Summary
GATA3 plays an integral role in breast luminal cell differentiation and is implicated in breast cancer progression. GATA3 immunohistochemistry is a useful marker of breast cancer; however, its use in specific subtypes is unclear. Here, we evaluate GATA3 expression in 86 invasive ductal carcinomas including triple-negative, Her-2, and luminal subtypes, in addition to 13 metaplastic carcinomas and in 34 fibroepithelial neoplasms. In addition, we report GATA3 expression in matched primary and metastatic breast carcinomas in 30 patients with known estrogen receptor (ER), progesterone receptor (PR), and Her-2 status, including 5 with ER and/or PR loss from primary to metastasis. Tissue microarrays containing 5 to 10 cores per tumor were stained for GATA3, scored as follows: 0 (0–5%), 1+ (6%–25%), 2+ (26%–50%), 3+ (51%–75%), and 4+ (>75%). GATA3 labeling was seen in 67% (66/99) of primary ductal carcinomas including 43% of triple-negative and 54% of metaplastic carcinomas. In contrast, stromal GATA3 labeling was seen in only 1 fibroepithelial neoplasm. GATA3 labeling was seen in 90% (27/30) of primary breast carcinomas in the paired cohort, including 67% of triple-negative carcinomas. GATA3 labeling was overwhelmingly maintained in paired metastases. Notably, GATA3 was maintained in all “luminal loss” metastases, which showed ER and/or PR loss. In conclusion, GATA3 expression is maintained between matched primary and metastatic carcinomas including ER-negative cases. GATA3 can be particularly useful as a marker for metastatic breast carcinoma, especially triple-negative and metaplastic carcinomas, which lack specific markers of mammary origin. Finally, GATA3 labeling may help distinguish metaplastic carcinoma from malignant phyllodes tumors.
Keywords: GATA3, Breast carcinoma, Metastatic breast carcinoma, Metaplastic carcinoma
1. Introduction
GATA3 is a member of the GATA family of zinc-finger binding transcription factors that regulates the specification and differentiation of many tissue types [1,2] including the breast [3–5], kidney [6], T cells [7], nervous system [8], and hair follicles [9]. Although GATA3 is expressed in a wide variety of tissues, the expression of transcription factors in many tissues is at low levels that are not detectable by immunohistochemistry (IHC). Immunohistochemical labeling for GATA3 in normal tissues is far more restricted, and GATA3 labeling has been demonstrated to be a highly specific marker for breast carcinomas [10,11] and urothelial carcinomas [11,12].
Although the high specificity of GATA3 expression in breast carcinoma has been well established, several key questions remain that impact the clinical use of IHC for GATA3. First, although multiple studies have suggested that GATA3 is more frequently expressed in estrogen receptor (ER)–positive tumors compared with ER-negative tumors [10,13,14], few studies have examined the expression of GATA3 in primary breast carcinomas (PBCs) subdivided by IHC surrogate profiles of molecular subtypes [15]. Specifically, expression of GATA3 in “triple-negative” (ER, progesterone receptor [PR], and Her-2 negative) breast carcinomas is most relevant because mammary origin of that subtype is most difficult to prove by IHC. Second, expression of GATA3 in metaplastic (sarcomatoid) carcinomas, which must be distinguished from other spindle cell malignancies in the breast, has not been assessed. Third, although the main clinical use of GATA3 IHC would be to establish the diagnosis of metastatic carcinoma, the expression of GATA3 in breast cancer metastases has not been well studied. Moreover, no study has compared the expression of GATA3 in metastatic breast carcinomas (MBCs) related to their matched primary carcinomas to determine if these results are concordant.
Here, we systematically evaluated GATA3 expression in 86 invasive ductal carcinomas subdivided by IHC-defined molecular subtypes, including triple-negative carcinomas (TNCs), Her-2 carcinomas, and luminal carcinomas. We evaluated GATA3 expression in 13 metaplastic (sarcomatoid) carcinomas and, for comparison, in 34 fibroepithelial neoplasms of the breast. Finally, we report the first study of GATA3 expression in matched primary and MBCs harvested at surgery or autopsy in 30 patients with known ER, PR, and Her-2 status, including 5 cases where ER and/or PR expression was lost from the primary to the metastasis.
2. Materials and methods
2.1. Tissue microarray construction and case selection
2.1.1. Primary invasive ductal carcinomas
This study was approved by the institutional review board of the Johns Hopkins Medical Institutions. We evaluated a series of tissue microarrays (TMAs) constructed from archived paraffin tissue blocks of 86 primary invasive ductal carcinomas, as previously described [16]. Each TMA consisted of 99 cores measuring 1.4 mm in diameter. Five cores were taken per case to minimize sampling error, including 1 core per case containing benign lobules. The cases were subdivided by established IHC surrogate markers of gene expression profiles [17,18] into the categories of luminal A, luminal B, Her-2, and TNC, as described below.
