Abstract
An experimental strategy to facilitate correction of single-base mutations of episomal targets in mammalian cells has been developed. The method utilizes a chimeric oligonucleotide composed of a contiguous stretch of RNA and DNA residues in a duplex conformation with double hairpin caps on the ends. The RNA/DNA sequence is designed to align with the sequence of the mutant locus and to contain the desired nucleotide change. Activity of the chimeric molecule in targeted correction was tested in a model system in which the aim was to correct a point mutation in the gene encoding the human liver/bone/kidney alkaline phosphatase. When the chimeric molecule was introduced into cells containing the mutant gene on an extrachromosomal plasmid, correction of the point mutation was accomplished with a frequency approaching 30%. These results extend the usefulness of the oligonucleotide-based gene targeting approaches by increasing specific targeting frequency. This strategy should enable the design of antiviral agents.
Full text
PDF
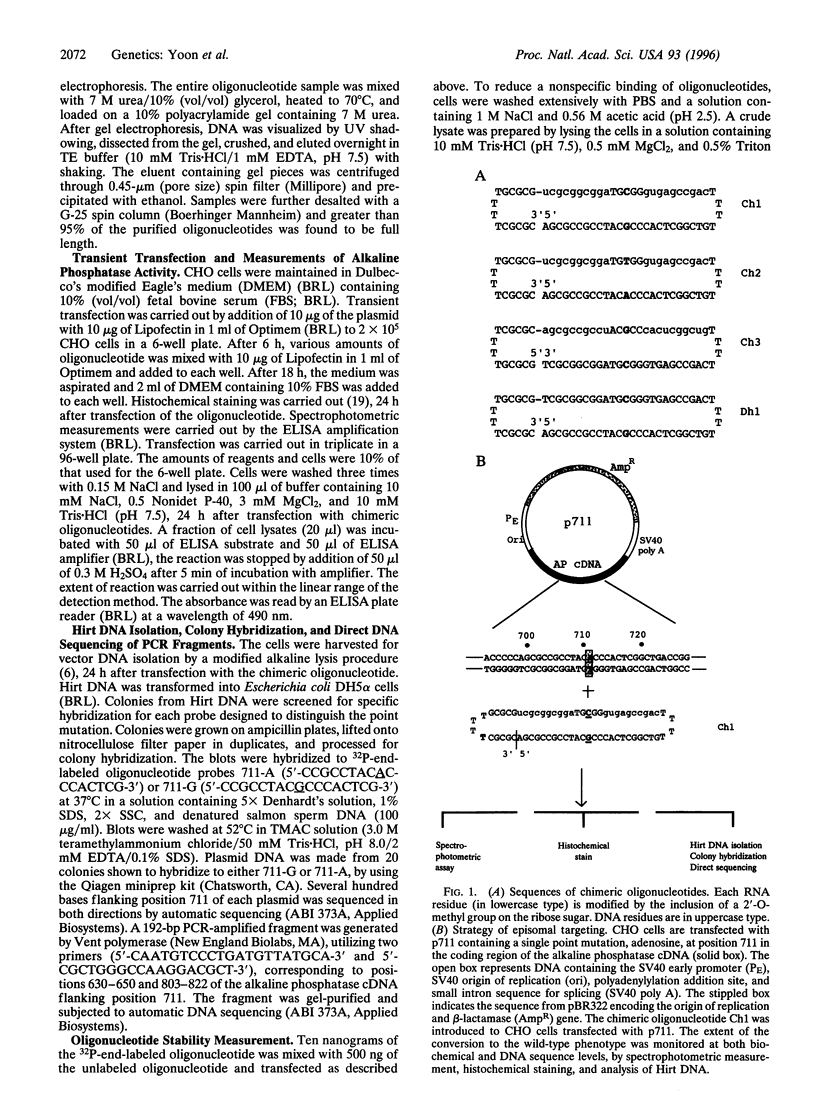

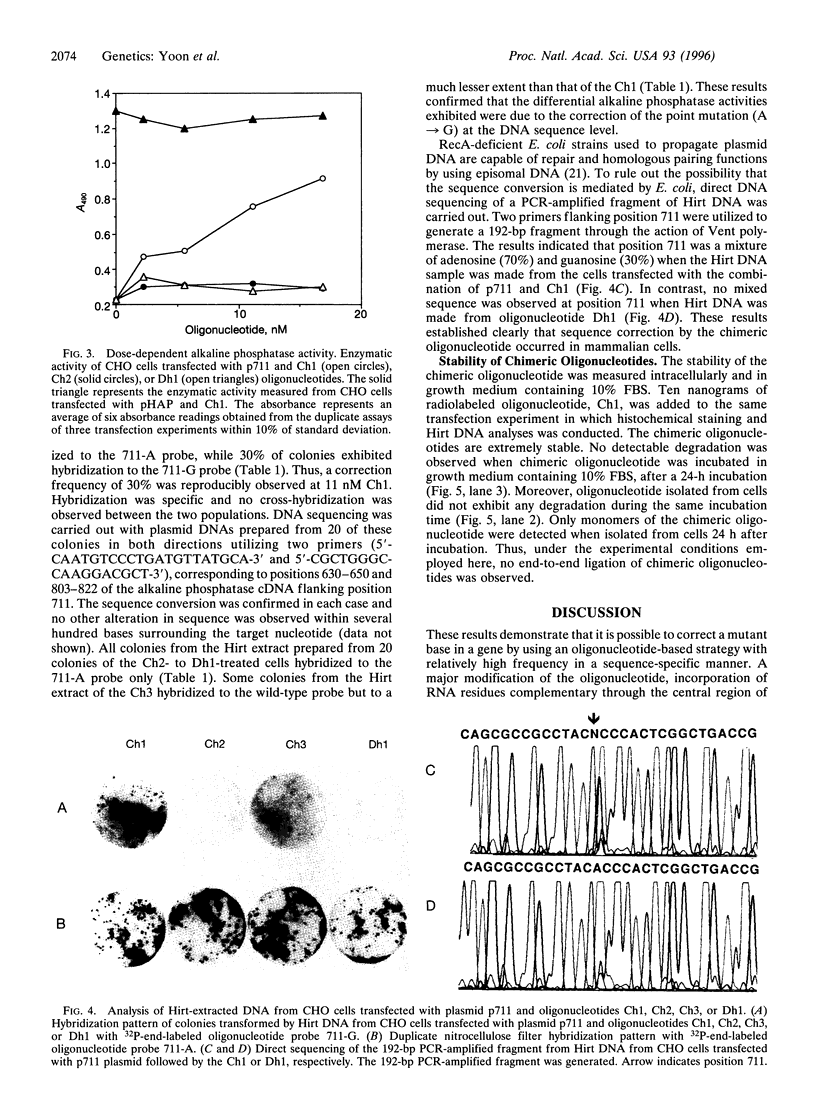
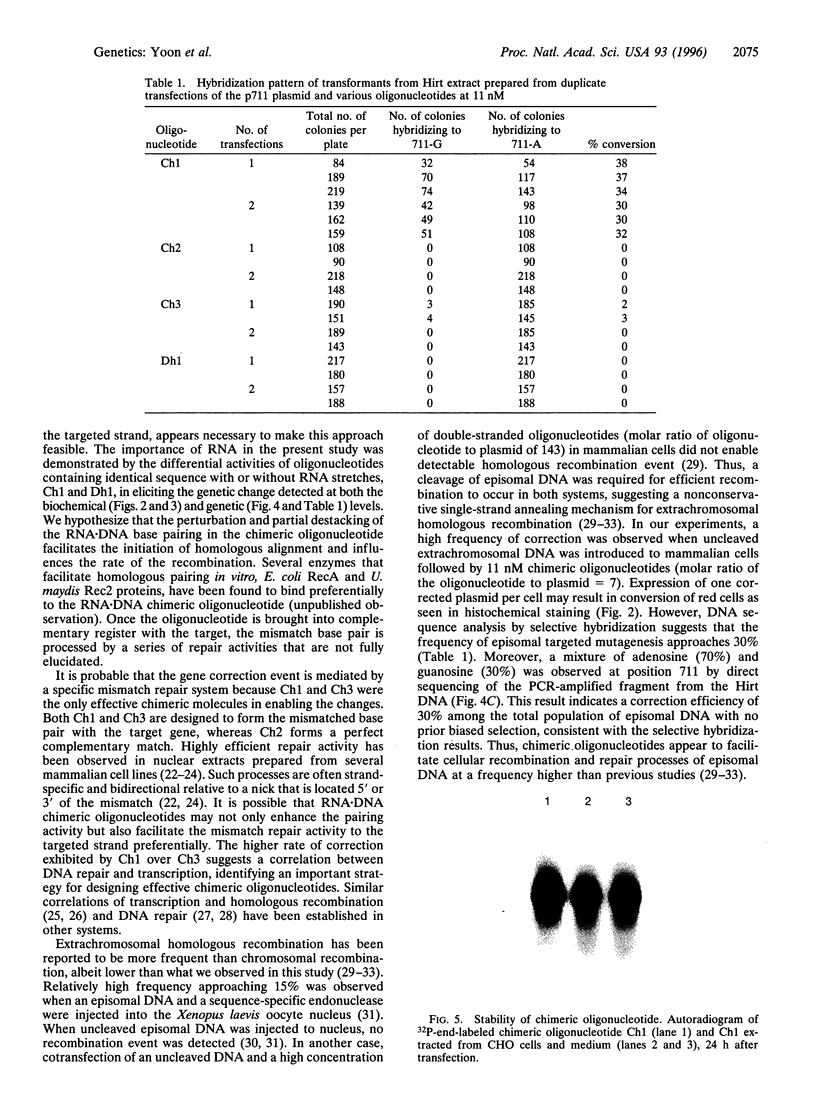

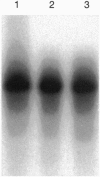
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Bootsma D., Hoeijmakers J. H. DNA repair. Engagement with transcription. Nature. 1993 May 13;363(6425):114–115. doi: 10.1038/363114a0. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Daniels G. A., Lieber M. R. Strand specificity in the transcriptional targeting of recombination at immunoglobulin switch sequences. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5625–5629. doi: 10.1073/pnas.92.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derr L. K., Strathern J. N. A role for reverse transcripts in gene conversion. Nature. 1993 Jan 14;361(6408):170–173. doi: 10.1038/361170a0. [DOI] [PubMed] [Google Scholar]
