 2009 | Vaccination through the routine activities or the October 2008 intervention | |
| Routine immunization + October 2008 intervention + January 2009 campaign | Registered cases from19 February to 17 April 2009 | Vaccination through the routine activities, the October intervention, or the January 2009 campaign |
NOTE. a The inclusion and exclusion criteria were applied in each substudy: age between 9 mo and 15 y, resident of Maroua, known vaccination status.
Ascertainment of Vaccination Status and Potential Confounding Variables
We followed the same procedure described for controls and we used the surveillance-system register for cases. We cross-checked the information in the surveillance database with the registers of the health centers when possible. The variables considered as possible confounders were age, place of residency (matching variables), and sex.
Statistical Analysis
We assessed associations between vaccination status in the 3 different periods (Table 1) and case–control status through conditional logistic regression, with case–control status as the dependent variable and the exposure variable of interest as the independent variable. The exponential of the coefficient for the vaccination variable in these models was computed to estimate the adjusted odds ratio, and the standard error of the coefficient was used to estimate the P value and 95% confidence interval (CI). To estimate the adjusted level of vaccine protection, we computed the following value for the vaccination variable: (1 – adjusted odds ratio) × 100.
We interpreted all P values and 95% CIs in a 2-tailed fashion. We defined statistical significance as a P value < .05.
Ethical Considerations
This study adhered to the principles that govern biomedical research involving human participants. We followed the Declaration of Helsinki, aiming to provide assurance that the rights, integrity, and confidentiality of participants were protected [14]. We obtained oral consent from participants or their parents or guardians. We ensured privacy and confidentiality in the data collected from the participants both during and after the conduct of the study. We entered and analyzed all information anonymously. To verify vaccination status reported through the surveillance data (health center registers), the names of all the children were recorded in one separate sheet of paper. After the dedicated survey teams inspected the medical records to verify information, this sheet of paper was destroyed by the supervision team. This was the only point during the survey that we recorded information on individual children.
We implemented the study in collaboration with the Ministry of Health after obtaining authorization to perform the survey from the Division of Operational Research of the Ministry of Health of Cameroon.
RESULTS
Description of the Children Included in the Survey
The survey was conducted between 13 and 17 April 2009. The vaccine-coverage survey included 2963 children. The mean age of these children was 6.9 years (standard deviation [SD], 4.02 y), and there were slightly fewer boys (49.9%) than girls. Most of the children did not report traveling outside Cameroon in the preceding 6 months (98.6%).
There were no statistical differences in the age or the sex of the children by strata. We observed a higher percentage of illiterate caregivers in the high–measles-incidence strata, but without statistical differences (Table 2).
Table 2.
Description of the Children Included in the Coverage Survey by Incidence Strata, Maroua, Cameroon, 2009
| High Incidence |
Middle Incidence |
Low Incidence |
||||
| Variable | % | (95% CI) | % | (95% CI) | % | (95% CI) |
| Age | ||||||
| ≤5 y | 43.5 | (40.2–46.8) | 42.4 | (39.7–45.1) | 41.4 | (36.5–46.3) |
| >5 y | 56.5 | (53.2–59.8) | 57.6 | (54.9–60.3) | 58.6 | (53.7–63.5) |
| Gender | ||||||
| Male | 52.3 | (49.3–55.4) | 48.3 | (44.4–52.2) | 48.6 | (44.4–52.8) |
| Female | 47.7 | (44.6–50.7) | 51.7 | (47.8–55.6) | 51.4 | (47.2–55.6) |
| Main caregiver | ||||||
| Mother | 87.8 | (83.1–92.6) | 83.2 | (78.2–88.2) | 79.7 | (73.0–86.5) |
| Father | 4.0 | (1.1–6.9) | 8.6 | (4.2–13.0) | 6.6 | (2.5–10.7) |
| Other | 8.2 | (4.7–11.6) | 8.2 | (5.7–10.7) | 13.7 | (8.7–18.6) |
| Literacya | ||||||
| No | 77.8 | (70.4–85.2) | 72.3 | (63.4–81.1) | 65.7 | (52.0–79.4) |
| Yes | 22.2 | (14.8–29.6) | 27.7 | (18.9–36.6) | 34.3 | (20.6–48.0) |
NOTE. CI, confidence interval. 95% confidence intervals calculated considering the design effect.
Literacy of the caregiver defined as the ability to read and write.