2.1.2. Metaplastic carcinomas and fibroepithelial neoplasms
We evaluated a TMA containing 13 primary breast metaplastic carcinomas, with 5 cores per case, as previously described [19]. Metaplastic carcinomas were defined by the presence of sarcomatoid differentiation including spindled, squamous, chondroid, or osseous components, as defined by established criteria [20]. In this series, 12 metaplastic carcinomas were high grade, and 1 was intermediate grade. In addition, because phyllodes tumors of the breast are in the differential diagnosis of metaplastic carcinomas of the breast with spindle cell differentiation (particularly on core needle biopsy), we evaluated for comparison a series of TMAs constructed from archived paraffin tissue blocks of 34 fibroepithelial neoplasms, as previously described [19]. These fibroepithelial neoplasms included 14 malignant phyllodes, 10 borderline phyllodes, 10 benign phyllodes, and 10 fibroadenomas, which were classified based on established criteria [21,22] including tumor circumscription, the presence of a leaflike architecture, stromal overgrowth, stromal cellularity, stromal pleomorphism, and stromal mitoses. Five cores were taken per phyllodes tumor and 2 cores per fibroadenoma, with benign lobules as an internal control.
2.1.3. Matched primary and metastatic mammary carcinomas
We evaluated a series of TMAs constructed from archived paraffin tissue blocks of matched PBCs and surgically resected MBCs from 15 patients (surgical pathology cases, Table 1), as previously described [23]. Briefly, each TMA consisted of 99 cores measuring 1.4 mm in diameter. Five to 10 cores per PBC and MBC tumor sample were taken to minimize sampling error, including 1 core per case containing benign breast lobules as control tissue. In addition, we evaluated a series of previously described single-patient TMAs constructed from paraffin tissue blocks of archived PBCs and from multiple MBCs sampled at rapid autopsies on 15 patients who died of widely MBC (autopsy pathology cases, Table 1) [24,25]. In brief, the metastases harvested from autopsy were formalin fixed and processed similarly to surgical breast specimens at our institution. All but 1 autopsy were performed within a 4-hour postmortem interval, with an average of 3.6 hours postmortem. The single-patient autopsy TMAs contained 5 to 10 cores per PBC and MBC, with several cores per case of benign breast lobules and benign visceral tissue (eg, adrenal and liver).
Table 1.
Clinicopathologic characteristics of paired primary and MBCs sampled at surgery (SPC) and harvested at autopsy (APC)
| SPC | APC | |
|---|---|---|
| No. of cases | 15 | 15 |
| Tumor morphology | ||
| Ductal | 12 | 14 |
| Lobular | 3 | 1 |
| Molecular subtype | ||
| Luminal A | 8 | 5 |
| Luminal B | 0 | 0 |
| Luminal loss | 0 | 5 |
| Her-2 | 1 | 2 |
| TNC | 6 | 3 |
| Hormone receptors | ||
| ER | ||
| Positive | 8 | 11 |
| Negative | 7 | 4 |
| PR | ||
| Positive | 7 | 9 |
| Negative | 8 | 6 |
| Her-2/Neu | ||
| Overexpressed | 1 | 2 |
| Not overexpressed | 14 | 13 |
| Elston grade | ||
| Grade 1 | 0 | 0 |
| Grade 2 | 4 | 9 |
| Grade 3 | 11 | 6 |
| TNM stage at first diagnosis | ||
| I | 4 | 1 |
| II | 7 | 10 |
| III | 1 | 2 |
| IV | 3 | 2 |
| Lymph node status at diagnosis | ||
| Positive | 6 | 12 |
| Negative | 7 | 2 |
| Not assessed | 2 | 1 |
| Metastasis location | ||
| Brain only | 6 | 0 |
| Lung only | 5 | 0 |
| GI only | 3 | 0 |
| GYN only | 1 | 0 |
| Multiple sites | 0 | 15 |
Abbreviations: SPC, surgical pathology cases; APC, autopsy pathology cases; TNM, tumor node metastasis staging, American Joint Committee on Cancer, 7th ed; GI, gastrointestinal tract; GYN, gynecologic tract.
