Abstract
The differential diagnosis of low–nuclear grade intraductal epithelial proliferations of the breast includes atypical ductal hyperplasia (ADH) and ductal carcinoma in situ (DCIS). This distinction can be difficult on core needle biopsy (CNB) but can have significant clinical ramifications. We examined the clinical course of patients diagnosed on CNB with borderline ADH/DCIS lesions [marked ADH (MADH)] at our institution. A total of 74 patients were diagnosed with MADH on CNB and underwent an excisional biopsy (EB). The majority of these CNBs reviewed at outside hospitals had been classified as DCIS. Twenty patients (27%) had benign findings or lobular neoplasia in their EB, 18 (24%) had ADH, 33 (45%) had DCIS, and 3 (4%) had DCIS and invasive ductal carcinoma (IDC). Among the 38 patients who were not diagnosed with DCIS or IDC on EB, no patient underwent further surgery or radiation post-operatively. Thirty-seven of these 38 patients had no recurrences, whereas 1 patient developed a “recurrence” that on our review was likely residual localized MADH. The mean follow-up for these patients was 54 months. Of the 36 patients diagnosed with DCIS or IDC on EB, < 20% required mastectomy. On review, MADH involving an intermediate-sized duct on CNB and the amount of residual lesion on imaging was significantly associated with DCIS or IDC on EB. Conversely, MADH involving columnar cell lesions and the presence of calcification on CNB were significantly associated with benign pathology on EB. In conclusion, our study provides preliminary data that justify a conservative approach to borderline ADH/DCIS lesions on CNB: that is, diagnose as MADH and treat by conservative excision.
Keywords: breast, core biopsy, atypical duct hyperplasia, ductal carcinoma in situ
The differential diagnosis of monotonous, low–nuclear grade intraductal epithelial proliferations of the breast includes atypical ductal hyperplasia (ADH) and low-grade ductal carcinoma in situ (LGDCIS). Although molecular data suggest that these 2 lesions are related,1 these 2 diagnoses have vastly different clinical implications. Patients with incompletely excised DCIS are at 10-fold–increased risk of developing invasive ductal carcinoma (IDC) at the involved site2; therefore, standard of care is either conservative excision with adjuvant radiation therapy (XRT) with or without chemopreventive hormone therapy or mastectomy if the lesion is extensive. In contrast, ADH confers a lower (4- to 5-fold) generalized increased risk of invasive breast cancer and is managed by close observation with or without chemopreventive hormone therapy.3–5 Although ADH and LGDCIS may or may not represent biologically different entities, lesions classified as DCIS are themselves clearly heterogenous, ranging from small, estrogen receptor–positive lesions of low nuclear grade to larger, estrogen receptor–negative lesions of high nuclear grade. Many have suggested that small, LGDCIS lesions can be adequately treated by conservative but complete excision alone without the need for adjuvant XRT,5–8 although others disagree.9–11
Although both lesions have similar cytology, ADH and LGDCIS are usually readily distinguished in excision specimens using size criteria. Categorization is more difficult on core needle biopsy (CNB) due in part to lesional fragmentation and incomplete lesional sampling. However, the distinction between ADH and DCIS on CNB can have significant clinical ramifications. It is well recognized that lesions interpreted as ADH on CNB may not be representative of the entire lesion, and thus conservative excisional biopsy (EB) of the entire mammographic abnormality is recommended.12–15 By contrast, a diagnosis of DCIS on CNB can commit the patient to adjuvant XRT even if the resulting EB is negative, and the word “carcinoma” can even prompt an anxious patient to opt for bilateral mastectomy.
