Abstract
These NCCN Guidelines Insights highlight the important updates specific to the management of HER2-positive metastatic breast cancer in the 2013 version of the NCCN Clinical Practice Guidelines in Oncology for Breast Cancer. These include new first-line and subsequent therapy options for patients with HER2-positive metastatic breast cancer.
Overview
Breast cancer is the most common malignancy in women in the United States and is second only to lung cancer as a cause of cancer death. The American Cancer Society estimates that 234,580 Americans will be diagnosed with breast cancer and 40,030 will die of the disease in the United States in 2013.1 The therapeutic options for patients with noninvasive or invasive breast cancer are complex and varied. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Breast Cancer include up-to-date guidelines for the clinical management of patients with carcinoma in situ, invasive breast cancer, Paget disease, phyllodes tumor, inflammatory breast cancer, and breast cancer during in pregnancy (to view the complete and most recent version of these guidelines, visit NCCN.org). These NCCN Guidelines Insights highlight the important updates/changes specific to the management of HER2-positive metastatic breast cancer in the 2013 version of the NCCN Guidelines. These include clinical data and NCCN recommendations regarding the new therapeutic options, pertuzumab and ado-trastuzumab emtansine (T-DM1), available for patients with HER2-positive metastatic breast cancer.
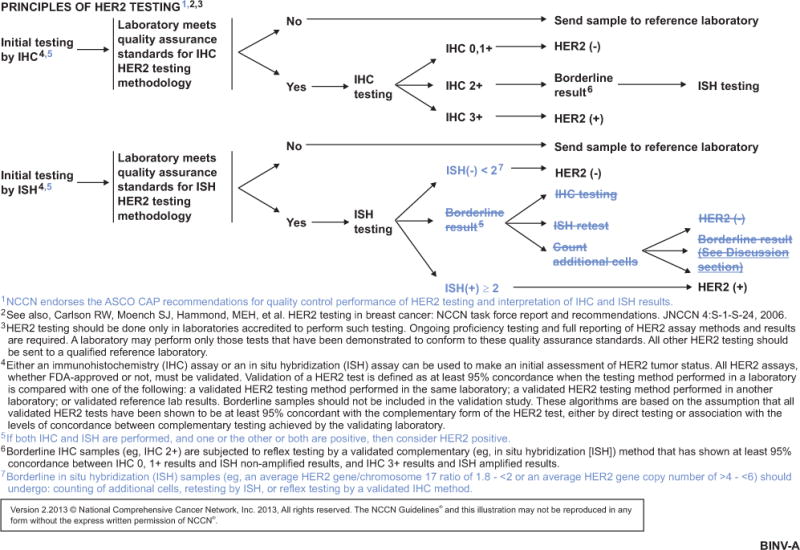
NCCN Guidelines Insights, Breast Cancer, Version 3.2013.
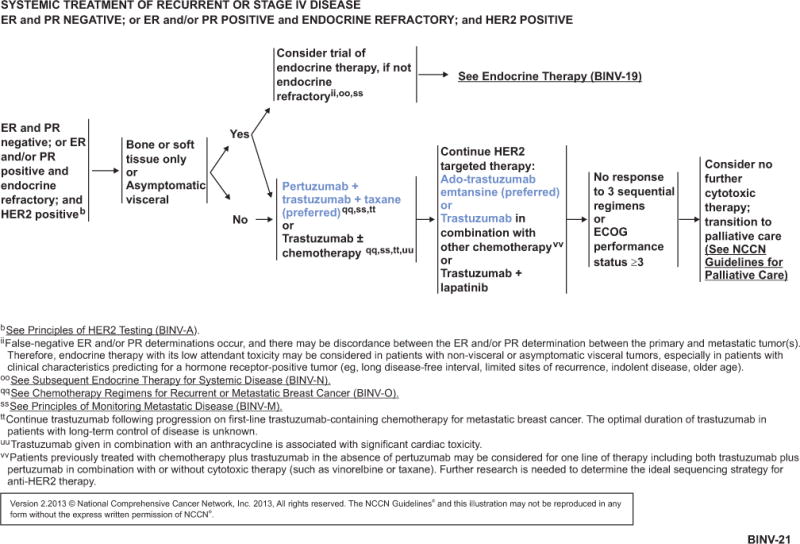
NCCN Guidelines Insights, Breast Cancer, Version 3.2013.