2.1.4. IHC and expression scoring
TMAs were labeled by IHC for ER, PR, and Her-2 and classified using established criteria [17,18] into the following categories: luminal A (ER/PR+, Her-2−), luminal B (ER/PR+, Her-2+), Her-2 (ER/PR−, Her-2+), luminal loss (ER and/or PR loss from PBC to MBC), and TNC (ER/PR/Her-2−), as previously described [16,23,24]. Briefly, hormone expression was scored by labeling intensity (none, weak, moderate, or strong) and percentage nuclear labeling (0–100%), with any labeling more than 1% considered a positive result. Her-2 expression was scored using established criteria from 0 to 3+ using labeling intensity and proportion of complete membranous staining. To qualify as HER-2 positive, a case had to demonstrate either a 3+ IHC score or HER-2 fluorescence in situ hybridization amplification ratio greater than 2.2. The hormone and Her-2 expression was scored individually on each TMA spot, and an average of all spots per site was calculated. We intentionally chose unequivocal luminal and Her-2–positive cases to include on the TMA, so that all ER-positive cases included in the TMAs in this series showed more than 70% ER labeling, and all cases with Her-2 amplification included in the TMAs in this series showed an amplification ratio greater than 4.0, as previously described [16]. All of our cases that were classified as ER and PR negative showed 0% nuclear labeling for these markers.
The TMAs were labeled by IHC for GATA3 with a mouse monoclonal anti-GATA3 antibody (1:100 dilution, clone L50–823; Biocare Medical, Concord, CA), using the Benchmark XT automated slide stainer (Ventana Medical Systems, Inc, Tucson, AZ). Briefly, unstained 5-μm sections were cut from paraffin TMA blocks; slides were deparaffinized by routine techniques and subjected to antigen retrieval with Cell Conditioning Solution (high pH CC1 standard) for 60 minutes. Primary antibody incubation was performed for 44 minutes, followed by amplification, and the reaction was developed with a biotin-free, polymer detection system (Ultra-view; Ventana Medical Systems, Inc), as per manufacturer’s instructions. Slides were counterstained with hematoxylin.
GATA3 labeling was scored on a scale of 0 to 4+ similar to that previously reported [10], with the extent of nuclear labeling graded as follows: 0 (0–5%), 1+ (6%–25%), 2+ (26%–50%), 3+ (51%–75%), and 4+ (>75%). We considered less than or equal to 5% staining as negative (score 0), as has also been reported [11,26]. Intensity of staining was recorded separately as weak, moderate, or strong, as previously reported [14]. Any intensity of staining with greater than 5% distribution was considered positive. Ductal epithelial cells in benign lobules were considered positive internal controls for GATA3 staining. The scoring was reviewed independently by 2 board-certified pathologists (A.C.M. and P.A.).
3. Results
3.1. Clinicopathologic characteristics
The cohort of 86 primary invasive ductal carcinomas consisted of 21 luminal A carcinomas, 7 luminal B carcinomas, 14 Her-2 carcinomas, and 44 TNCs. The 13 primary invasive metaplastic carcinomas were all triple negative for ER, PR, and Her-2; were intermediate to high grade; and contained spindled, squamous, chondroid, and/or osseous metaplastic components.
The clinicopathologic characteristics of the patients in the cohorts of paired PBCs and MBCs are seen in Table 1. In the cohort of surgically resected metastases (surgical pathology cases, n = 15), the age at diagnosis ranged from 33 to 58 years (mean, 44 years). Three patients had invasive lobular carcinoma, and the remaining 12 had invasive ductal carcinoma. The cohort was composed of 8 luminal A cases, 1 Her-2 case, 6 TNC cases, and no luminal B or luminal loss cases. The sites of metastasis were solitary and were brain (n = 6), lung (n = 5), gastrointestinal tract (n = 3), and ovary (n = 1). Three patients presented with metastatic disease at the time of initial diagnosis, and the average time to development of metastasis in the remaining 12 patients was 4.1 years. Excluding the patients who presented with metastases at the time of diagnosis, the remaining 12 patients received chemotherapy before the development of their metastases, including adriamycin, cyclophosphamide, taxols, and carboplatin. All but 1 patient with ER+ disease received either tamoxifen or an aromatase inhibitor.
In the cohort of metastases harvested at autopsy (autopsy pathology cases, n = 15), the age at diagnosis ranged from 28 to 59 years (mean, 44 years). One patient had invasive lobular carcinoma, and the remaining 14 had invasive ductal carcinoma. The cohort was composed of 5 luminal A cases, 5 luminal loss cases, 2 Her-2 cases, 3 TNC cases, and no luminal B cases. The metastatic sites at autopsy were multiple, and the number of sites per patient ranged from 2 to 18 (average, 11). All patients were refractory to multiple rounds of hormonal therapy and chemotherapy at the time of autopsy. The results of the ER, PR, and Her-2 staining of the PBCs performed on both surgical pathology and autopsy pathology TMAs were concordant with the results of the staining performed on whole sections at the time of diagnosis and documented in the surgical pathology reports.
