Abstract
Background
Postoperative nausea and vomiting (PONV) are commonly feared after general anesthesia and can impact results. The primary aim of our study was to examine incidence and severity of PONV by investigating complete response, or absence of PONV, to prophylaxis used in patients undergoing DIEP flaps. Our secondary aims were definition of the magnitude of risk, state of the art of interventions, clinical sequelae of PONV, and interaction between these variables, specifically for DIEP patients.
Methods
A retrospective chart review occurred for 29 patients undergoing DIEP flap breast reconstruction from September 2007 to February 2008. We assessed known patient and procedure-specific risks for PONV after DIEPs, prophylactic antiemetic regimens, incidence, and severity of PONV, postoperative antiemetic rescues, and effects of risks and treatments on symptoms.
Results
Three or more established risks existed in all patients, with up to seven risks per patient. Although 90% of patients received diverse prophylaxis, 76% of patients experienced PONV, and 66% experienced its severe form, emesis. Early PONV (73%) was frequent; symptoms were long lasting (average 20 hours for nausea and emesis); and multiple rescue medications were frequently required (55% for nausea, 58% for emesis). Length of surgery and nonsmoking statistically significantly impacted PONV.
Conclusion
We identify previously undocumented high risks for PONV in DIEP patients. High frequency, severity, and refractoriness of PONV occur despite standard prophylaxis. Plastic surgeons and anesthesiologists should further investigate methods to optimize PONV prophylaxis and treatment in DIEP flap patients.
Postoperative nausea and vomiting (PONV) is overall a well-recognized entity among perioperative health care providers and also among patients.1,2 Anesthesia literature is rife with studies of PONV risks.2–4 Severe consequences of PONV have been documented repeatedly.3–7 Many antiemetic prevention and treatment regimens have been studied and proven as efficacious.8–13 However, patients express high levels of preoperative anxiety over potential PONV5 most simply because PONV persists.1,2 Multiple investigations into PONV have not translated reliably into clinically efficacious eradication of the disease. A unification of well-documented anesthesia principles regarding PONV risks and therapies with specific experiences of potentially special needs cohorts of surgical patients is necessary to improve overall perioperative experiences.
Risk factors, consequences, prevention and treatment regimens, and refractory PONV in other patient populations have been heavily studied in other surgical subspecialties. However, PONV in patients undergoing deep inferior epigastric perforator (DIEP) flap breast reconstruction has not been heavily studied. PONV risk is expected to be high at baseline. These surgeries tend to be lengthy, requiring identification, and dissection of blood vessels through the rectus abdominis muscle, preparation of recipient vessels in the chest, microsurgical anastomoses, abdominal donor site closure, and breast shaping, and almost all patients require postoperative opiates.14–17 Women are up to three times more susceptible to PONV than men,6,18,19 and breast surgeries are associated with PONV incidences ranging between 51 and 84% in the absence of prophylactic treatment.20–22 However, magnitude of risk, actual incidence of disease, severity of manifestations, current interventions, and clinical consequences of PONV are not well documented for DIEP patients. This may hinder efforts to decrease potentially debilitating PONV, especially since it has been shown to be refractory, even in well-defined situations.
The primary aim of our study was to examine incidence and severity of PONV disease by investigating complete response (CR), or absence of PONV, to prophylaxis used in patients undergoing DIEP flap breast reconstruction. Our secondary aims were definition of the magnitude of risk, state of the art of interventions, clinical sequelae of PONV, and interaction between these variables, specifically for DIEP patients. With ongoing improvements in surgical techniques, the management of potentially preventable negative sequelae such as PONV has become a rate-limiting step in patient choice of, recovery from, and satisfaction with microsurgical breast reconstruction. By addressing our goals, we hope to establish a foundation for further efforts to improve overall success in breast reconstruction.
METHODS
Patient Population
After obtaining approval from our Institutional Review Board, a retrospective chart review was completed for 30 consecutive patients meeting inclusion criteria having undergone immediate, staged, or delayed DIEP flap breast reconstruction by a single surgeon.
All patients were preoperatively counseled about a full range of reconstructive options including immediate, staged, or delayed prosthetic or autologous tissue based reconstruction. In the absence of contraindications, all patients opting for autologous reconstruction were planned for perforator based abdominal flap harvest without muscle sacrifice. Preoperative CT scanning was used 100% of the time to facilitate perforator choice, which was augmented intraoperatively with clinical data including perforator size, position, visual and Doppler examination of pulse and signal, and dermal bleeding and capillary refill with differential vessel clamping. Abdominal tissue dissection was performed without loupes. Amount and relative zone of abdominal tissue transferred was tailored based on perforator location and goal breast size and shape. Preservation of crossing motor nerves during flap harvest was pursued when at all feasible. Internal mammary artery and veins were used as recipient vessels, and all anastomoses were performed with the use of the operating microscope. Abdominal fasciotomies were closed with interrupted permanent, braided suture with or without biologic or prosthetic mesh onlay.
Exclusion criteria included use of more than one perforator or muscle during flap harvest, use of recipient vessels other than internal mammary, additional abdominal procedures such as hernia repair or rectus plication, and use of donor site other than the abdomen. One patient included in the study was excluded from analysis because she underwent a second general anesthetic in the early postoperative period for flap salvage.