HER2-Targeted Therapy for Stage IV or Recurrent Metastatic Disease
HER2 is a proto-oncogene located on chromosome 17 and is amplified in 15% to 20% of breast carcinomas.2 Before the approval of trastuzumab, amplification of HER2 was considered a poor prognostic factor in patients with metastatic breast cancer. With the introduction of trastuzumab, the outcomes of patients with HER2-postive metastatic breast cancer dramatically improved.3 However, in most of these patients, the disease ultimately develops resistance to trastuzumab; therefore, effective targeted therapies are needed. In an attempt to further improve the outcomes of these patients, newer drugs targeting the HER2 pathway, including lapatinib, pertuzumab, and ado-trastuzumab (T-DM1), have been developed and added to the current standard of care.
HER2 Testing
Adequate standardization and validation of HER2 assays used in clinical practice is a concern, and data suggest that false-positive determinations are common.2,4–7 The NCCN Breast Cancer Panel endorses the ASCO/College of American Pathologists recommendations for quality control performance of HER2 testing and interpretation of results. The panel recommends that HER2 testing be performed only in laboratories accredited to perform such testing.
Either the immunohistochemistry (IHC) with the anti-HER2 antibodies or in situ hybridization (ISH) assay can be used to make an initial assessment of HER2 status. The NCCN Breast Cancer Panel recommends selecting patients for HER2-targeted therapy if their tumors are positive for HER2 by either ISH or IHC. The NCCN Guidelines consider IHC 3+ and HER2 gene/chromosome 17 ratio of 2 or greater as HER2-positive. According to the guidelines, borderline IHC samples (eg, IHC 2+) should be subjected to reflex testing by a validated complementary method, such as ISH, that has shown at least 95% concordance between IHC 0, 1+ results and ISH nonamplified results, and immunohistochemistry 3+ results and ISH amplified results. Also, it is recommended that borderline ISH results (average HER2/chromosome 17 ratio of 1.8 to <2 or average HER2 gene copy number >4 to <6) should undergo counting of additional cells, retesting by ISH, or reflex testing by a validated immunohistochemistry method (see BINV-A, page 755).

NCCN Guidelines Insights, Breast Cancer, Version 3.2013.
Pertuzumab
Pertuzumab is a recombinant humanized monoclonal antibody that inhibits the ligand-dependent dimerization of HER2 and its downstream signaling. Pertuzumab and trastuzumab bind to different epitopes of the HER2 receptor and have complementary mechanisms of action. Therefore, the rationale for administering the drugs together is to achieve a more powerful blockage of the HER2 pathway. This was demonstrated to be true in tumor models and in humans, wherein combining pertuzumab with trastuzumab provided a greater overall antitumor effect than either alone.8,9
In a randomized, double-blind, phase III study (CLEOPATRA), 808 women with HER2-positive metastatic breast cancer were randomized to receive trastuzumab and docetaxel with or without pertuzumab as their first-line treatment.10 The results demonstrated a 6.1-month improvement in median progression-free survival with the addition of pertuzumab (12.4 vs 18.5 months; hazard ratio [HR] for progression or death, 0.62; 95% CI, 0.51–0.75; P<.001). In addition, a strong trend was seen toward an overall survival benefit with pertuzumab, with a 34% reduction in the risk of death (HR, 0.66; 95% CI, 0.52–0.84; P=.0008). The median overall survival was 37.6 months in the nonpertuzumab group and had not yet been reached at the time of analysis in the group treated with pertuzumab.11 Notably, no significant difference was seen in health-related quality of life or toxicities between the treatment arms, including no increase in either symptomatic or asymptomatic cardiac dysfunction.12,13

NCCN Guidelines Insights, Breast Cancer, Version 3.2013.
