Abstract
Background
The stability of biomarkers in stored biomedical samples is crucial, especially when storage is for extended periods of time. High-sensitivity CRP (Hs-CRP) is a biomarker of low grade inflammation that is extensively used to identify and study cardiovascular and/or inflammatory processes in clinical care and large epidemiologic studies. Therefore, assessing Hs-CRP stability in archived samples at a given temperature is important to insure precision of measurements over time and the validity of studies using archived samples.
Methods
We evaluated the stability of Hs-CRP in 30 randomly selected human serum samples by measuring Hs-CRP concentrations in freshly collected sample [Hs-CRP (0)] and in the same set of samples after 7–11 years of storage at −80°C [Hs-CRP (LT)].
Results
Hs-CRP did not significantly change up to 11 years of storage at −80°C as shown by a negligible median difference between Hs-CRP (0) and Hs-CRP (LT), delta(Hs-CRP (0)- Hs-CRP (LT)=−0.01, p=0.45. There was a good concordance and agreement between Hs-CRP (0) and Hs-CRP (LT) as measured respectively by Lin's coefficient of correlation (ρC= 0.98) and Bland-Altman analysis (mean difference=−0.02, 95% CI [−0.04–0.0045] p=0.107). In addition, the data also suggest that the time elapsed between collection and Hs-CRP measurement does not affect Hs-CRP stability over time when samples are kept under the appropriate conditions.
Conclusions
Long-term storage at −80°C for up to 11 years did not significantly affect the stability of serum Hs-CRP. Given the cost and time for collecting fresh samples, this observation represents an important finding for biomedical research and clinical care.
Keywords: C-reactive protein, archived samples, long-term storage, stability, biomedical research
Background
Studies often use archived samples to retrospectively measure the concentrations of biomarkers in plasma or serum [1–3]. This practice is particularly common among large research consortia that combine biomarkers data assayed from samples stored in freezers over varying time intervals, sometimes for several years after initial sample collection[4]. The increasing use of large biobanks to procure samples for research studies also means that more investigators than ever are using samples stored at low temperatures for periods ranging from months to years, even up to decades [5, 6]. These practices in biomedical research raise the problem of stability of serum biomarkers over time as well as the validity of the results of such studies.
C-reactive protein (CRP) is an important acute phase reactant protein in many epidemiologic studies designed to investigate the relationship between inflammation and several chronic conditions including heart disease and metabolic diseases. High-sensitivity CRP (Hs-CRP) is routinely used in conjunction with lipid panels to access cardiovascular risk in clinics and population studies [7, 8]. Like many other biomarkers, Hs-CRP concentrations may be affected by preclinical handling and storage conditions. While manufacturers of the reagents used to measure such biomarkers given some information about biomarkers stability in biological fluids in the accompanying laboratory specifications, most of them do not have data on stability that exceed periods of few months at −20°C [9, 10]. This leaves a tremendous gap between what is known about the stability period of samples stored for few months and samples stored for few years or decades. For example, issues surrounding the long-term stability of serum Hs-CRP remain unresolved. One study reported that Hs-CRP was stable in samples stored for a couple of years at −20°C [11] while others have reported contradictory results from samples stored over long periods of time i.e. up to 10 years [12, 13]. Ishikawa S et al. found that Hs-CRP concentrations increased with time whereas Nilsson, TK et al found that Hs-CRP concentrations were barely affected by long-term storage at low temperature [13]. The two studies used different study designs, outcome measurements, and also had different overall objectives, which could explain the observed inconsistency. Therefore, the issue of Hs-CRP stability in human samples stored over several years remains an open question. In contrast to most previous studies that have assessed Hs-CRP stability, the focus of our study is to determine the effect of long-term storage on Hs-CRP stability in one type of sample, namely serum. Evaluating the effects of sample type (plasma, serum) or anticoagulant (citrate, EDTA) on Hs-CRP stability is outside the scope of this study. Evaluating the expected stability of Hs-CRP after years of storage is of potential utility to epidemiologic studies and biobanks that often conduct assays on serum samples that have been banked for years to decades.
Materials and Methods
Subjects
The participants of this study were drawn from the Howard University study (HUFS), a large population-based genetic epidemiology study of African Americans in Washington DC, USA. The individuals in HUFS were healthy volunteers who were not ascertained on any phenotype and were recruited between 2001 and 2008 [14]. All subjects included in this study provided written informed consent. The study was approved by the Howard University Institutional Board Review [15]. The thirty (30) participants included in this analysis were randomly selected from a subset of HUFS (N=1174) that consists of individuals that have baseline Hs-CRP measured and have more than one serum aliquots archived.