Description of Vaccination Status
Regarding routine immunization, 74.1% of the children (95% CI, 70.0%–78.3%) were vaccinated through routine service delivery. It was reported that 28.1% of the children (95% CI, 22.3%–33.9%) had been vaccinated through the targeted vaccination intervention in October 2008. A high percentage of children were vaccinated in the January 2009 mass campaign: 79.7% (95% CI, 76.4%–82.9%).
We computed the immunization coverage of children who had received ≥1 dose of measles vaccine following routine vaccination, after the targeted vaccination in October 2008, and after the January 2009 campaign (Table 3). We found a statistical difference (P < .001) in the routine coverage among strata. Only after the January 2009 campaign was the coverage >90% in all 3 measles-incidence strata. The stratified analysis by age showed lower routine coverage in the youngest children, as low as 66% in children aged 9–24 months. The coverage was lower in those children with an illiterate caregiver (coverage ratio, 1.26; 95% CI, 1.17–1.33) (Table 3).
Table 3.
Vaccine Coverage Through the Routine Activities and After Each Intervention, Maroua, Cameroon, April 2009
| Categories | Vaccine coverage | 95% CI | Deff | CR | 95% CI | ACR | 95% CI |
| Routine immunization | |||||||
| Strata | |||||||
| High incidence | 70.6 | (64.4–78.6) | 5.9 | Ref | Ref | ||
| Intermediate incidence | 74.1 | (66.4–81.8) | 7.5 | 1.05 | (.91–1.21) | 1.05 | (.95–1.16) |
| Low incidence | 84.4 | (80.1–88.7) | 1.6 | 1.20 | (1.10–1.30) | 1.16 | (1.09–1.24) |
| Age | |||||||
| 9–23 mo | 66.1 | (59.2–73.1) | 1.3 | Ref | |||
| 24–59 mo | 74.4 | (70.2–78.7) | 1.8 | 1.12 | (.01–1.25) | 1.14 | (1.02–1.26) |
| 5–9 y | 74.8 | (70.1–79.5) | 2.8 | 1.13 | (1.02–1.25) | 1.14 | (1.03–1.25) |
| 10–15 y | 75.7 | (70.2–81.2) | 2.9 | 1.14 | (1.05–1.25) | 1.15 | (1.05–1.26) |
| Sex | |||||||
| Male | 73.7 | (69.0–78.4) | 3.9 | Ref | |||
| Female | 74.4 | (70.1–78.7) | 3.3 | 1.01 | (.95–1.07) | ||
| Caregiver | |||||||
| Mother | 73.8 | (69.8–77.8) | 4.8 | Ref | |||
| Father | 81.2 | (68.8–93.7) | 4.3 | 1.10 | (.97–1.24) | ||
| Other | 72.3 | (53.6–91.0) | 7.7 | .98 | (.79–1.22) | ||
| Literacy of the caregiver | |||||||
| Illiterate | 69.7 | (65.3–74.2) | 4.7 | Ref | Ref | ||
| Literate | 87.8 | (84.0–91.5) | 2.3 | 1.26 | (1.17–1.35) | 1.25 | (1.17–1.33) |
| Number of children | |||||||
| 1–2 children | 73.8 | (70.01–77.5) | 3.3 | Ref | |||
| 3–5 children | 75.2 | (69.4–80.8) | 3.5 | 1.02 | (.98–1.07) | ||
| > 5 children | 71.6 | (53.2–90.0) | 2.5 | .98 | (.80–1.20) | ||
| After October 2008 intervention | |||||||
| Strata | |||||||
| High incidence | 75.4 | (68.7–82.1) | 7.7 | Ref | Ref | ||
| Intermediate incidence | 84.8 | (77.0–92.1) | 11.5 | 1.13 | (.97–1.30) | 1.13 | (1.02–1.25) |
| Low incidence | 88.8 | (84.7–93.0) | 1.9 | 1.18 | (1.06–1.31) | 1.16 | (1.05–1.27) |
| Age | |||||||
| 9–23 mo | 71.3 | (63.6–78.9) | 1.7 | Ref | Ref | ||
| 24–59 mo | 81.6 | (77.2–86.0) | 2.4 | 1.14 | (1.03–1.27) | 1.15 | (1.04–1.27) |