Data Collection
Preoperative history and physical examination documents, anesthesia data records, postanesthesia care unit (PACU) records, and inpatient hospital charts were obtained and reviewed. Information on the cost of the medications was collected. Demographic data were recorded. Patient-specific and procedure-specific risk factors already well-established in the literature were identified in our patients including age, nonsmoking status for at least six months, history of PONV, inhalational anesthetic use, opioid use, and surgical duration. All preoperative and intraoperative prophylactic antiemetics, anesthetics, and rescue antiemetics were documented regarding dosages and timing of administrations. The presence, frequency, and timing of PONV or side effects of treatment were recorded. The primary efficacy endpoint, CR, was defined as no rescue administration of antiemetics postoperatively and no reported symptoms of PONV.
Surgical time was used as the clinically relevant surrogate for the full variety of DIEP procedures included in the study. Length of surgery was calculated using the surgery start and surgery end times from the anesthesia data record; the total time was rounded to the nearest half hour. Postoperative antiemetic dose timing was calculated as the number of hours from the surgery end time, rounding to the nearest integer. Nausea or vomiting within 6 hours of surgery end time was considered “early” PONV; otherwise, it was termed “delayed” PONV. This breakpoint was chosen to coincide largely with discharge from the PACU and is well supported in the anesthesia literature as a logical breakpoint.3,23
Postoperative Routine Care
Postoperative monitoring for flap viability included clinical and Doppler evaluation hourly for 24 hours, every other hour for an additional 24 hours, and every four hours during the remainder of the hospital stay. This was performed in an inpatient, non-ICU setting by experienced nursing and resident staff. All patients were advanced to a clear liquid diet in the early morning of postoperative day 1 when the DIEP flap was confirmed to appear viable. Patients were further advanced to a solid diet on postoperative day 2 or as tolerated, unless experiencing PONV, in which case solids were held until PONV resolved. In all patients, pain was controlled initially with a patient-controlled analgesia intravenous narcotic infusion pump with conversion to oral narcotics upon reliable tolerance of oral intake. Narcotics were continued for the entire observation period in all patients. All patients were out of bed to chair on postoperative day 1 and ambulated on postoperative day 2. Patients were only discharged with oral antiemetics if they had residual nausea and requested an antiemetic on discharge. Patients returned to the clinic 1 week after discharge for follow-up.
Statistical Analysis
Descriptive statistics (medians, means, and frequencies) were calculated. Time-related data was analyzed using Kaplan Meier methods.24 All analyses reported were performed with the use of SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Incidence and Severity of PONV
Patients underwent a full spectrum of unilateral and bilateral DIEP surgeries, including immediately after mastectomy, staged (with tissue expanders), and delayed (Table 1). Operative time ranged from 6 to 14.5 hours, with an average case length of 10 hours. There were no flap failures, and the one patient requiring return to the operating room for flap salvage was excluded from the analyses.
Table 1.
Patient, Procedural, and Pharmacological Variables
| N (%) | Range | Mean ±sd | Median [IQR] | ||
|---|---|---|---|---|---|
| Risk factors per patient | 3 | 2 (6.9%) | 2–6 | 3.6 ±0.9 | 3 [3–4] |
| 4 | 13 (44.8%) | ||||
| 5 | 10 (34.5%) | ||||
| 6 | 3 (10.3%) | ||||
| 7 | 1 (3.5%) | ||||
| Risk Factors | Female | 29 (100%) | |||
| Breast surgery | 29 (100%) | ||||
| Opioid therapy | 29 (100%) | ||||
| History of nausea/motion sickness | 12 (41.4%) | ||||
| Nonsmoker | 19 (65.5%) | ||||
| Age <40 years | 6 (20.7%) | 31–72 | 49.5 ±11.9 | 48 [40–58] | |
| Surgery >12 hour long | 9 (31.0%) | 6–14.5 | 10.1 ±2.5 | 10 [8–12.5] | |
| Inhalational anesthetic use | 23 (79.0%) | ||||
| Procedure type | Bilateral delayed | 1 (3.5%) | |||
| Bilateral immediate | 8 (27.6%) | ||||
| Bilateral staged | 3 (10.3%) | ||||
| Immediate and delayed | 3 (10.3%) | ||||
| Unilateral delayed | 4 (13.8%) | ||||
| Unilateral immediate | 7 (24.1%) | ||||
| Unilateral staged | 3 (10.3%) | ||||
| Preoperative antiemetic use | 12 (41.4%) | ||||
| Intraoperative antiemetic medication types | None | 6 (20.7%) | 0–2 | 1.1 ±0.7 | 1 [1–2] |
| 1 | 14 (48.3%) | ||||
| 2 | 9 (31.0%) | ||||
| Intraoperative antiemetic doses | None | 6 (20.7%) | 0–3 | 1.2 ±0.9 | 1 [1–2] |
| 1 | 13 (44.8%) | ||||
| 2 | 8 (27.6%) | ||||
| 3 | 2 (6.9%) | ||||
| Any prophylaxis | No | 3 (10.3%) | |||
| Yes | 26 (89.7%) | ||||
| Preop only | 3 (10.3%) | ||||
| Intraop only | 14 (48.3%) | ||||
| Both | 9 (31.0%) |
The large majority of patients (75.9%) experienced PONV, and most patients experienced the more severe presentation of emesis, not just nausea (65.5%). Most patients with PONV experienced it shortly after surgery, “early” PONV (72.7%), but most (68.2%) also experienced this in delayed fashion, “late” PONV, with symptoms beginning as late as postoperative day 2 (9.1% for nausea, 5.3% for emesis). Most patients experiencing PONV suffered multiple instances of the symptoms (54.7% for nausea, 58.1% for emesis), and symptoms lasted on average close to a whole day (mean 20 hours for both nausea and emesis). Over half of patients (54.5%) required multiple doses of medications to treat PONV symptoms when they occurred, and just under half (45.5%) required more than one type of medication for effect.