When encountering low-grade lesions that are borderline and difficult to classify as ADH or DCIS on core biopsy, we have favored a conservative approach by diagnosing such lesions as marked ADH (MADH) and recommending EB as initial treatment. Our main concerns have been to avoid what might be unnecessary mastectomy or XRT for a low-grade lesion that may not be universally regarded as DCIS and seems likely to be cured by conservative excision. However, we know of no formal outcome studies to support this approach. In this study, we review 74 patients diagnosed with MADH on CNB who underwent EB at our institution, examine their clinical treatment and outcome in comparison to patients with conventional ADH and DCIS on CNB, and review their CNB pathology to identify features on CNB that predict whether or not there will be cancer in the EB.
MATERIALS AND METHODS
MADH Definition
At our institution, lesions diagnosed as MADH on core biopsy generally fall into 1 of 3 categories and generally lack a sufficient quantity of the lesion for an out-right diagnosis of DCIS. The first are monotonous and uniform low–nuclear grade intraductal proliferations that completely fill duct spaces over a length approaching but not exceeding 3 mm. The second are similar monotonous low-grade intraductal proliferations that extend over lengths > 3 mm but only partially fill the involved ducts. The third are limited (roughly < 2 mm) intraductal proliferations including some cells with nuclei similar to those of grade 2 DCIS. Lesions with grade 3 nuclei are excluded from this definition as such lesions are classified as DCIS regardless of their extent. We emphasize that the term MADH is not intended to be a distinctive diagnostic category like conventional ADH or LGDCIS; instead, it is a working diagnosis primarily intended for difficult-to-classify core biopsy specimens that almost always become clarified after a definitive conservative excision of the lesion.
Study Design
We first obtained approval to perform this study from the Johns Hopkins Hospital Institutional Review Board. We searched our computerized hospital database from the period of January 1, 1998 to January 1, 2009 for all breast CNBs with the diagnosis of MADH. The time period was chosen to assure the potential of at least 3 years of follow-up. Not all lesions were removed in a standard manner. As the gauge of needles used at Johns Hopkins changed over this time period, these biopsies were not of uniform gauge. Moreover, the number of passes was not identical, and only some biopsies were performed with vacuum assistance. Finally, many of these cases were consultations from outside hospitals, and the specifics of the biopsy procedure were not provided.
The search identified 179 specimens from 177 patients. Patients who had a subsequent CNB showing DCIS or IDC before EB were excluded, as were consult cases in which the patient did not receive follow-up at our medical center. After applying the exclusion criteria, 107 specimens remained. In addition, using similar search criteria we created 2 control cohorts of patients diagnosed on CNB with lesions in the differential diagnosis of MADH. One was a cohort of patients with low–nuclear grade or intermediate–nuclear grade DCIS [LGDCIS (38%) or IGDCIS (62%); nuclear grade 1 or 2], whereas the second was a cohort of patients diagnosed with conventional ADH. We included IGDCIS in the first cohort because we include limited lesions with some grade 2 nuclei on CNB in the MADH group and because of the relative paucity of nuclear grade 1 DCIS cases diagnosed on CNB at our institution.
Clinical follow-up data were obtained from electronic medical records (EMR) (98 patients) or by consented telephone survey when EMR data were insufficient (< 36 mo follow-up from initial CNB) (14 patients). Follow-up date was determined by either the most recent clinical EMR note or the date of the telephone survey. A database was created that tracked the following information: patient age at biopsy, sex, race, follow-up pathology and dates, size of lesion on excision, margins on excision, treatment, deaths, and any clinical follow-up and relevant radiologic information.
Pathology Review
Available core biopsy slides were reviewed from patients diagnosed with MADH who underwent follow-up EB. The slide review was conducted by 2 authors blind to the EB outcome (P.A. and C.J.V.). The MADH lesions were evaluated for the following: lesional nuclear grade, size, number of lesional foci, associated pathology (eg, columnar or papillary lesions), lesional involvement of an intermediate-sized duct (defined as a duct space unaccompanied by smaller acini and larger than that of a normal lobular structure), apocrine features, micro-calcifications, and/or necrosis.