- Fang W. H., Modrich P. Human strand-specific mismatch repair occurs by a bidirectional mechanism similar to that of the bacterial reaction. J Biol Chem. 1993 Jun 5;268(16):11838–11844. [PubMed] [Google Scholar]
- Fang W. H., Modrich P. Human strand-specific mismatch repair occurs by a bidirectional mechanism similar to that of the bacterial reaction. J Biol Chem. 1993 Jun 5;268(16):11838–11844. [PubMed] [Google Scholar]
- Giovannangéli C., Rougée M., Garestier T., Thuong N. T., Hélène C. Triple-helix formation by oligonucleotides containing the three bases thymine, cytosine, and guanine. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8631–8635. doi: 10.1073/pnas.89.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havre P. A., Glazer P. M. Targeted mutagenesis of simian virus 40 DNA mediated by a triple helix-forming oligonucleotide. J Virol. 1993 Dec;67(12):7324–7331. doi: 10.1128/jvi.67.12.7324-7331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs C. A., Yoon K. Differential regulation of gene expression in vivo by triple helix-forming oligonucleotides as detected by a reporter enzyme. Antisense Res Dev. 1994 Spring;4(1):1–8. doi: 10.1089/ard.1994.4.1. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Cole A., Holloman W. K. The REC2 gene encodes the homologous pairing protein of Ustilago maydis. Mol Cell Biol. 1994 Nov;14(11):7163–7172. doi: 10.1128/mcb.14.11.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H., Kmiec E. B. A role for RNA synthesis in homologous pairing events. Mol Cell Biol. 1994 Sep;14(9):6097–6106. doi: 10.1128/mcb.14.9.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H., Kmiec E. B. Transcription activates RecA-promoted homologous pairing of nucleosomal DNA. Mol Cell Biol. 1994 Mar;14(3):1949–1955. doi: 10.1128/mcb.14.3.