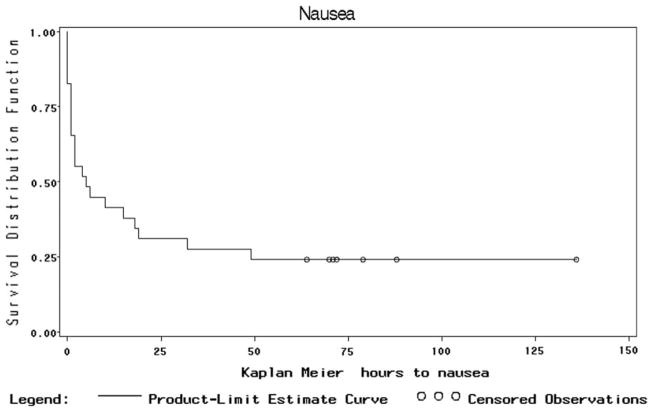
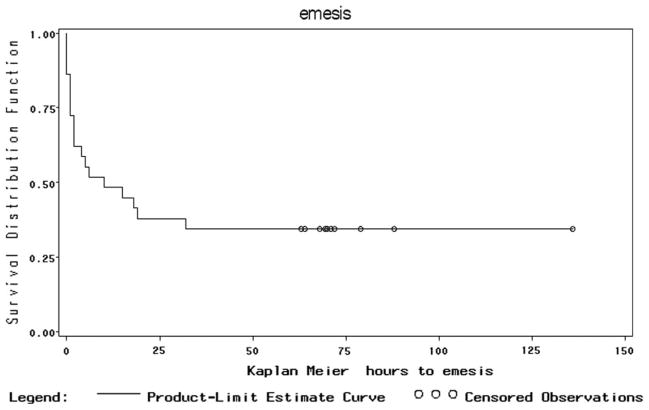
Figure 1 shows the Kaplan-Meier curve for cumulative percent of patients with CR (no documented PONV symptoms or rescue medication treatment of episodes of PONV), decreasing over time. Figure 2 shows similar data for the more severe form of PONV, emesis.
Figure 1.
Kaplan Meier plot of proportion without nausea or antiemesis medications (i.e., CR) demonstrating decrease in CR as episodes of nausea occur over time.
Figure 2.
Kaplan Meier Plot of Proportion without Emesis demonstrating decrease in CR as episodes of emesis occur over time.
Magnitude of Risk
Hundred percent of patients had the following three risk factors: were female, underwent breast surgery, and received opioid therapy; thus, all patients demonstrated at least three critical, known risk factors for PONV. Substantial percentages of patients demonstrated other known risk factors (Table 1). In fact, 93.1% of patients demonstrated four or more risk factors, and one patient demonstrated seven independent known risk factors for PONV.
State of the Art of Interventions
Antiemetic prophylaxis of some variety was very common and occurred in 89.7% of the study sample. However, the prophylactic regimen varied widely and in fact, was never the same between any of the patients. Few patients (10.3%) received only preoperative prophylaxis. Roughly, half of the patients received only intraoperative prophylaxis (48.3%), but nearly a third (31.1%) received both preoperative and intraoperative prophylactic medications (Table 1).
As is standard in adult patients, all 29 patients (100%) had an intravenous induction of anesthesia with propofol, but this was excluded from further analyses because the practice shown to decrease PONV in other studies was use of ongoing intraoperative propofol infusions not use during induction. Roughly, one third of patients received more than one type of prophylactic medication intraoperatively (31%), and roughly, one third of patients received more than one dose of prophylactic medication intraoperatively (34.5%). See Table 1.
Antiemetic drug costs were also investigated. Hospital cost of dexamethosone (4 mg) is $0.48, droperidol (5 mg) is $3.05, promethazine (6.25 mg) is $2.77, and transdermal scopolamine (3-day patch) is $10.58. Typically, patient costs are marked up approximately by a factor of 2–3.
Most patients required more than one dose of rescue medications to address PONV when it occurred (54.5%), and almost half of patients required use of more than one type of antiemetic to treat breakthrough PONV (47.5%). See Table 2.
Table 2.