Radiology Review
Available mammograms from patients diagnosed with MADH on CNB were reviewed by a dedicated breast radiologist (N.K.) who was blinded to the EB outcome. The imaging studies were evaluated for the following: indication for core biopsy (calcium, mass, architectural distortion), suspicion for DCIS or IDC (high, intermediate, low), residual lesion after biopsy [none, small (< 20%), moderate (20% to 50%), abundant (> 50%)], and characteristics of the biopsy (needle gauge, number of cores taken, vacuum assisted or not).
Statistics
Statistical analysis was performed with the Fisher exact probability test. A P-value of < 0.05 was considered statistically significant.
RESULTS
MADH Cases on Core Biopsy
A total of 107 specimens meeting the study criteria were diagnosed as MADH on CNB during an 11-year period (Fig. 1). Thirty-three patients did not have documented follow-up at our institution, whereas 74 (67%) patients underwent EB. No patient underwent mastectomy as initial treatment. It is noteworthy that among the 74 patients in this group, 19 were “confirming consults” or cases in which the patient came to our institution to see a surgeon with an established diagnosis by the contributing pathologist. Of these 19 cases, 11 had been classified by the contributing pathologist as DCIS, 3 as ADH, and 2 as MADH. The contributor’s diagnosis was not available in 3 cases.
FIGURE 1.
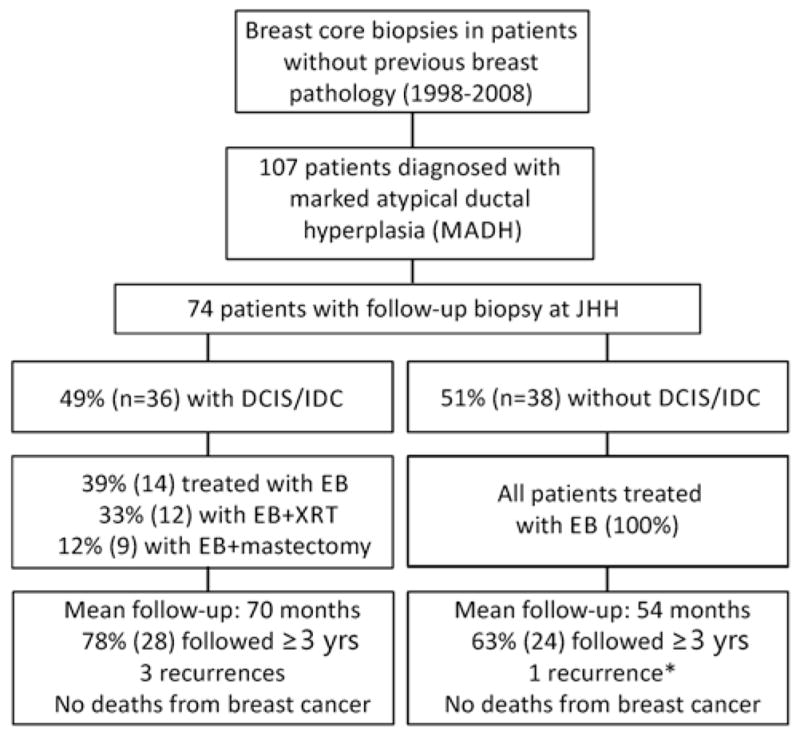
Flow chart diagramming the EB and clinical outcomes of patients with a diagnosis of MADH on CNB. One patient with DCIS on EB had unknown treatment. *Upon review, this “recurrence” was most likely residual MADH incompletely excised on initial EB.
Of the 74 patients receiving follow-up EB, 20 (27%) had benign pathology or lobular neoplasia in their EB, 18 (24%) had ADH, 33 (45%) had DCIS, and 3 (4%) had DCIS and IDC (Table 1). These EBs are separated below into cases without cancer (20+18 = 38 cases) and those with cancer (33+3 = 36 cases)
TABLE 1.