Measurement of baseline Hs-CRP
Blood samples were obtained from participants in plain vacutainer tubes and allowed to clot at room temperature for 1 hour and then spun to separate serum. Serum samples were stored in externally threaded 2-ml cryovials. Each cryovial was filled with about 1.8 ml of serum leaving about 0.2 ml of space to allow for liquid expansion during freezing.
Hs-CRP was measured on Cobas Integra 400 Plus using a latex particle-enhanced immunoturbidimetric assay following the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN). The measuring range for this assay is 0.01–20 mg/dL. The baseline Hs-CRP was obtained the day of collection when possible or samples are stored following manufacturer's recommendations (stored at 4°C for 8 days or at −20°C for up to 3 years) [10] and batch- assayed thereafter. The average storage time at −20°C before the baseline Hs-CRP [Hs-CRP (0)] measurement was 3 months. After the baseline measurements, all samples were stored at −80°C.
Measurement of Hs-CRP on archived samples
The 30 samples randomly selected for this study were slowly thawed at 4°C and then thoroughly mixed before long-term Hs-CRP (Hs-CRP (LT)) was measured using the same method as described above for the baseline measurement (Roche Diagnostics, Indianapolis, IN). While the methodology remained the same throughout the study, the reagents were slightly modified by the manufacturer to improve the assay performance. The accuracy remains similar with a measuring range of 0.03–35mg/dL.
Statistical methods
All statistical analyses were run in SPSS version 19 and SAS package. Agreement between Hs-CRP (0) and Hs-CRP (LT) was assessed using: Bland –Altman limits of agreement analysis, Lin's concordance coefficient [16, 17] and the Wilcoxon signed rank test (a non-parametric test for related samples test). Non-parametric correlation between the two endpoints was also determined by Kendall's tau-b coefficient. To visualize the difference between Hs-CRP (0) and Hs-CRP (LT) for each sample and across all samples, line plots and Bland-Altman plots [16] were generated. To determine if the relationship between Hs-CRP (0) and Hs-CRP (LT) is affected by the length of time elapsed between samples collection and Hs-CRP measurement, the data was split into 3 groups: 1) samples assayed between 0–8 days after collection; 2) samples assayed between 9–80 days and 3) samples assayed more than 80 days after collection. The Wilcoxon signed rank test was used to determine if the differences between the medians are statistically significant.
Results
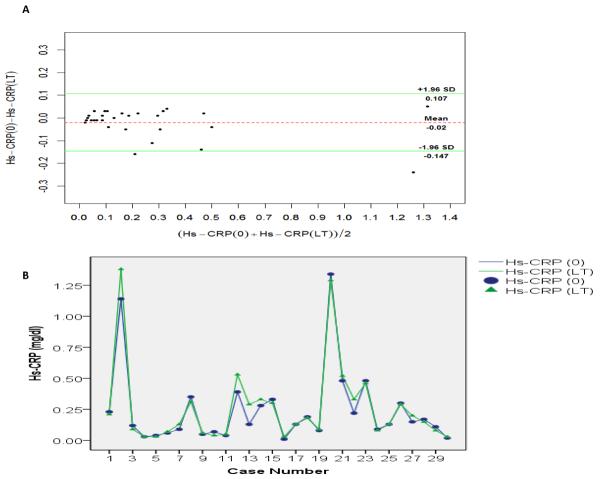
The mean age for the 30 individuals from which the samples were collected is 35.7 years. Mean± STD for Hs-CRP at baseline (Hs-CRP (0)) is 0.24 ±0.3 mg/dL with a range of 0.01–1.34 mg/dL and a median value of 0.13 mg/dl. Upon storage at −80°C for an average of 8.7 years (range: 7–11 years), we observed a slight increase in Hs-CRP concentrations (Mean ± STD= 0.26±0.33 mg/dl, range [0.03–1.35 mg/dl] and median=0.14 mg/dL). However, the increase was not statistically significant as shown by the Wilcoxon signed rank test statistics (Z=−0.76, p= 0.44). Furthermore, Bland-Altman analysis, a measure of agreement, showed no bias between the two measurements of Hs-CRP i.e. at baseline and after long-term storage (mean difference= −0.02, p= 0.10 and 95% limits of agreement [−0.15–0.11]) (Figure 1A) and the absence of difference between the two measurements is also confirmed by the two line plots representing Hs-CRP (0) and Hs-CRP (LT) respectively in the 30 samples (Figure 1B).
Figure 1. Stability of serum Hs-CRP concentrations after long-term storage.
A. Bland and Altman plot; green lines represent the 95% limits of agreement, dotted red line represents the mean difference
B. Change in serum Hs-CRP among the 30 participants after long-term storage: Line plot
(_) green line represents serum Hs-CRP variation at baseline among the study participants.