Characteristics of PONV Episodes and Rescue Therapy
| N (%) | Range | Mean ±Std | Median [IQR] | ||
|---|---|---|---|---|---|
| Early PONV (<6 hour)a | 16 (72.7%) | ||||
| Late PONV (>6 hour)a | 15 (68.2%) | ||||
| Nausea | 22 (75.9%) | ||||
| Number of episodes of nauseaa | 1 | 10 (45.5%) | 1–11 | 2.6 ±2.5 | 2 [1–4] |
| 2 | 5 (22.7%) | ||||
| 3 | 1 (4.6%) | ||||
| 4 | 4 (18.2%) | ||||
| 8 | 1 (4.6%) | ||||
| 11 | 1 (4.6%) | ||||
| Timeb of first nausea | 0–49 | 7.7 ±12.4 | 2 [1–10] | ||
| Postop day of first nauseaa | 0 | 16 (72.7%) | 0–2 | 0.4 ±0.7 | 0 [0–1] |
| 1 | 4 (18.2%) | ||||
| 2 | 2 (9.1%) | ||||
| Timeb of LAST nausea | 0.01–97 | 28.1 ±26.1 | 23 [4–45] | ||
| Postop day of last nauseaa | 0 | 7 (31.8%) | |||
| 1 | 6 (27.3%) | 0.01–4 | 1.3 ±1.2 | 1 [0.01–2] | |
| 2 | 6 (27.3%) | ||||
| 3 | 2 (9.1%) | ||||
| 4 or more | 1 (4.6%) | ||||
| Nausea duration (hour) | 0–97 | 20.4 ±28.1 | 1.5 [0–38] | ||
| Emesis | 19 (65.5%) | ||||
| Number of episodes of emesisc | 1 | 8 (42.1%) | 1–9 | 2.7 ±2.4 | 2 [1–4] |
| 2 | 4 (21.1%) | ||||
| 3 | 1 (5.3%) | ||||
| 4 | 4 (21.1%) | ||||
| 8 | 1 (5.3%) | ||||
| 9 | 1 (5.3%) | ||||
| Timeb of first emesis | 0.01–32 | 6.3 ±8.8 | 2 [1–10] | ||
| Postop day of first emesisc | 0 | 14 (73.7%) | 0.01–2 | 0.3 ±0.6 | 0.01 [0.01–1] |
| 1 | 4 (21.1%) | ||||
| 2 | 1 (5.3%) | ||||
| Timeb of LAST emesis | 0.01–97 | 26.5 ±25.1 | 19 [4–45] | ||
| Postop day of LAST emesisc | 0 | 6 (31.6%) | 0.01–4 | 1.2 ±1.1 | 1 [0.01–2] |
| 1 | 6 (31.6%) | ||||
| 2 | 5 (26.3%) | ||||
| 3 | 1 (5.3%) | ||||
| 4 | 1 (5.3%) | ||||
| Emesis duration (hour) | 0–97 | 20.2±27.3 | 2 [0–44] | ||
| Number of doses of rescue medsa | 1 | 10 (45.5%) | 1–11 | 2.7 ±2.5 | 2 [1–4] |
| 2 | 3 (13.6%) | ||||
| 3 | 3 (13.6%) | ||||
| 4 | 4 (18.2%) | ||||
| 8 | 1 (4.6%) | ||||
| 11 | 1 (4.6%) | ||||
| Number of rescue medsa | 1 | 12 (54.6%) | |||
| 2 | 9 (40.9%) | ||||
| 3 | 1 (4.6%) | ||||
| Discharged w/antiemetic meds | Yes | 1 (3.5%) | |||
| Discharge Day | 3 | 17 (58.6%) | 3–8 | 3.9 ±1.4 | 3 [3–4] |
| 4 | 5 (17.2%) | ||||
| >4 | 7 (24.2%) |
Percent of the 22 patients with PONV.
Hours after end of surgery.
Percent of the 19 patients with emesis.
Clinical Sequelae of PONV
PONV delayed discharge beyond the standard three to four days in roughly one quarter of patients (24.2%). Evaluation of the reason for delay in these patients confirmed that this was due to persistent, refractory PONV. One patient still had symptoms upon discharge. Table 2 demonstrates these characteristics of PONV episodes.
Interaction Between Variables
Established general risk factors and DIEP specific potential risk factors were assessed independently and in multiple logistic regressions for impact on outcomes broken down into overall PONV, early PONV, late PONV, and the severe form of PONV-emesis. Of all assessed interactions, the only statistically significant finding was that surgery greater than twelve hours increased early PONV (Table 3). Factors found not to have a significant effect on CR in our patients included history of motion sickness/PONV, nonsmoking, age <40, number of risk factors possessed by each patient, and immediate reconstruction at time of mastectomy. Likewise, no prophylactic regimen statistically significantly affected CR, including preoperative antiemetics, intraoperative antiemetics, and intraoperative propofol use.
Table 3.