Phase II trials have also assessed the activity and tolerability for pertuzumab, pertuzumab with trastuzumab, and other regimens combining pertuzumab and trastuzumab together with other active cytotoxics, such as paclitaxel and vinorelbine14,15 (ClinicalTrials.gov identifier: NCT01276041). Phase III trials of pertuzumab plus chemotherapy without trastuzumab have not been reported.
Pertuzumab has antitumor activity in patients beyond the first-line setting. The results of a multicenter, open-label, single-arm, phase II study show that the combination of pertuzumab and trastuzumab is active and well tolerated in patients with HER2-positive metastatic breast cancer that has progressed on prior trastuzumab therapy. The trial reported an objective response rate of 24.2% and a clinical benefit rate of 50%.16 To determine whether the clinical benefit seen in the study was from pertuzumab alone or was a result of the combined effect of pertuzumab and trastuzumab, a cohort of 29 patients whose disease progressed during prior trastuzumab-based therapy received pertuzumab monotherapy until progressive disease or unacceptable toxicity. Of these, 17 patients with disease progression continued to receive pertuzumab with the addition of trastuzumab. In the 29 patients who received pertuzumab monotherapy, the objective response and clinical benefit rates reported were 3.4% and 10.3%, respectively, whereas in the 17 patients who received dual blockade after progression on pertuzumab, the objective response rate and clinical benefit rate were 17.6% and 41.2%, respectively.17

NCCN Guidelines Insights, Breast Cancer, Version 3.2013.
Further research is expected to determine the ideal sequencing strategy for anti-HER2 therapy.
NCCN Recommendations
Based on the available data, the NCCN Breast Cancer Panel recommends pertuzumab plus trastuzumab in combination with a taxane as a preferred option for first-line treatment of patients with HER2-positive metastatic breast cancer (see BINV-21, page 756). Pertuzumab plus trastuzumab in combination with docetaxel is a category 1 recommendation, and in combination with paclitaxel is a category 2A recommendation (see BINV-O 1 of 7, page 757, and BINV-O 4 of 7, page 758, for dosing schedule).
For patients with disease progression after treatment with trastuzumab-based therapy without pertuzumab, the NCCN Breast Cancer Panel recommends considering a line of therapy containing both trastuzumab plus pertuzumab with or without a cytotoxic agent (eg, vinorelbine or taxane; see BINV-21, page 756).
T-DM1
T-DM1 is a first-in-class antibody–drug conjugate. Through a stable linker, the HER-2 targeting antitumor portion of trastuzumab is conjugated with the microtubule-inhibitory agent DM1 (derivative of maytansine). Therefore, T-DM1 delivers its antitumor activity to HER2-overexpressing cells through combining the specificity of trastuzumab with cytotoxicity of maytansine, thus increasing the therapeutic index.18
A recent randomized, multicenter, open-label, phase III study (EMILIA) showed overall and progression-free survival benefits for T-DM1 compared with the combination of lapatinib and capecitabine in patients with HER2-positive or metastatic breast cancer previously treated with a taxane-trastuzumab regimen.19 The median progression-free survival (assessed by independent review) with T-DM1 was 9.6 months versus 6.4 months with lapatinib plus capecitabine; the HR for progression or death from any cause was 0.65 (95% CI, 0.55–0.77; P<.001). The stratified HR for death from any cause with T-DM1 versus lapatinib plus capecitabine was 0.62 (95% CI, 0.48–0.81; P=.0005).19 Rates of grade 3 or 4 adverse events were higher with lapatinib plus capecitabine than with T-DM1 (57% vs 41%). The incidences of thrombocytopenia and increased serum aminotransferase levels were higher with T-DM1 (frequency >25%), whereas the incidences of diarrhea, nausea, vomiting, and palmar-plantar erythrodysesthesia were higher with lapatinib plus capecitabine.19
NCCN Recommendations
Based on the available data, the NCCN Breast Cancer Panel recommends T-DM1 as a preferred option for treatment of patients with HER2-positive metastatic breast cancer who were previously treated with a trastuzumab-based regimen (see BINV-21, page 756, and BINV-O 5 of 7, page 759, for dosing schedule).