| 5–9 y | 81.7 | (77.1–86.0) | 3.5 | 1.14 | (1.03–1.26) | 1.15 | (1.04–1.26) |
| 10–15 y | 82.6 | (77.0–88.2) | 3.9 | 1.16 | (1.04–1.29) | 1.16 | (1.04–1.28) |
| Sex | |||||||
| Male | 80.7 | (75.8–85.5) | 5.2 | Ref | |||
| Female | 81.2 | (76.8–85.5) | 4.1 | 1.01 | (.96–1.06) | ||
| Caregiver | |||||||
| Mother | 80.6 | (76.3–84.8) | 6.7 | Ref | |||
| Father | 90.4 | (80.3–100.5) | 5.0 | 1.12 | (.99–1.26) | ||
| Other | 78.1 | (60.9–95.4) | 8.1 | .97 | (.83–1.14) | ||
| Literacy of the caregiver | |||||||
| Illiterate | 77.3 | (72.7–82.0) | 6.0 | Ref | Ref | ||
| Literate | 92.4 | (89.6–95.2) | 2.0 | 1.19 | (1.10–1.30) | 1.18 | (1.10–1.27) |
| Number of children | |||||||
| 1–2 children | 80.4 | (76.5–84.4) | 4.6 | Ref | |||
| 3–5 children | 82.7 | (77.0–88.5) | 4.7 | .93 | (.98–1.09) | ||
| > 5 children | 74.7 | (55.5–94.0) | 3.0 | .93 | (.77–1.13) | ||
| After January–February 2008 intervention | |||||||
| Strata | |||||||
| High incidence | 92.3 | (88.4–96.2) | 7.3 | Ref | |||
| Intermediate incidence | 95.0 | (92.8–97.1) | 2.5 | 1.03 | (.98–1.08) | 1.03 | (.98–1.07) |
| Low incidence | 95.8 | (94.1–97.6) | .9 | 1.04 | (.99–1.08) | 1.03 | .99–1.07 |
| Age | |||||||
| 9–23 mo | 84.5 | (79.4–89.5) | 1.2 | Ref | Ref | ||
| 24–59 mo | 93.9 | (91.0–96.8) | 2.9 | 1.12 | (1.05–1.19) | 1.11 | (1.05–1.17) |
| 5–9 y | 95.3 | (93.3–97.4) | 2.5 | 1.14 | (1.08–1.20) | 1.11 | (1.06–1.77) |
| 10–15 y | 94.7 | 92.5–96.9 | 1.8 | 1.13 | (1.06–1.20) | 1.09 | (1.03–1.16) |
| Sex | |||||||
| Male | 93.7 | (90.7–96.7) | 5.4 | Ref | |||
| Female | 94.0 | (92.3–95.6) | 1.8 | 1.00 | (.97–1.03) | ||
| Caregiver | |||||||
| Mother | 93.3 | (90.9–95.7) | 5.6 | Ref | Ref | ||
| Father | 96.5 | (92.7–100.2) | 1.9 | 1.04 | (.99–1.09) | 1.01 | (.97–1.06) |
| Other | 97.6 | (95.5–99.8) | 1.2 | 1.05 | (1.01–1.08) | 1.05 | (1.01–1.08) |
| Literacy of the caregiver | |||||||
| Illiterate | 92.7 | (90.0–95.4) | 5.6 | Ref | Ref | ||
| Literate | 92.7 | (95.4–99.0) | 2.2 | 1.05 | (1.02–1.08) | 1.05 | (1.02–1.08) |
| Number of children | |||||||
| 1–2 children | 92.8 | (90.7–94.9) | 3.2 | Ref | Ref | ||
| 3–5 children | 95.9 | (93.6–98.2) | 3.0 | 1.03 | (1.02–1.05) | 1.03 | (1.01–1.05) |
| > 5 children | 99.2 | (97.5–100.8) | .5 | 1.07 | (1.04–1.10) | 1.07 | (1.04–1.10) |
NOTE. 95% CI, 95% confidence interval; Deff, design effect; CR, coverage ratio; ACR, adjusted coverage ratio; Ref, reference value.
Written records of vaccination were available to ascertain vaccination status for only 20% of the children vaccinated through the routine immunization and <2% of those vaccinated during the mass campaigns. Nonetheless, >95% of respondents reported the geographical location where the vaccine was administered for the 3 vaccination activities, and most identified the shoulder as the site of injection (routine, 86.0%; October 2008 = 95.7%; January 2009 = 94.6%). The exact date of the routine vaccination was unknown for most of the children who reported oral vaccination (98%).