Associations Between Risk Factors and Outcome =Early Nausea
| Risk factor | N (%) CR | N (%) early nausea | P-value | |
|---|---|---|---|---|
| Number of risk factors per patient | 2 | 0 | 2 (100) | 0.7610 |
| 3 | 6 (46) | 7 (54) | ||
| 4 | 5 (50) | 5 (50) | ||
| 5 | 1 (33) | 2 (67) | ||
| 6 | 1 (100) | 0 | ||
| History of motion sickness/PONV | No | 7 (41) | 10 (59) | 0.7163 |
| Yes | 6 (50) | 6 (50) | ||
| Nonsmoker | No | 4 (40) | 6 (60) | 1.0 |
| Yes | 9 (47) | 10 (53) | ||
| Age <40 years | No | 12 (52) | 11 (48) | 0.1834 |
| Yes | 1 (17) | 5 (83) | ||
| Surgery >12 hours | No | 6 (30) | 14 (70) | 0.0405a |
| Yes | 7 (78) | 2 (22) | ||
| Immediate reconstruction | No | 4 (36) | 7 (64) | 0.7021 |
| Yes | 9 (50) | 9 (50) | ||
| Preoperative antiemetic use | No | 9 (53) | 8 (47) | 0.4515 |
| Yes | 4 (33) | 8 (67) | ||
| Intraoperative antiemetic medication types | 0 | 3 (50) | 3 (50) | 0.7929 |
| 1 | 7 (50) | 7 (50) | ||
| 2 | 3 933) | 6 (67) | ||
| Intraoperative antiemetic numbers of doses | 0 | 3 (50) | 3 (50) | 0.7947 |
| 1 | 6 (46) | 7 (54) | ||
| 2 | 4 (50) | 4 (50) | ||
| 3 | 0 | 2 (100) | ||
| Intraoperative propofol use | No | 12 (50) | 12 (50) | 0.3432 |
| Yes | 1 (20) | 4 (80) | ||
| Any prophylaxis | No | 2 (67) | 1 (33) | 0.5731 |
| Yes | 11 (42) | 15 (58) |
Statistically significant, P <0.05.
We also evaluated the same known and potential risk factors for their impacts on what we considered markers for refractoriness of the nausea and emesis symptoms, namely number of episodes of symptoms, numbers of doses of treatment medications, and numbers of different antiemetics used. Statistically significant effects of non-smoking status on number of episodes of nausea and number of doses of antiemetics to treat nausea were noted (P =0.039 and 0.035, respectively). Additionally, surgeries >12 hours in length affected the day of onset of emesis (P =0.037), pushing back the onset of emesis from postoperative day 0 (for the majority of patients with <or =12 hour surgeries) to postoperative day 1 (for the majority of patients with longer surgeries). See Tables 4 and 5.
Table 4.
P-Values of Associations Between Risk Factors and Outcomes Including Number of Episodes of Nausea, Number of Doses of Antiemetics, and Number of Different Antiemetic Medications
| Risk factor | Episodes of nausea | Doses of antiemetics | Number of different medications |
|---|---|---|---|
| Number of risk factors per patient | 0.6107 | 0.2292 | 1.0 |
| History of motion sickness | 0.9065 | 0.9158 | 0.8200 |
| Nonsmoker | 0.0394a | 0.0347a | 1.0 |
| Age <40 years | 0.3962 | 0.4395 | 0.6992 |
| Surgery >12 hours | 0.6984 | 0.3406 | 0.2959 |
| Immediate reconstruction | 0.9696 | 0.1330 | 1.0 |
| Preoperative antiemetic use | 0.7872 | 0.8085 | 0.5096 |
| Intraoperative antiemetic medication types | 0.7295 | 0.8553 | 0.9345 |
| Intraoperative antiemetic numbers of doses | 0.4725 | 0.7206 | 1.0 |
| Intraoperative propofol use | 0.8770 | 0.7314 | 0.6752 |
| Any prophylaxis | 0.5887 | 0.8052 | 1.0 |
Statistically significant, P <0.05.
Table 5.
P-Values of Associations Between Risk Factors and Outcomes Defined as Number of Episodes of Emesis, Postoperative Day of First Emesis, Postoperative Day of Last Emesis, Need for Antiemetic Discharge Medications, and Postoperative Day of Discharge
| Risk factor | Episodes of emesis | First day of emesis | Last day of emesis | Discharge medications | Day of discharge |
|---|---|---|---|---|---|
| Number of risk factors per patient | 0.5278 | 0.5201 | 0.0981 | 0.5517 | 0.6628 |
| History of motion sickness | 0.3428 | 0.2621 | 0.0940 | 0.4138 | 1.0 |
| Nonsmoker | 0.1219 | 0.5800 | 0.4278 | 1.0 | 0.0627 |
| Age <40 years | 0.4840 | 0.6244 | 1.0 | 1.0 | 0.7595 |
| Surgery >12 hours | 0.8844 | 0.0374a | 0.7678 | 1.0 | 0.1939 |
| Immediate reconstruction | 1.0 | 1.0 | 0.0546 | 1.0 | 0.8383 |
| Preop antiemetic use | 0.8933 | 1.0 | 0.7470 | 1.0 | 0.1162 |
| Intraop antiemetic medication types | 0.4487 | 1.0 | 0.6609 | 1.0 | 0.5172 |
| Intraop antiemetic numbers of doses | 0.2650 | 0.9422 | 0.2937 | 1.0 | 0.1845 |
| Intraop propofol use | 0.8679 | 1.0 | 0.8142 | 1.0 | 0.8139 |
| Any prophylaxis | 1.0 | 0.4678 | 0.2047 | 1.0 | 0.1300 |
Statistically significant, P <0.05. Of those with longer surgery >12 hours, 1 (25%) vomit on postoperative day 0, 2 (50%) on day 1, and 1 (25%) on day 2. Of those with shorter surgery 12 hours or less, 87% vomit on day 0, 13% on day 1, 0% on day 2.