3.2. GATA3 labeling
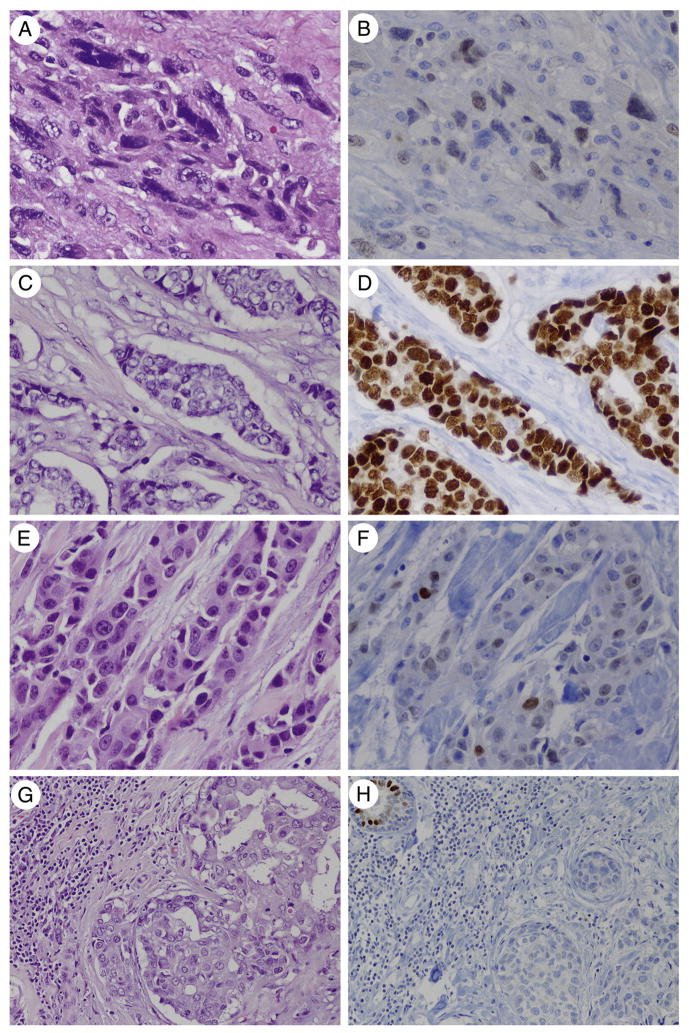
Among the cohort of nonmetastatic primary invasive ductal carcinomas, GATA3 labeling was seen overall in 67% (66/99) of cases (Table 2), including 100% of luminal A and luminal B carcinomas, 86% of Her-2 carcinomas, 43% of TNCs, and 54% of metaplastic carcinomas. Most positive cases (65%) showed 4+ staining. The intensity of staining ranged from moderate to strong in the luminal A and luminal B subgroups, and weak to moderate/strong in the Her-2 and triple-negative subgroups. Among the 7 metaplastic carcinomas with GATA3 labeling, most cases (86%) had weak labeling, with 1 case showing moderate labeling (Table 3). GATA3 positivity was seen in spindled (Fig. 1A, B), chondroid, and squamous metaplastic components. In comparison, stromal nuclear GATA3 labeling was seen in only 1 (3%) fibroepithelial neoplasm, which was a malignant phyllodes tumor with 1+, weak staining. GATA3 labeling was present in the benign luminal epithelial cells, serving as a positive internal control.
Table 2.
Immunohistochemical labeling of GATA3 in primary invasive ductal carcinomas (n = 99)
| Tumor type | n | GATA3
|
Total positive | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | |||
| Total | 99 | 33 | 8 | 7 | 8 | 43 | 66/99 (67%) |
| Luminal A–type carcinoma | 21 | 0 | 0 | 0 | 1 | 20 | 21/21 (100%) |
| Luminal B–type carcinoma | 7 | 0 | 0 | 0 | 0 | 7 | 7/7 (100%) |
| Luminal loss carcinoma | 0 | – | – | – | – | – | – |
| HER-2–type carcinoma | 14 | 2 | 2 | 1 | 2 | 7 | 12/14 (86%) |
| TNC | 44 | 25 | 3 | 5 | 3 | 8 | 19/44 (43%) |
| Metaplastic carcinomas | 13 | 6 | 3 | 1 | 2 | 1 | 7/13 (54%) |
NOTE. Nuclear GATA3 labeling is defined as 0 (0–5%), 1+ (6%–25%), 2+ (25%–50%), 3+ (50%–75%), and 4+ (>75%).
Table 3.
Immunohistochemical labeling of GATA3 in triple-negative metaplastic (sarcomatoid) breast carcinomas
| Case no. | Tumor size (cm) | Tumor grade | Metaplastic component(s) | GATA3 score (0–4+) |
|---|---|---|---|---|
| Case 1 | 3.5 | III | Spindled and squamous | 3 |
| Case 2 | 4 | III | Chondroid | 0 |
| Case 3 | 2.7 | III | Spindled and squamous | 0 |
| Case 4 | 13 | III | Spindled | 2 |
| Case 5 | 4.2 | II | Spindled | 0 |
| Case 6 | 2.3 | III | Chondroid | 1 |
| Case 7 | 5.5 | III | Spindled | 0 |
| Case 8 | 2.8 | III | Chondroid | 0 |
| Case 9 | 3 | III | Chondroid and osseous | 1 |
| Case 10 | 10.1 | III | Squamous | 3 |
| Case 11 | 1.6 | III | Chondroid and squamous | 4 |
| Case 12 | 5.5 | III | Chondroid | 1 |
| Case 13 | 3 | III | Spindled and squamous | 0 |
NOTE. Nuclear GATA3 labeling is defined as 0 (0–5%), 1+ (6%–25%), 2+ (25%–50%), 3+ (50%–75%), and 4+ (>75%).