(_) blue line represents serum Hs-CRP variation after long-term storage among the study participants.
(▲) green triangle represents serum Hs-CRP at baseline for each study participant and (•) blue dot represents serum Hs-CRP after long-term storage for each study participant.
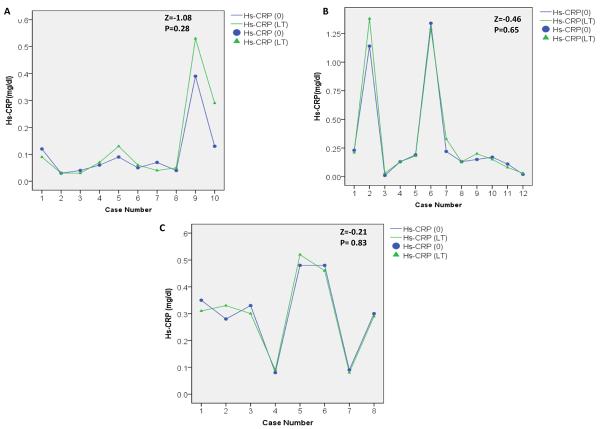
The correlation between Hs-CRP (0) and Hs-CRP (LT) is strong (Kendall's tau-b=0.87, p<0.0001). Since high correlation does not always mean agreement or concordance, we also estimated Lin's coefficient of correlation, ρc, which shows a high concordance between Hs-CRP (0) and Hs-CRP (LT) (ρc = 0.98). Subset analysis based on the time elapsed between sample collection and Hs-CRP measurement showed that the duration between collection and testing did not significantly affect Hs-CRP concentrations (Figure 2).
Figure 2. Change in serum Hs-CRP concentrations measured at baseline and after long-term storage based on the time elapsed between sample collection and Hs-CRP (0) test.
(_) green line represents serum HS-CRP variation at baseline among the study participants.
(_) blue line represents serum HS-CRP variation at after long term storage among the study participants.
(▲) green triangle represents serum Hs-CRP at baseline for each study participant and (•) blue dot represents serum Hs-CRP after long-term storage for each study participant.
(A) Samples assayed between 0–8 days after collection (n=10). (B) Samples assayed between 9–80 days (n=12) and (C), samples assayed more than 80 days after collection (n=8). Z, Wilcoxon signed rank test statistics, P is p-value.
Discussion
This study shows that serum Hs-CRP concentrations remain stable for up to 11 years at −80°C. There was a slight increase (2%) in Hs-CRP level overtime that was not significantly meaningful. Similar to the findings of this study, Ishikawa S et al. observed an increase in Hs-CRP concentrations, but in contrast to this study's findings, the increase they reported was statistically significant and more pronounced especially for samples that have low baseline Hs-CRP[12]. The discrepancy between these two studies could be due to several factors including the freeze/thaw cycle which can affect Hs-CRP stability by disrupting CRP pentameric structure resulting in monomers that can be detected and thus lead to overestimation of Hs-CRP concentrations.
While the effects of long -term storage on Hs-CRP have been investigated by others [12, 13, 18, 19], our study design is unique. In fact, we used a sample randomization procedure to select our samples unlike other studies which either used randomly selected samples from groups stratified based on Hs-CRP concentrations or used different type of samples for the stability study (serum at baseline and plasma after long term storage). Secondly, samples used in this study were fasting samples which is important for precision especially when the assay principle, turbidimetry, depends on optical clarity. Thirdly, in this study we only focused on the effect of long -term storage on a single type of sample, serum, to avoid the intrinsic variability that may be introduced as confounder due to the use of different type of samples. A change in analytical methods or change in reagent lots can influence the accuracy of measurements. To minimize the effect of such change on our results, we have used strict quality control processes such as retaining the same analytical methodology and manufacturer for all assays, re-assaying samples previously assayed with earlier reagent lots using newly procured lots and accessing concordance between reagent lots.
A limitation of this study is the relatively small sample size. The increase in sample size in such experiment may further narrow the limits of agreement interval between the before and after storage Hs-CRP measurements and allow an enhanced generalization of our findings.
In conclusion, our study shows that Hs-CRP concentration is remarkably stable after long-term storage in −80°C freezer for up to 11 years. These findings are significant for investigators involved in international collaborations in which samples are shipped between countries or studies involving the use of biobank samples.
Published Highlights
We evaluate the expected stability of CRP in serum samples banked for several years.
Long-term storage at −80°C has no apparent effect on CRP concentrations.
The time elapsed between sample collection and CRP assay has no effect on CRP stability.
These findings provide useful operational data for epidemiological studies and biobanks.