DISCUSSION
Risk factors have been heavily studied. Patient-specific risk factors for PONV include young age (<40 years), nonsmoking status since nicotine has antiemetic properties, history of PONV or motion sickness, and female gender.2 Procedure-specific risk factors for PONV include use of specific classes of anesthetic agents, intraoperative and postoperative opioid use, certain operative sites, and increased duration of anesthesia.25,26 Certain types of surgery such as intraabdominal, major gynecologic, laparoscopic, ear, nose, throat, ophthalmologic, and breast surgery increase PONV risk through a variety of mechanisms such as promotion of ileus, disruption of cochlear equilibrium, and interruption of visual-ocular coordination.2,3,27
Our data demonstrate that patients undergoing DIEP breast reconstruction are, as expected, at very high risk for PONV. Ninety-three percent of patients had double the number of established risk factors to be considered “high risk” at our institution based on anesthesiology protocols. This highlights a need to refine further stratifications of “high risk” categories since no subdivisions of “high risk” currently exist.
Prevention and treatment regimens have been heavily studied. Since patient-specific and surgical-procedure-associated risk factors are largely unchangeable, pharmacological interventions have been the focus of study for decreasing PONV. Less PONV in the early postoperative period is associated with total intravenous anesthesia with propofol, a known antiemetic, compared to inhalational agents.10,12,13,27 Guideline-recommended antiemetic agents include serotonin (5-HT3) receptor antagonists, dexamethosone, droperidol, phenothiazines, and transdermal scopolamine, and “high risk” patients (those with at least 2 risk factors) are expected to receive multimodal pharmacological perioperative prevention.3,8,9 Yet specific combination preoperative and intraoperative regimens have not demonstrated relative superiority.1,3
Our patients received standard of care prophylaxis.1,3,10,12,13 Our data supports the previously documented variability of prophylactic regimens, and therefore, does not demonstrate a bias among our anesthesia teams toward particular regimens. With 76% prevalence of PONV symptoms despite widespread (90%) use of well-recognized prophylaxis, these patients may be part of one of the most refractory to prophylaxis populations yet defined when compared globally to other surgical populations.20–22,28–30
Refractory PONV has been heavily studied. Despite, the widespread use of established antiemetics with documented efficacy, nausea, and vomiting continue to be common symptoms experienced by patients undergoing general anesthesia for surgical procedures.1,2 Reported incidences of PONV range between 25 and 30% in general surgical populations despite routine prophylaxis 4,31,32 and have been found to be as high as 70% in high-risk patients.28–30 PONV can be a more dreaded postoperative complication by patients than severe pain.5
Our data demonstrated both severity as well as tenacity of PONV with high rates of emesis rather than just nausea, early onset of symptoms, high numbers of episodes of symptoms, and late resolution of symptoms despite multiple prophylaxis and treatment doses and medication types documented as effective in the existing body of literature.3,8–10,12,13,27
Consequences have been heavily studied. PONV can delay discharge of surgical patients since it occurs most frequently during the first 72 postoperative hours.3 Additionally, persistent retching and vomiting can cause venous hypertension, tension on suture lines, and bleeding under skin flaps, which are particularly unwanted events after microsurgical breast reconstruction. Other serious complications that have been attributed to PONV include pulmonary aspiration, dehydration, and electrolyte imbalance.4,6,24,33 Equally important are the high levels of patient dissatisfaction and discomfort, especially related to elective abdominal surgery, associated with PONV.5 White et al. defined functional interference with psychosocial activities from nausea and vomiting, showing adverse function over a three-day period with relation to patient appetite, sleep, physical activities, and enjoyment of life.7
We also confirmed previous reports that PONV has direct clinical implications,3–7 specifically delay of discharge from the hospital in our sample. In addition to the intangible distress to patients, this can be a very expensive consequence of unchecked PONV disease. All of these findings emphasize the increased magnitude of the problem as well as its urgency, specifically in the DIEP flap population, compared with more generalized surgical populations previously studied.20–22,28–30
Even with a small sample size, the extreme nature of our findings lends credibility to their significance. We have established the DIEP flap population as extraordinarily susceptible to and affected by PONV. We began preliminary investigations into risk stratification of this special needs group. Our logistic regressions for the most part did not support statistically significant conclusions in this regard. It may be that an association does not exist, or that our small sample size limited our analyses. The variety of treatments also makes analysis of data less powerful. Since our data demonstrates that we are clearly losing the battle against this pervasive, pernicious disease, we must focus future efforts in this direction.