Initial Treatment of Patients After MADH on CNB, Subdivided by EB Findings
| EB Pathology | N (%) | Initial Treatment
|
|||
|---|---|---|---|---|---|
| EB, n (%) | EB+Unknown, n (%) | EB+XRT, n (%) | EB+Mastectomy, n (%) | ||
| Benign | 17 (23) | 17 (100) | — | — | — |
| ALH | 2 (3) | 2 (100) | — | — | — |
| LCIS | 1 (1) | 1 (100) | — | — | — |
| ADH | 18 (24) | 18 (100) | — | — | — |
| DCIS | 33 (45) | 13 (40) | 1 (3) | 11 (33) | 8 (24) |
| IDC | 3 (4) | 1 (33) | — | 1 (33) | 1 (33) |
| Total | 74 (100) | 52 (70) | 1 (1) | 12 (16) | 9 (12) |
ALH indicates atypical lobular hyperplasia; LCIS, lobular carcinoma in situ.
Cases Without Cancer on Excision
Among the 38 patients who were not diagnosed with DCIS or IDC on EB, no patient underwent further surgery initially or received adjuvant XRT. Only 4 (10.5%) took chemopreventive hormone therapy. Thirty-seven patients had no recurrences. The mean follow-up for these patients was 54 months; 24 (63%) had at least 36 months follow-up. One patient (who did not receive hormone therapy) was diagnosed with a small “recurrence” of LGDCIS 3 years after EB. Review of her postoperative and follow-up mammograms showed the continued presence of stable background category 3 calcifications after the original EB, until larger category 4 calcifications were detected at the original EB site, prompting a reexcision. On our retrospective review of the EB and the reexcision, we interpreted this lesion, which remained confined to lobules and did not involve intermediate-sized ducts, as residual persistent MADH that was incompletely excised on original EB (Fig. 2). However, given a diagnosis of DCIS on reexcision, the patient then opted for bilateral mastectomy, which was completely benign and showed no evidence of ADH or DCIS.
FIGURE 2.
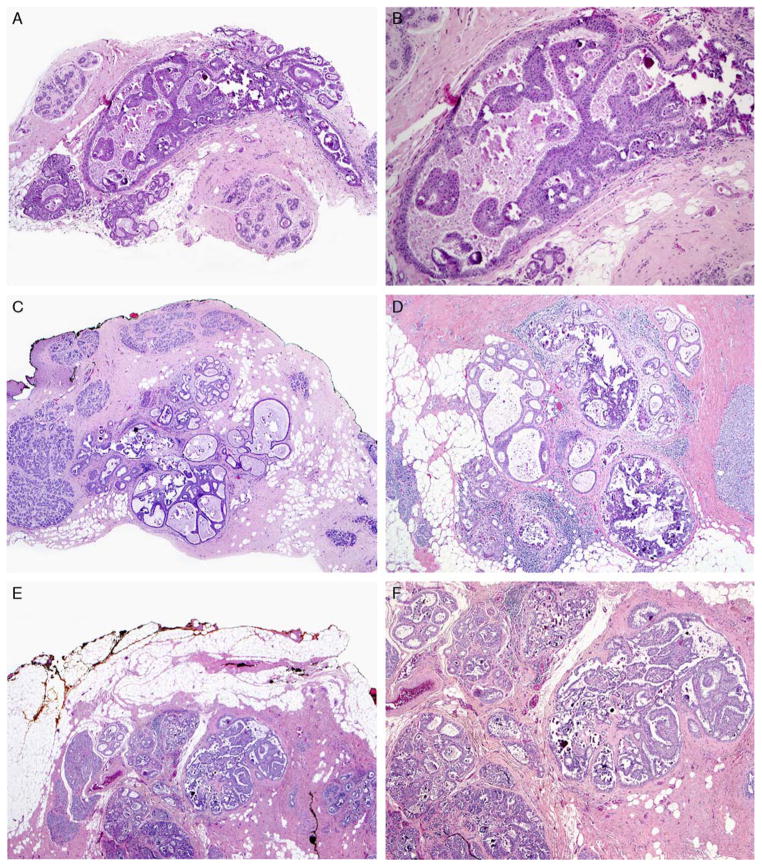
“Recurrence” of conservatively managed MADH. A and B, MADH on CNB (H&E). The lesion consists of monotonous cells with low-grade nuclei that did not completely fill the duct. C and D, The resulting EB (H&E) had multiple foci of ADH near margins. E and F, Two years later, a second EB (H&E) was diagnosed as DCIS not involving the margins, leading to a follow-up bilateral mastectomy with no residual disease. H&E indicates hematoxylin and eosin.