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Repair of double-stranded DNA breaks by homologous DNA fragments during transfer of DNA into mouse L cells. Mol Cell Biol. 1990 Jan;10(1):113–119. doi: 10.1128/mcb.10.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi-DeLuca C., Kolodner R. D. Effect of terminal non-homology on intramolecular recombination of linear plasmid substrates in Escherichia coli. J Mol Biol. 1992 Sep 5;227(1):72–80. doi: 10.1016/0022-2836(92)90682-a. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Modrich P. Mismatch repair, genetic stability, and cancer. Science. 1994 Dec 23;266(5193):1959–1960. doi: 10.1126/science.7801122. [DOI] [PubMed] [Google Scholar]
- Monia B. P., Lesnik E. A., Gonzalez C., Lima W. F., McGee D., Guinosso C. J., Kawasaki A. M., Cook P. D., Freier S. M. Evaluation of 2'-modified oligonucleotides containing 2'-deoxy gaps as antisense inhibitors of gene expression. J Biol Chem. 1993 Jul 5;268(19):14514–14522. [PubMed] [Google Scholar]
- Morgan R. A., Anderson W. F. Human gene therapy. Annu Rev Biochem. 1993;62:191–217. doi: 10.1146/annurev.bi.62.070193.001203. [DOI] [PubMed] [Google Scholar]
- Parsons R., Li G. M., Longley M. J., Fang W. H., Papadopoulos N., Jen J., de la Chapelle A., Kinzler K. W., Vogelstein B., Modrich P. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993 Dec 17;75(6):1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- Rouet P., Smih F., Jasin M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6064–6068. doi: 10.1073/pnas.91.13.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. J., Carroll D. Endonuclease-induced, targeted homologous extrachromosomal recombination in Xenopus oocytes. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):806–810. doi: 10.1073/pnas.92.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Umar A., Boyer J. C., Kunkel T. A. DNA loop repair by human cell extracts. Science. 1994 Nov 4;266(5186):814–816. doi: 10.1126/science.7973637. [DOI] [PubMed] [Google Scholar]
- Wang G., Levy D. D., Seidman M. M., Glazer P. M. Targeted mutagenesis in mammalian cells mediated by intracellular triple helix formation. Mol Cell Biol. 1995 Mar;15(3):1759–1768. doi: 10.1128/mcb.15.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Cole D. E., Ray K., Whyte M. P., Lafferty M. A., Mulivor R. A., Harris H. A missense mutation in the human liver/bone/kidney alkaline phosphatase gene causing a lethal form of hypophosphatasia. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7666–7669. doi: 10.1073/pnas.85.20.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Fallon M. D., Whyte M. P., Fedde K. N., Lafferty M. A., Mulivor R. A., Harris H. Analysis of liver/bone/kidney alkaline phosphatase mRNA, DNA, and enzymatic activity in cultured skin fibroblasts from 14 unrelated patients with severe hypophosphatasia. Am J Hum Genet. 1989 May;44(5):686–694. [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H., Leung W. Y., Bosco G., Dieu D., Haber J. E. The frequency of gene targeting in yeast depends on the number of target copies. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):177–181. doi: 10.1073/pnas.91.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K., Thiede M. A., Rodan G. A. Alkaline phosphatase as a reporter enzyme. Gene. 1988 Jun 15;66(1):11–17. doi: 10.1016/0378-1119(88)90220-x. [DOI] [PubMed] [Google Scholar]
- Zheng H., Wilson J. H. Gene targeting in normal and amplified cell lines. Nature. 1990 Mar 8;344(6262):170–173. doi: 10.1038/344170a0. [DOI] [PubMed] [Google Scholar]