In our study, operative time did demonstrate statistically significant impacts on PONV. Length of surgery appears to affect the timing of PONV symptoms, rather than the presence or absence of these. Surgeries over 12 hours predispose to early nausea but delayed onset of emesis. This is in contrast to shorter surgeries where nausea onset is more commonly later but is more closely followed by emesis on the same day. We assessed whether immediate reconstruction at the time of mastectomy, because of the amount of surgery performed on the breast, might predispose to PONV and actually be an avoidable risk. With the exception of predisposition to longer surgical times, our data did not support this hypothesis.
Our data demonstrated that non-smoking predisposes to more refractory symptoms, demonstrating a statistically significant predisposition to increased numbers of episodes of nausea and increased numbers of doses needed to treat nausea symptoms. This relationship did not, however, translate to the more severe presentation, emesis.
For the most part, however, risk factors for PONV are relatively fixed and not able to be modified in any individual patient in preparation for upcoming surgery. Therefore, attention of future efforts may need to center on identifying which pharmacological interventions work best in which strata of our high risk population or on creative methods of risk modification.
These findings are likely generalizable to other series of patients undergoing DIEP surgeries, because full ranges of unilateral and bilateral procedures and sequencing (immediate, staged, and delayed) are represented by our population. Reported surgery lengths as well as postoperative care on the nursing floors is in keeping with widely accepted practices. Limitations of our study include the manner in which the patients were selected in retrospective fashion with a fairly small sample size. These factors, combined with the wide variability in the evaluated categories, make it difficult to standardize the patient population. PONV is subjective, unique to the individual, may have a strong psychosocial element, and is not guaranteed to be recorded in the medical record consistently. We had to rely upon a combination of reported symptoms and documented rescue therapy to determine postoperative episodes of PONV. We did not evaluate other types of autologous breast reconstruction or DIEP reconstructions of areas other than the breast.
CONCLUSION
To our knowledge, the common, potentially preventable, and definitely treatable complication of PONV has not been adequately studied in patients undergoing DIEP breast reconstructions. Multiple facets of our study demonstrate the absolute importance of obtaining increased understanding in this area. This retrospective patient chart review is clinically relevant in that it elucidates the need for nausea and vomiting prevention strategy for DIEP flap patients that is more comprehensive than the standard prophylactic mechanisms currently in use. Prospective, placebo-controlled, blinded studies comparing various pharmaceutical agents with antiemetic properties would be optimal. PONV need not be tolerated by patients or seen as inevitable by providers, and therefore, should be monitored and recorded, as are temperature, heart rate, blood pressure, respiratory rate, oxygen saturation, and pain. PONV should be actively identified and treated.
Acknowledgments
The authors thank Elaine P. Henze, BJ, ELS, Medical Editor and Director of Editorial Services, Department of Orthopaedic Surgery, The Johns Hopkins University, and Marta Gilson, Ph.D., formerly with The Johns Hopkins University Center for Surgical Trials and Outcomes Research, for their assistance with this manuscript.
References
- 1.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. NEJM. 2004;350:2441–2451. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White PF. Prevention of postoperative nausea and vomiting-a multimodal solution to a persistent problem. NEJM. 2004;350:2511–2512. doi: 10.1056/NEJMe048099. [DOI] [PubMed] [Google Scholar]
- 3.Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107:439–444. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]
- 4.Layeeque R, Siegel E, Kass R, Henry-Tillman RS, Colvert M, Mancino A, Klimberg VS. Prevention of nausea and vomiting following breast surgery. Am J Surg. 2006;191:767–772. doi: 10.1016/j.amjsurg.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Vance JP, Neill RS, Norris W. The incidence and aetiology of postoperative nausea and vomiting in a plastic surgical unit. Br J Plast Surg. 1973;26:336–339. doi: 10.1016/s0007-1226(73)90035-0. [DOI] [PubMed] [Google Scholar]
- 7.White PF, O’Hara JF, Roberson CR, Wender RH, Candiotti KA. The impact of current antiemetic practices on patient outcomes: A prospective study on high-risk patients. Anesth Analg. 2008;107:452–458. doi: 10.1213/ane.0b013e31817b842c. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Anesthesiologists Task Force on Postanesthetic Care. Practice guidelines for postanesthetic care: A report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2002;96:742–752. doi: 10.1097/00000542-200203000-00033. [DOI] [PubMed] [Google Scholar]