Acknowledgements
This work was supported in part by the Intramural Research Program of the Center for Research on Genomics and Global Health (CRGGH). The CRGGH is supported by funds from the Office of the Director, National Institute of Diabetes and Digestive and Kidney diseases (NIDDK) and National Human Genome Research Institute (NHGRI) at National Institutes of Health [Z01HG200362]. The Howard University Family Study was supported by National Institutes of Health grants [S06GM008016-320107 to C. R., S06GM008016-380111 to A.A.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bays HE, Stein EA, Shah AK, Maccubbin DL, Mitchel YB, Mercuri M. Effects of simvastatin on C-reactive protein in mixed hyperlipidemic and hypertriglyceridemic patients. Am J Cardiol. 2002;90(9):942–6. doi: 10.1016/s0002-9149(02)02658-9. [DOI] [PubMed] [Google Scholar]
- [2].Bea JW, Wright NC, Thompson P, Hu C, Guerra S, Chen Z. Performance evaluation of a multiplex assay for future use in biomarker discovery efforts to predict body composition. Clin Chem Lab Med. 2011;49(5):817–24. doi: 10.1515/CCLM.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of Metabolomic Profiles among Men and Women in 2 Large Cohort Studies. Clin Chem. 2013;59(11):1657–67. doi: 10.1373/clinchem.2012.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in N80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123(7):731–8. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Floegel A, von Ruesten A, Drogan D, Schulze MB, Prehn C, Adamski J, et al. Variation of serum metabolites related to habitual diet: a targeted metabolomic approach in EPIC-Potsdam. Eur J Clin Nutr. 2013;67(10):1100–8. doi: 10.1038/ejcn.2013.147. [DOI] [PubMed] [Google Scholar]
- [6].Mercuri A, Turchi S, Borghini A, Chiesa MR, Lazzerini G, Musacchio L, et al. Nitrogen biobank for cardiovascular research. Curr Cardiol Rev. 2013;9(3):253–9. doi: 10.2174/1573403X113099990035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. 2003;92(4B):17K–22K. doi: 10.1016/s0002-9149(03)00774-4. [DOI] [PubMed] [Google Scholar]
- [8].Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148(1 Suppl.):S19–26. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- [9].Banfi G, Bauer K, Brand W, Buchberger M, Deom A, Ehret W, et al. Use of Anticoagulants in Diagnostic Laboratory Investigations, 2002. World Health Organization; Geneva: 2002. pp. 1–64. [Google Scholar]
- [10].Diagnostics R. Cobas Integra 400/8002009. Roche Diagnostics; 2009. Cardiac C-Reactive Protein (Latex) High Sensitive; pp. 1–5. [Google Scholar]
- [11].Brindle E, Fujita M, Shofer J, O'Connor KA. Serum, plasma, and dried blood spot highsensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362(1–2):112–20. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ishikawa S, Kayaba K, Gotoh T, Nakamura Y, Kario K, Ito Y, et al. Comparison of Creactive protein levels between serum and plasma samples on long-term frozen storage after a 13.8 year interval: the JMS Cohort Study. J Epidemiol. 2007;17(4):120–4. doi: 10.2188/jea.17.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nilsson TK, Boman K, Jansson JH, Thogersen AM, Berggren M, Broberg A, et al. Comparison of soluble thrombomodulin, von Willebrand factor, tPA/PAI-1 complex, and high-sensitivity CRP concentrations in serum, EDTA plasma, citrated plasma, and acidified citrated plasma (Stabilyte) stored at −70 degrees C for 8–11 years. Thromb Res. 2005;116(3):249–54. doi: 10.1016/j.thromres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- [14].Doumatey AP, Bentley AR, Zhou J, Huang H, Adeyemo A, Rotimi CN. Paradoxical Hyperadiponectinemia is Associated With the Metabolically Healthy Obese (MHO) Phenotype in African Americans. J Endocrinol Metab. 2012;2(2):51–65. doi: 10.4021/jem95W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cutting CW, Hunt C, Nisbet JA, Bland JM, Dalgleish AG, Kirby RS. Serum insulin-like growth factor-1 is not a useful marker of prostate cancer. BJU Int. 1999;83(9):996–9. doi: 10.1046/j.1464-410x.1999.00088.x. [DOI] [PubMed] [Google Scholar]
- [16].Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- [17].Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–68. [PubMed] [Google Scholar]
- [18].Ledue TB, Rifai N. Preanalytic and analytic sources of variations in C-reactive protein measurement: implications for cardiovascular disease risk assessment. Clin Chem. 2003;49(8):1258–71. doi: 10.1373/49.8.1258. [DOI] [PubMed] [Google Scholar]
- [19].Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43(1):52–8. [PubMed] [Google Scholar]