Nonvaccinated Children and Reasons for Nonvaccination
Of the 662 children not vaccinated through the routine system, most were vaccinated during the January–February 2009 campaign (52.0%; 95% CI, 41.9%–62.0%); 159 remained unvaccinated at the date of the survey (6.1%; 95% CI, 4.1%–8.2%). The main reason for non vaccination of children in the routine activities was refusal (25.1%; 95% CI, 15.3%–34.9%). The second most frequent was “lack of information” (22.8%; 95% CI, 15.1%–30.5%). For those children not vaccinated during the October intervention or the January campaign, the main reason was “lack of information” (36.2%; 95% CI, 29.4%–43.0%). Some mothers reported not being allowed to make a decision regarding vaccination in both routine and campaigns (6.7%).
Vaccine Effectiveness Study
The vaccine effectiveness was close to 95% in the 3 periods (prior to the October 2008 intervention, October 2008–January 2009 intervention, and post-January 2009 intervention), without statistical differences between them (Table 4). The percentage of controls vaccinated progressively increased over the 3 periods in line with the increase in citywide coverage. Cases exhibited a similar trend with the percent vaccinated higher at the end of the study period.
Table 4.
Total Numbers of Cases and Controls, Percentage of Children Vaccinated, and Vaccine Effectiveness for Each Vaccination Period
| Cases |
Controls |
|||||
| Intervention | n | % vaccinated | n | % vaccinated | VEa | 95% CI |
| Routine | 54 | 13.0 | 170 | 73.5 | 96.4 | (88.0–98.9) |
| Routine + October 2008 | 361 | 8.6 | 870 | 80.7 | 97.7 | (95.9–98.7) |
| Routine + October 2008 + January 2009 | 72 | 44.4 | 240 | 94.2 | 94.2 | (86.7–97.4) |
NOTE. VE, vaccine effectiveness; 95% CI, 95% confidence interval.
Vaccine effectiveness: adjustment was performed using a conditional logistic regression matched by age and residency place and adjusted by sex.
DISCUSSION
The results of this investigation suggest that the measles epidemic observed from October 2008 to April 2009 in Maroua was due to insufficient vaccination coverage. The most plausible explanation for the epidemic is that routine coverage was not extensive enough to contain the epidemic. In addition, the study demonstrates high vaccine effectiveness, in keeping with low routine coverage as the main causative factor. The routine coverage differed among the geographical strata examined in the study and was inversely correlated with the measles incidence in each area. This reinforces the hypothesis that low routine vaccine coverage was the main reason for the epidemic. Vaccine coverage was >90% only after the mass vaccination campaign of January–February 2009, with a subsequent decrease in the number of reported measles cases. The coverage of the target population with ≥1 dose of measles vaccine reached after the mass campaign conducted by the Ministry of Health was >90% even though the ORI covered ≤80% of the targeted children. This experience demonstrates that such campaigns can help to control outbreaks and to increase the vaccine coverage to desirable levels.
The recently revised WHO guidelines for response to measles outbreaks in mortality-reduction settings advise the use of measles vaccine as a control measure [6]. This recommendation was revised based on evidence suggesting that there is enough time to perform vaccination campaigns before the natural end of the outbreak, thereby reducing cases and subsequent deaths [15–17]. Our work documents one of the first interventions performed following the new WHO recommendation. Time is important when conducting ORIs, and it was a limitation of the mass campaign implemented in Maroua. The campaign was performed late in the epidemic, and its potential impact would have been higher with earlier implementation. This highlights the importance of supporting governments to revise existing measles control and elimination plans to include ORI as a key strategy to reduce measles related mortality. The WHO, the United Nations Children's Fund (UNICEF), Médecins Sans Frontières, and other health actors should continue to advocate for the use of measles vaccine as soon as possible in outbreak situations.
Even with the presence of routine immunization and the 2 supplemental vaccination interventions in Maroua, 6% of all children never received measles-containing vaccine. This emphasizes the need for strategies specifically addressing those children who have little or no contact with health structures and are missed in mass campaigns. Although the reported reasons for nonvaccination are subject to recall bias and interviewer bias, and should be taken only as a rough indication, the principal reason cited was lack of information. The second reported cause for nonvaccination was refusal to accept vaccination. Many hypotheses consider why caregivers may refuse vaccination—restrictions on female heads of households accompanying their children without the male heads of households; fear of side effects; lack of understanding of the potential gravity of nonvaccination; mistrust of the health system; and lack of means if they are required to pay for services. However, these hypotheses remain untested and require a specifically designed study to investigate the different reasons for vaccine refusal in Maroua.