Fig. 1.
A and B, GATA3 labeling in seen in ER-positive and ER-negative breast carcinomas. Weak-moderate and focal (2+) GATA3 labeling is seen in this primary metaplastic (sarcomatoid) carcinoma (×400), which was negative for ER, PR, and Her-2. C and D, Strong and diffuse (4+) GATA3 labeling is seen in this primary luminal A carcinoma (×400) from the surgical pathology cohort, as well as in the patient’s paired metastasis. E and F. Moderate and diffuse (3+) GATA3 labeling is seen in this primary TNC (×400) from the surgical pathology cohort, as well as in the patient’s paired metastasis. G and H, No GATA3 labeling is seen in this primary TNC (×200). F, A normal lobule is present as an internal control for GATA3 on the level used for the immunostain (hematoxylin and eosin and GATA3 immunostain).
GATA3 labeling was seen overall in 90% (27/30) of the PBCs in the paired PBC-MBC cohorts (Table 4), including 100% of luminal A (Fig. 1C–D), luminal loss, and Her-2 carcinomas and in 67% of TNCs (Fig. 1E–H). Most positive cases (78%) showed 4+ staining distribution. The intensity of staining ranged from weak to strong in the luminal A and Her-2 subgroups, from moderate to strong in the luminal loss subgroup, and from weak to moderate in the triple-negative subgroup. All 4 invasive lobular carcinomas were strongly and diffusely positive for GATA3.
Table 4.
Immunohistochemical labeling of GATA3 in primary mammary carcinomas from matched cohorts (SPC and APC, n = 30)
| Tumor type | n | GATA3
|
Total positive | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | |||
| Total | 30 | 3 | 3 | 2 | 1 | 21 | 27/30 (90%) |
| Luminal A–type carcinoma | 13 | 0 | 1 | 0 | 0 | 12 | 13/13 (100%) |
| Luminal B–type carcinoma | 0 | – | – | – | – | – | – |
| Luminal loss carcinoma | 5 | 0 | 0 | 0 | 0 | 5 | 5/5 (100%) |
| HER-2–type carcinoma | 3 | 0 | 0 | 0 | 0 | 3 | 3/3 (100%) |
| TNC | 9 | 3 | 2 | 2 | 1 | 1 | 6/9 (67%) |
NOTE. Nuclear GATA3 labeling is defined as 0 (0–5%), 1+ (6%–25%), 2+ (25%–50%), 3+ (50%–75%), and 4+ (>75%).
Abbreviations: SPC, surgical pathology cases; APC, autopsy pathology case.
GATA3 labeling was overwhelmingly maintained in the paired MBCs (Table 5). Overall, GATA3 labeling was seen in 87% (26/30) of the MBCs in the paired PBC-MBC cohorts, including 100% of luminal A, luminal loss, and Her-2 metastases and 56% of triple-negative metastases (Table 5). Most notably, GATA3 labeling was maintained with strong and diffuse staining in all of the luminal loss metastases (5/5), which had complete loss of ER and/or PR expression from the primary to the metastasis (Fig. 2). Loss of GATA3 labeling was seen in the metastases in 2 (7%) of 27 cases where the primary was positive. Both of these were triple negative and had 1+ labeling in the primary. Gain of GATA3 labeling was also seen in 1 case, which was triple negative and had 1+ labeling in the metastasis. Most positive cases (77%) showed 4+ staining distribution. The intensity of staining ranged from moderate to strong in the luminal A and luminal loss subgroups and from weak to moderate in the Her-2 and triple-negative subgroups.
Table 5.
Immunohistochemical labeling of GATA3 in metastatic mammary carcinomas from matched cohorts (SPC and APC, n = 30)
| Tumor type | n | GATA3
|
Total positive | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | |||
| Total | 30 | 4 | 2 | 4 | 0 | 20 | 26/30 (87%) |
| Luminal A–type carcinoma | 13 | 0 | 0 | 0 | 0 | 13 | 13/13 (100%) |
| Luminal B–type carcinoma | 0 | – | – | – | – | – | – |
| Luminal loss carcinoma | 5 | 0 | 0 | 0 | 0 | 5 | 5/5 (100%) |
| HER-2–type carcinoma | 3 | 0 | 0 | 2 | 0 | 1 | 3/3 (100%) |
| TNC | 9 | 4 | 2 | 2 | 0 | 1 | 5/9 (56%) |
NOTE. Nuclear GATA3 labeling is defined as 0 (0–5%), 1+ (6%–25%), 2+ (25%–50%), 3+ (50%–75%), and 4+ (>75%).