- 9.American Society of PeriAnesthesia Nurses PONV/PDNV Strategic Work Team. ASPAN’S evidence-based clinical practice guideline for the prevention and/or management of PONV/PDNV. J Perianesth Nurs. 2006;21:230–250. doi: 10.1016/j.jopan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Doze VA, Shafer A, White PF. Propofol-nitrous oxide versus thiopental-isoflurane-nitrous oxide for general anesthesia. Anesthesiology. 1988;69:63–71. doi: 10.1097/00000542-198807000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Habib AS, White WD, Eubanks S, Pappas TN, Gan TJ. A randomized comparison of a multimodal management strategy versus combination antiemetics for the prevention of postoperative nausea and vomiting. Anesth Analg. 2004;99:77–81. doi: 10.1213/01.ANE.0000120161.30788.04. [DOI] [PubMed] [Google Scholar]
- 12.Lebenbom-Mansour MH, Pandit SK, Kothary SP, Randel GI, Levy L. Desflurane versus propofol anesthesia: A comparative analysis in outpatients. Anesth Analg. 1993;76:936–941. doi: 10.1213/00000539-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Price ML, Walmsley A, Swaine C, Ponte J. Comparison of a total intravenous anaesthetic technique using a propofol infusion, with an inhalational technique using enflurane for day case surgery. Anaesthesia. 1988;43(Suppl):84–87. doi: 10.1111/j.1365-2044.1988.tb09081.x. [DOI] [PubMed] [Google Scholar]
- 14.Blondeel PN. One hundred free DIEP flap breast reconstructions: A personal experience. Br J Plast Surg. 1999;52:104–111. doi: 10.1054/bjps.1998.3033. [DOI] [PubMed] [Google Scholar]
- 15.Nahabedian MY, Momen B, Galdino G, Manson PN. Breast Reconstruction with the free TRAM or DIEP flap: Patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475. doi: 10.1097/00006534-200208000-00015. discussion 476–467. [DOI] [PubMed] [Google Scholar]
- 16.Shafighi M, Constantinescu MA, Huemer GM, Olariu R, Bonel HM, Banic A, Ramakrishnan V. The extended diep flap: Extending the possibilities for breast reconstruction with tissue from the lower abdomen. Microsurgery. 2013;33:24–31. doi: 10.1002/micr.21975. [DOI] [PubMed] [Google Scholar]
- 17.Venkat R, Lee JC, Rad AN, Manahan MA, Rosson GD. Bilateral autologous breast reconstruction with deep inferior epigastric artery perforator flaps: Review of a single surgeon’s early experience. Microsurgery. 2012;32:275–280. doi: 10.1002/micr.21948. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: Assessing risk factors for nausea and vomiting. Anesth Analg. 1994;78:7–16. doi: 10.1213/00000539-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Myles PS, Hunt JO, Moloney JT. Postoperative ’minor’ complications. Comparison between men and women. Anaesthesia. 1997;52:300–306. doi: 10.1111/j.1365-2044.1997.89-az0091.x. [DOI] [PubMed] [Google Scholar]
- 20.Hammas B, Thorn SE, Wattwil M. Superior prolonged antiemetic prophylaxis with a four-drug multimodal regimen - comparison with propofol or placebo. Acta Anaesthesiol Scand. 2002;46:232–237. doi: 10.1034/j.1399-6576.2002.460302.x. [DOI] [PubMed] [Google Scholar]
- 21.Oddby-Muhrbeck E, Jakobsson J, Andersson L, Askergren J. Postoperative nausea and vomiting. A comparison between intravenous and inhalation anaesthesia in breast surgery. Acta Anaesthesiol Scand. 1994;38:52–56. doi: 10.1111/j.1399-6576.1994.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 22.Sadhasivam S, Saxena A, Kathirvel S, Kannan TR, Trikha A, Mohan V. The safety and efficacy of prophylactic ondansetron in patients undergoing modified radical mastectomy. Anesth Analg. 1999;89:1340–1345. doi: 10.1097/00000539-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Wu CL, Elkassabany N, Krug CE, Parker SD, Fleisher LA. Does the routine prophylactic use of antiemetics affect the incidence of postdischarge nausea and vomiting following ambulatory surgery? A systematic review of randomized controlled trials. Anesthesiology. 2003;99:488–495. doi: 10.1097/00000542-200308000-00033. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–184. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Leslie K, Myles PS, Chan MT, Paech MJ, Peyton P, Forbes A, McKenzie D. Risk factors for severe postoperative nausea and vomiting in a randomized trial of nitrous oxide-based vs nitrous oxide-free anaesthesia. Br J Anaesth. 2008;101:498–505. doi: 10.1093/bja/aen230. [DOI] [PubMed] [Google Scholar]
- 27.Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: A retrospective database analysis. Curr Med Res Opin. 2006;22:1093–1099. doi: 10.1185/030079906X104830. [DOI] [PubMed] [Google Scholar]
- 28.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Gan TJ. Postoperative nausea and vomiting-can it be eliminated? JAMA. 2002;287:1233–1236. doi: 10.1001/jama.287.10.1233. [DOI] [PubMed] [Google Scholar]
- 30.Gan TJ, Ginsberg B, Grant AP, Glass PS. Double-blind, randomized comparison of ondansetron and intraoperative propofol to prevent postoperative nausea and vomiting. Anesthesiology. 1996;85:1036–1042. doi: 10.1097/00000542-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–243. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 32.Watcha MF. Postoperative nausea and emesis. Anesthesiol Clin North Am. 2002;20:709–722. doi: 10.1016/s0889-8537(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 33.Pan PH, Lee SC, Harris LC. Antiemetic prophylaxis for postdischarge nausea and vomiting and impact on functional quality of living during recovery in patients with high emetic risks: A prospective, randomized, double-blind comparison of two prophylactic antiemetic regimens. Anesth Analg. 2008;107:429–438. doi: 10.1213/ane.0b013e318172f992. [DOI] [PubMed] [Google Scholar]