It is similarly important to note the limitations of this investigation. First, because caregivers may not accurately recall history, children without vaccination cards reporting nonvaccination may have indeed been vaccinated. To minimize misclassification, we asked parents to describe in which clinic their child received the vaccine and by way of what part of the body the vaccine was delivered (eg, shoulder, elbow, mouth) to check whether the parent correctly remembered a vaccine consistent with measles-vaccine delivery. The possibility of response bias is always present; however, previous studies in areas of high measles incidence have shown parental recall to be highly reliable [18]. To increase the sensitivity and specificity, we also used the local term for measles during the interviews [19]. In addition, some vaccinations received through countrywide SIAs (2002 and 2006) could have been classified as routine vaccinations, which could explain the higher routine coverage in older children.
Second, our study design relied on available population data for each of the administrative areas. Because a cluster randomized design, our allocation of clusters was based on population size. If there were large differences between actual and estimated population, this may have led to a less-than-optimal sample selection. However, becasuse we included a large population size and covered the entire urban area, estimates of vaccine coverage should be robust to differences in the true population sizes. Further, we used the best available maps of the administrative areas to design our survey. However, these maps may misrepresent the true boundaries of the areas. Thus, we may have erroneously included individuals in one cluster when they should have been allocated to another.
Because there is limited serological confirmation of the cases in this outbreak, we relied on respondent-reported history of previous measles episodes and health-center registries. Using a case definition of clinically confirmed cases, rather than laboratory-confirmed cases, introduces the possibility of inadvertently including cases of rubella and other exanthemas [20]. This is a difficult limitation to overcome without performing concurrent serological testing. If misclassification is present, it could lead to an increase in the number of vaccinated children among reported cases, thereby decreasing the effectiveness estimate [21]. A similar bias, in the same direction, may result from health care–seeking behavior, if vaccinees sought care more frequently than nonvaccinees. Alternatively, an overestimation of the vaccine effectiveness would be observed if mild, vaccinated cases did not attend the clinics, resulting in a higher estimate of the proportion of unvaccinated individuals among reported cases; in this event, the effectiveness estimate would reflect the vaccine's ability to prevent severe cases. Moreover, it is also likely that unvaccinated children may have had greater exposure to the measles virus, as vaccine coverage was inversely related to incidence strata. We have tried to reduce this bias by adjusting the analysis for place of residence. Because ascertainment was obtained mostly through oral reporting, misclassification of vaccination status may have occurred, but it is unlikely that this bias would affect cases more than controls [21].
In light of the results of this investigation and considering the afore-mentioned key limitations, we can still conclude that the measles outbreak in Maroua was principally due to insufficient routine coverage, especially in the youngest children of some areas of the city. We found vaccination coverage differed within geographic strata, which warrants an additional analysis of the catchment population and their experiences with the health-care system. Data concerning differences in access to care and health-center operations (eg, opening hours, vaccine availability, staffing) may reveal how to better serve this population. Although the January 2009 campaign was successful, strategies to ensure that absent residents participate in future campaigns could be considered. Measles control in Maroua is largely a success story; nonetheless, this outbreak shows the importance of keeping high routine vaccine coverage and maintaining effective supplemental activities.
Acknowledgments
We wish to thank the residents of the city of Maroua for their support and participation in the conduct of this survey, Dr M. Koblela of the EPI Program in Yaoundé for facilitating this study, Thomas Roederer for his support in data management, the survey teams and the MSF-France team in Yaoundé for the permanent collaboration. We are greatly indebted to the Head of Mission, Claude Galinier, for his patient and enthusiastic support of this survey.
Funding
This work was supported by the World Health Organization; and Médecins Sans Frontières France.