Abbreviations: SPC, surgical pathology cases; APC, autopsy pathology case.
Fig. 2.
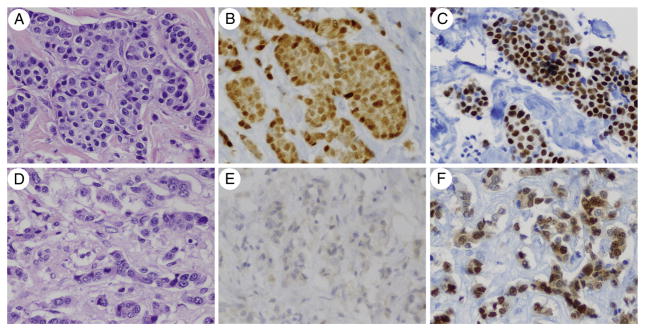
GATA3 labeling is seen in MBCs that have loss of ER and/or PR expression from the primary to the metastasis. The primary invasive ductal carcinoma (A) is diffusely positive for ER (B) and GATA3 (C). The paired MBC to the liver harvested at autopsy (D) shows complete loss of nuclear ER labeling (E), but intact and diffuse GATA3 labeling (F). Normal ovarian stroma harvested at autopsy showed positive nuclear ER labeling and served as a positive control for ER labeling in autopsy tissue (not shown) (hematoxylin and eosin, ER immunostain, and GATA3 immunostain; ×400).
4. Discussion
The GATA family of zinc-finger binding transcription factors regulates the lineage determination and differentiation of many tissue types including the mammary gland [1]. GATA3 plays an integral role in the differentiation of breast luminal epithelial cells and in the morphogenesis of the normal breast [2–5]. The expression of GATA3 in murine breast cancers models inhibits the transition from an epithelial to mesenchymal phenotype [27,28] and the development of metastases [29–31]. Human breast cancers contain recurrent GATA3 somatic mutations [32,33], and microarray data have linked GATA3 to the ER signaling pathway [34–38]. As expected for an ER-associated gene, GATA3 expression has been associated with improved prognosis and survival in patients with invasive breast carcinoma [39–41]. Intact GATA3 expression in PBC has been correlated with responsiveness to hormonal therapy [13,42] and a lack to response to cytotoxic chemotherapy [43]. However, GATA3 expression has not reliably been shown to be a prognostic factor independent from the tumor’s ER status [26,39].
IHC for GATA3 has been used in surgical pathology to support the diagnosis of urothelial carcinoma [11,12] or PBC [10,11]. GATA3 labeling in breast carcinomas has been reported primarily in ER-positive carcinomas [10,13,14], with varying degrees of expression reported in ER-negative carcinomas. Of the 2 studies that have specifically evaluated expression of GATA3 in triple-negative (ER/PR/Her-2 negative) carcinomas, Yang and Nonaka [10] found expression in 5% of cases, and Albergaria et al [14] found expression in 16% of cases. No prior study has evaluated GATA3 expression in MBCs and spindle cell carcinomas with full characterization of the ER, PR, and Her-2 status of the tumors.
As mentioned previously, reports of GATA3 expression in ER-negative breast carcinomas ranged from 5% [10] to 16% [14]. Our study has the highest percentage positivity yet reported of approximately 50%. The varying degrees of expression reported may be caused by the differing TMA methodology used (eg, core size and number of cores per tumor), as well as the varied cutoffs used to define GATA3 positivity in previous studies. The nuclear labeling cutoffs used in the literature to define GATA3 positivity have ranged from greater than 1% [10], 5% [11,26], 10% [15,43], 20% [13,40], and 30% [14]. In our study, we used a relatively low cutoff (5%) but also used multiple relatively large cores of each tumor on the TMAs, which likely accounts for the high percentage of labeling we obtained. In our clinical practice, we have noted that GATA3 immunohistochemical staining can be focal in whole sections of high-grade, ER-negative PBCs. Parenthetically, even focal staining for markers such as gross cystic disease fluid protein and mammaglobin is considered a positive result and supports a breast primary [44,45], and the same appears to be true for GATA3.