Conclusions
The NCCN Guidelines for Breast Cancer are in continuous evolution. They are updated annually or sometimes more often, if new high-quality clinical data become available in the interim. The recommendations in the NCCN Guidelines, with few exceptions, are based on the evidence from clinical trials. Expert medical clinical judgment is required to apply these guidelines in the context of an individual patient to provide optimal care. The physician and the patient have the responsibility to jointly explore and select the most appropriate option from among the available alternatives. When possible, consistent with NCCN philosophy, the NCCN Breast Cancer Panel strongly encourages patient/physician participation in prospective clinical trials. The full version of the 2013 NCCN Guidelines for Breast Cancer is available online (NCCN.org).
NCCN Categories of Evidence and Consensus.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Acknowledgments
Supported by educational grants from Eisai, Inc.; Millennium: The Takeda Oncology Company; Teva Pharmaceuticals; Bayer HealthCare Pharmaceuticals Inc.; Celgene Corporation; Endo Pharmaceuticals and HealthTronics; Genentech; and ARIAD Pharmaceuticals, Inc.
Disclosure of Affiliations and Significant Relationships: NCCN Breast Cancer Panel
The following authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products or devices discussed in this report or their competitors: Dr. Theriault, Dr. Allred, Dr. Burstein, Dr. Edge, Dr. Farrar, Dr. Giordano, Dr. Hudis, Dr. Mayer, Dr. McCormick, Dr. Ward, and Dr. Zellars.
The following authors have disclosed that they have financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products or devices discussed in this report or their competitors:
Dr. Carlson: Investigator for Genentech, Inc., and a PI for sanofi-aventis U.S.
Dr. Anderson: PI for General Electric and sanofi-aventis U.S.
Dr. Forero: Research support from Abbott Laboratories; Biogen Idec; Celgene Corporation; Daiichi- Sankyo Co.; Eisai Inc.; Eli Lilly and Company; Genentech, Inc.; BioCryst Pharmaceuticals, Inc.; Immunomedics, Inc.; and Seattle Genetics, Inc.
Dr. Goldstein: PI for Novartis Pharmaceuticals Corporation and Dompé. Advisory board member for Celgene Corporation; Eisai Inc.; and Genomic Health, Inc. Data monitoring committee for Genentech, Inc. and Novartis Pharmaceuticals Corporation.
Dr. Gradishar: Consultant for Bayer HealthCare. Advisory board member for Eisai Inc.; Genentech, Inc.; Genomic Health, Inc.; Myriad Genetic Laboratories, Inc.; and Onyx Pharmaceuticals, Inc.
Dr. Hayes: PI for Novartis Pharmaceuticals Corporation; Janssen R&D, LLC; Pfizer Inc.; and Veridex, LLC.
Dr. Isakoff: Research funding from Abbott Laboratories. Advisory board member for Myriad Genetic Laboratories, Inc.
Dr. Ljung: PI for National Cancer Institute.
Dr. Mankoff: Research support from Merck & Co., Inc. and Pfizer Inc. Speakers’ bureau member for Genzyme Corporation.
Dr. Marcom: Research support from Genentech, Inc. and Novartis Pharmaceuticals Corporation. PI for DoD/CDMRP. Speakers’ bureau member for Genentech, Inc.
Dr. Pierce: Advisory board member for Genomic Health, Inc.
Dr. Reed: PI for Novartis Pharmaceuticals Corporation. Advisory board member for UnitedHealthcare.
Dr. Schwartzberg: PI for Bristol-Myers Squibb Company. Advisory board member for Amgen Inc. Speakers’ bureau member for Eisai Inc.; Genentech, Inc.; and GlaxoSmithKline.