References
- 1.World Health Organization. Progress towards measles control in WHO's African Region, 2001–2008. Wkly Epidemiol Rec. 2009;84:397–404. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Progress in global measles control and mortality reduction, 2000–2006. MMWR Morb Mortal Wkly Rep. 2007;56:1237–41. [PubMed] [Google Scholar]
- 3.World Health Organization Regional Office for Africa. Regional strategic plan for the expanded programme on immunization, 2006–2009. Geneva, Switzerland: World Health Organization Regional Committee for Africa; 2006. WHO publication AFR/RC56. [Google Scholar]
- 4.World Health Organization, UNICEF, United Nations Children's Fund. Measles: Mortality reduction and regional elimination—strategic plan, 2001–2005. Geneva, Switzerland: World Health Organization, Department of Immunizations, Vaccines and Biologicals; 2001. WHO publication WHO/V&B/01.13. [Google Scholar]
- 5.Waters HR, Dougherty L, Tegang SP, et al. Coverage and costs of childhood immunizations in Cameroon. Bull World Health Organ. 2004;82:668–75. [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Response to measles outbreaks in measles mortality reduction settings. Geneva, Switzerland: World Health Organization, Department of Immunizations, Vaccines and Biologicals; 2009. WHO publication WHO/IVB/09/03. [PubMed] [Google Scholar]
- 7.Goodson JL, Wiesen E, Perry RT, et al. Impact of measles outbreak response vaccination campaign in Dar es Salaam, Tanzania. Vaccine. 2009;27:5870–4. doi: 10.1016/j.vaccine.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 8.Sniadack DH, Moscoso B, Aguilar R, Heath J, Bellini W, Chiu MC. Measles epidemiology and outbreak response immunization in a rural community in Peru. Bull World Health Organ. 1999;77:545–52. [PMC free article] [PubMed] [Google Scholar]
- 9.Aylward RB, Clements J, Olivé JM. The impact of immunization control activities on measles outbreaks in middle and low income countries. Int J Epidemiol. 1997;26:662–9. doi: 10.1093/ije/26.3.662. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Immunization coverage cluster survey. Geneva, Switzerland: World Health Organization, Dept. of Immunization, Vaccines and Biologicals, Vaccine Assessment and Monitoring Team; 2005. WHO publication WHO/IVB/04.23. [Google Scholar]
- 11.Lowther SA, Curriero FC, Kalish BT, Shields TM, Monze M, Moss WJ. Population immunity to measles virus and the effect of HIV-1 infection after a mass measles vaccination campaign in Lusaka, Zambia: A cross-sectional survey. Lancet. 2009;373:1025–32. doi: 10.1016/S0140-6736(09)60142-2. [DOI] [PubMed] [Google Scholar]
- 12.Katz J, Zeger SL. Estimation of design effects in cluster surveys. Ann Epidemiol. 1994;4:295–301. doi: 10.1016/1047-2797(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 13.de Alencar Ximenes RA, Martelli CM, Merchán-Hamann E, et al. Multilevel analysis of hepatitis A infection in children and adolescents: A household survey in the Northeast and Central-west regions of Brazil. Int J Epidemiol. 2008;37:852–61. doi: 10.1093/ije/dyn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Grais RF, Conlan AJ, Ferrari MJ, et al. Time is of the essence: Exploring a measles outbreak response vaccination in Niamey, Niger. J R Soc Interface. 2008;5:67–74. doi: 10.1098/rsif.2007.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubray C, Gervelmeyer A, Djibo A, et al. Late vaccination reinforcement during a measles epidemic in Niamey, Niger (2003–2004) Vaccine. 2006;24:3984–9. doi: 10.1016/j.vaccine.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Grais RF, de Radiguès X, Dubray C, Fermon F, Guerin PJ. Exploring the time to intervene with a reactive mass vaccination campaign in measles epidemics. Epidemiol Infect. 2006;134:845–9. doi: 10.1017/S0950268805005716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samb B, Aaby P, Whittle H, Seck AM, Simondon F. Decline in measles case fatality ratio after the introduction of measles immunization in rural Senegal. Am J Epidemiol. 1997;145:51–7. doi: 10.1093/oxfordjournals.aje.a009031. [DOI] [PubMed] [Google Scholar]
- 19.Anker M, Black RE, Coldham C, et al. A standard verbal autopsy method for investigating causes of death in infants and children. Geneva, Switzerland: World Health Organization, Dept. of Communicable Disease Surveillance and Response; 1999. WHO publication WHO/CDS/CSR/ISR/99/4. [Google Scholar]
- 20.Helfand RF, Chibi T, Biellik R, Shearley A, Bellini WJ. Negative impact of clinical misdiagnosis of measles on health workers' confidence in measles vaccine. Epidemiol Infect. 2004;132:7–10. doi: 10.1017/s0950268803001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orenstein WA, Bernier RH, Hinman AR. Assessing vaccine efficacy in the field. Further observations. Epidemiol Rev. 1988;10:212–41. doi: 10.1093/oxfordjournals.epirev.a036023. [DOI] [PubMed] [Google Scholar]