Further studies are needed to fully understand the role of GATA3 in breast carcinomas, particularly in the different molecular subtypes. For instance, although we and others have shown that GATA3 is expressed in TNCs [10,14], other murine models have suggested that GATA3 expression inhibits the triple-negative phenotype [28,31]. Furthermore, one previous study [11] suggested that GATA3 expression is decreased in luminal B carcinomas, whereas we and others [15] found strong and diffuse GATA3 expression, albeit in a small sample size. Additional studies are needed to assess the expression of GATA3 in other breast cancer subtypes such as invasive lobular carcinomas and apocrine carcinomas, as well as in other neoplasms in the differential diagnosis of breast carcinoma such as adnexal tumors. In addition, future studies could analyze GATA3 gene expression in the different molecular subtypes of breast cancer.
Our study demonstrates that GATA3 labeling is seen in more than 50% of metaplastic (sarcomatoid) carcinomas. Because GATA3 labeling was minimal and weak in phyllodes tumors, our results suggest that GATA3 labeling may help distinguish metaplastic carcinoma from phyllodes tumors, which are in the main differential diagnosis of a malignant spindle cell neoplasm of the breast. Although this distinction is not usually difficult on the resection specimen, the diagnosis can be challenging on the limited material of a core needle biopsy. Adding GATA3 to a panel that includes cytokeratins and p63 (for metaplastic carcinoma) and CD34 (for phyllodes tumor) may thus be useful.
In addition, several points can be made regarding the use of GATA3 in metastatic carcinomas. First, GATA3 immunolabeling is seen in both initial metastases sampled at surgery and end-stage metastases harvested at autopsy, and GATA3 labeling is unrelated to metastatic site (eg, brain versus lung). Second, GATA3 expression is maintained in primary TNCs and even in metastases of luminal cancers where ER expression is lost, suggesting that at least in some breast carcinomas, GATA3 expression is dissociated from ER expression and signaling. This should not be surprising, given that GATA3 is consistently expressed in urothelial carcinomas that are ER negative. Third, GATA3 can be particularly useful as a marker for MBC or to support the diagnosis of breast carcinoma in the evaluation of a metastasis of unknown primary. For instance, GATA3 may be useful in the differential of a CK7+ CK20− carcinoma, where both lung and breast carcinomas are possibilities. This is especially noteworthy because 43% of high-grade ER, PR, and Her-2–negative (triple-negative) breast ductal carcinomas express GATA3, and these tumors are often negative for other breast-specific immunomarkers such as gross cystic disease fluid protein and mammaglobin [44,45].
In summary, GATA3 expression is overwhelmingly maintained between matched primary and metastatic carcinomas in both early and terminal metastatic disease. GATA3 labeling is seen in more than 90% of ER-positive and Her-2–positive cases but is also strongly expressed in primary and metastatic carcinomas that lack ER expression. The published literature has shown that GATA3 expression is largely limited to carcinomas of the breast and bladder, and GATA3 is therefore a sensitive and fairly specific marker for MBC, particularly for cases which otherwise lack specific markers of mammary origin such as luminal loss and TNCs.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Funding source: The study was supported by Johns Hopkins Hospital Breast Cancer Research Fund (A.C.M.).
References
- 1.Zheng R, Blobel GA. GATA transcription factors and cancer. Genes Cancer. 2010;1:1178–88. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou J, Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol. 2010;222:42–9. doi: 10.1002/jcp.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 4.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouros-Mehr H, Kim JW, Bechis SK, Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008;20:164–70. doi: 10.1016/j.ceb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grote D, Souabni A, Busslinger M, Bouchard M. Pax2/8-regulated Gata3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- 7.Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–8. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 8.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;25:209–12. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman CK, Zhou P, Pasolli HA, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–22. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23:654–61. doi: 10.1038/modpathol.2010.38. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138:57–64. doi: 10.1309/AJCP5UAFMSA9ZQBZ. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–80. doi: 10.1097/01.pas.0000213438.01278.5f. [DOI] [PubMed] [Google Scholar]