Ms. Smith: Data safety monitoring board for Genentech, Inc. Advisory board member for AstraZeneca Pharmaceuticals LP. Board member for Gateway for Cancer Research Foundation and National Accreditation Program for Breast Centers.
Dr. Soliman: PI for Agendia BV. Speakers’ bureau member for Agendia BV, Celgene Corporation, and NanoString Technologies, Inc. Advisory board member for Veridex, LLC.
Dr. Somlo: PI for AstraZeneca Pharmaceuticals LP, Celgene Corporation, and National Cancer Institute. Advisor for Veridex, LLC. dvisory board member, consultant, and member of the speakers’ bureau for Genentech, Inc.; Abraxane; and Roche Laboratories, Inc. dvisory board member for Celgene Corporation; Millennium Pharmaceuticals, Inc.; and Novartis Pharmaceuticals Corporation.
Dr. Wolff: PI for Bristol-Myers Squibb Company.
The NCCN Guidelines Staff have no conflicts to disclose.
Footnotes
Please Note
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. The NCCN Guidelines® Insights highlight important changes in the NCCN Guidelines® recommendations from previous versions. Colored markings in the algorithm show changes and the discussion aims to further understanding of these changes by summarizing salient portions of the panel’s discussion, including the literature reviewed.
The NCCN Guidelines Insights do not represent the full NCCN Guidelines; further, the National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding the content, use, or application of the NCCN Guidelines and NCCN Guidelines Insights and disclaims any responsibility for their applications or use in any way.
The full and most current version of these NCCN Guidelines are available at NCCN.org.
© National Comprehensive Cancer Network, Inc. 2013, All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 3.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson RW, Moench SJ, Hammond ME, et al. HER2 testing in breast cancer: NCCN Task Force report and recommendations. J Natl Compr Canc Netw. 2006;4(Suppl 3):1–22. [PubMed] [Google Scholar]
- 5.Paik S, Bryant J, Tan-Chiu E, et al. Real-world performance of HER2 testing—National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94:852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 Intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 7.Roche PC, Suman VJ, Jenkins RB, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002;94:855–857. doi: 10.1093/jnci/94.11.855. [DOI] [PubMed] [Google Scholar]
- 8.Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Cortes J, Im SA, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in HER2-positive, first-line metastatic breast cancer (MBC) Cancer Res. 2012;72(24 Suppl):S5–1. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain S, Kim S-B, Cortes J, et al. Confirmatory overall survival (OS) analysis of CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study with pertuzumab (P), trastuzumab (T), and docetaxel (D) in patients (pts) with HER2-positive first-line (1L) metastatic breast cancer (MBC) Cancer Res. 2012;72(24 Suppl):P5-18-26. [Google Scholar]
- 12.Cortés J, Baselga J, Im Y, et al. Quality of life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer [abstract] J Clin Oncol. 2012;30(Suppl 15) doi: 10.1093/annonc/mdt274. Abstract 598. [DOI] [PubMed] [Google Scholar]
- 13.Swain SM, Ewer MS, Cortes J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datko F, D’Andrea G, Dickler M, et al. Phase II study of pertuzumab, trastuzumab, and weekly paclitaxel in patients with metastatic HER2-overexpressing metastatic breast cancer. Cancer Res. 2012;72(24 Suppl):P5-18-20. [Google Scholar]
- 15.Perez E, Lopez-Vega J, Mastro L, et al. A combination of pertuzumab, trastuzumab, and vinorelbine for first-line treatment of patients with HER2-positive metastatic breast cancer: an open-label, two-cohort, phase II study (VELVET) [abstract] J Clin Oncol. 2012;30(Suppl 15) Abstract TPS653. [Google Scholar]
- 16.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:1594–1600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 18.Widdison WC, Wilhelm SD, Cavanagh EE, et al. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem. 2006;49:4392–4408. doi: 10.1021/jm060319f. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]