- 13.Ciocca V, Daskalakis C, Ciocca RM, Ruiz-Orrico A, Palazzo JP. The significance of GATA3 expression in breast cancer: a 10-year follow-up study. Hum Pathol. 2009;40:489–95. doi: 10.1016/j.humpath.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Albergaria A, Paredes J, Sousa B, et al. Expression of FOXA1 and GATA-3 in breast cancer: the prognostic significance in hormone receptor-negative tumours. Breast Cancer Res. 2009;11:R40. doi: 10.1186/bcr2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell–related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res. 2010;16:876–87. doi: 10.1158/1078-0432.CCR-09-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subhawong AP, Subhawong T, Nassar H, et al. Most basal-like breast carcinomas demonstrate the same Rb−/p16+ immunophenotype as the HPV-related poorly differentiated squamous cell carcinomas which they resemble morphologically. Am J Surg Pathol. 2009;33:163–75. doi: 10.1097/PAS.0b013e31817f9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 19.Cimino-Mathews A, Subhawong AP, Elwood H, et al. Neural crest transcription factor Sox10 is preferentially expressed in triple negative and metaplastic breast carcinomas. Hum Pathol. 2012 doi: 10.1016/j.humpath.2012.09.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis IO, Cornelisse CJ, Schnitt SJ, et al. Invasive breast carcinoma. In: Tavassoli FA, Devilee P, editors. World Health Organization classification of tumours: tumours of the breast and female genital organs. Lyon, France: IARC Press; 2003. p. 37. [Google Scholar]
- 21.Bellow JP, Magro G. Fibroepithelial tumors. In: Tavassoli FA, Devilee P, editors. World Health Organization classification of tumours: tumours of the breast and female genital organs. Lyon, France: IARC Press; 2003. pp. 99–103. [Google Scholar]
- 22.Rosen PP. Fibroepithelial neoplasms. In: Rosen PP, editor. Rosen’s breast pathology. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. pp. 187–229. [Google Scholar]
- 23.Cimino-Mathews A, Hicks J, Illei PB, et al. Androgen receptor expression is usually maintained in initial surgically-resected breast cancer metastases, but often lost in terminal metastases found at autopsy. Hum Pathol. 2012;43:1003–11. doi: 10.1016/j.humpath.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JM, Fackler MJ, Halushka MK, et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–46. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cimino A, Halushka M, Illei P, et al. Epithelial cell adhesion molecule (EpCAM) is overexpressed in breast cancer metastases. Breast Cancer Res Treat. 2010;123:701–8. doi: 10.1007/s10549-009-0671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voduc D, Cheang M, Nielsen T. GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev. 2008;17:365–73. doi: 10.1158/1055-9965.EPI-06-1090. [DOI] [PubMed] [Google Scholar]
- 27.Yan W, Cao QJ, Arenas RB, Bentley B, Shao R. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J Biol Chem. 2010;285:14042–51. doi: 10.1074/jbc.M110.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tkocz D, Crawford NT, Buckley NE, et al. BRCA1 and GATA3 corepress FOXC1 to inhibit the pathogenesis of basal-like breast cancers. Oncogene. 2012;31:3667–78. doi: 10.1038/onc.2011.531. [DOI] [PubMed] [Google Scholar]
- 29.Kouros-Mehr H, Bechis SK, Slorach EM, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–52. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dydensborg AB, Rose AA, Wilson BJ, et al. GATA3 inhibits breast cancer growth and pulmonary breast cancer metastasis. Oncogene. 2009;28:2634–42. doi: 10.1038/onc.2009.126. [DOI] [PubMed] [Google Scholar]
- 31.Chu IM, Michalowski AM, Hoenerhoff M, et al. GATA3 inhibits lysyl oxidase-mediated metastases of human basal triple-negative breast cancer cells. Oncogene. 2012;31:2017–27. doi: 10.1038/onc.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usary J, Llaca V, Karaca G, et al. Mutation of GATA3 in human breast tumors. Oncogene. 2004;23:7669–78. doi: 10.1038/sj.onc.1207966. [DOI] [PubMed] [Google Scholar]
- 33.Banerji S, Cibulskis K, Rangel-Escareno C, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–9. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoch RV, Thompson DA, Baker RJ, Weigel RJ. GATA-3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer. 1999;84:122–8. doi: 10.1002/(sici)1097-0215(19990420)84:2<122::aid-ijc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 36.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 37.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson BJ, Giguère V. Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Mol Cancer. 2008;7:49. doi: 10.1186/1476-4598-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehra R, Varambally S, Ding L, et al. Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res. 2005;65:11259–64. doi: 10.1158/0008-5472.CAN-05-2495. [DOI] [PubMed] [Google Scholar]
- 40.Parikh P, Palazzo JP, Rose LJ, Daskalakis C, Weigel RJ. GATA-3 expression as a predictor of hormone response in breast cancer. J Am Coll Surg. 2005;200:705–10. doi: 10.1016/j.jamcollsurg.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Yoon NK, Maresh EL, Shen D, et al. Higher levels of GATA3 predict better survival in women with breast cancer. Hum Pathol. 2010;41:1794–801. doi: 10.1016/j.humpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang SH, Chen Y, Weigel RJ. GATA-3 as a marker of hormone response in breast cancer. J Surg Res. 2009;157:290–5. doi: 10.1016/j.jss.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Tominaga N, Naoi Y, Shimazu K, et al. Clinicopathological analysis of GATA3-positive breast cancers with special reference to response to neoadjuvant chemotherapy. Ann Oncol. 2012;23:3051–7. doi: 10.1093/annonc/mds120. [DOI] [PubMed] [Google Scholar]
- 44.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–13. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 45.Lewis GH, Subhawong AP, Nassar H, et al. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol. 2011;135:587–91. doi: 10.1309/AJCPMFR6OA8ICHNH. [DOI] [PMC free article] [PubMed] [Google Scholar]