Cases With Cancer on Excision
Of the 36 patients diagnosed with DCIS or IDC on EB, 10 (28%) underwent observation alone, 4 (11%) took chemopreventive hormone therapy, 7 (19%) received XRT after EB, 5 (14%) received chemopreventive hormone therapy and XRT after EB, 9 (25%) underwent a mastectomy after EB, and 1 patient (3%) had unknown treatment after EB. Seven of the 9 patients who underwent mastectomy after EB had IDC or DCIS near or close to the EB margin, whereas 2 opted for completion mastectomy even though EB margins were negative. Hence, 29 of these 36 patients (80.5%) could have been and 27 of these 36 patients (75%) were managed by breast-conserving therapy.
The mean follow-up for these patients was 70 months; 28 (78%) had at least 36 months of follow-up. Four patients had additional breast pathology on long-term follow-up. One patient was diagnosed with Paget disease 2 years after CNB and received a mastectomy. A second patient was diagnosed with IDC at her CNB site after 1 year and received a mastectomy. A third patient was found to have DCIS at an adjacent site 1 year after CNB and received a mastectomy. The fourth patient developed DCIS in the contralateral breast after 8 years of follow-up; she did not receive further treatment secondary to advanced age and tenuous cardiovascular condition. No deaths secondary to breast cancer were reported.
Control Cohorts of ADH and DCIS on CNB
For comparison of treatment, we examined patient cohorts who were diagnosed with either conventional ADH or LGDCIS/IGDCIS on CNB (Tables 2, 3). Patients with MADH were treated similarly to those with conventional ADH. No patients with MADH or conventional ADH on CNB were initially treated with mastectomy. In contrast, 13% of patients with LGDCIS/IGDCIS on CNB (6 patients) were initially treated with mastectomy, despite the fact that 3 had disease localized to 1 quadrant on preoperative imaging. The localized nature of these lesions was confirmed in the resulting mastectomy specimens, as the DCIS was limited to 1 quadrant in each case.
TABLE 2.
Initial Treatment of Patients After DCIS on CNB, Subdivided by Initial EB Findings
| EB Pathology | N (%) | Initial Treatment
|
|||
|---|---|---|---|---|---|
| EB, n (%) | EB+XRT, n (%) | EB+Mastectomy, n (%) | Mastectomy, n (%) | ||
| Benign | 3 (9) | 2 (67) | 1 (33) | — | — |
| ADH | 3 (9) | 2 (67) | 1 (33) | — | — |
| DCIS | 24 (71) | 6 (25) | 10 (42) | 2 (8) | 6 (25) |
| IDC | 4 (12) | — | 2 (50) | 2 (50) | — |
| Total | 34 (100) | 10 (29) | 14 (41) | 4 (12) | 6 (18) |
TABLE 3.
Initial Treatment of Patients After ADH on CNB, Subdivided by EB Findings
| EB Pathology | N (%) | Initial Treatment
|
||
|---|---|---|---|---|
| EB, n (%) | EB+XRT, n (%) | EB+Mastectomy, n (%) | ||
| Benign | 11 (38) | 11 (100) | — | — |
| ALH | 3 (10) | 3 (100) | — | — |
| ADH | 10 (34) | 9 (90) | — | 1 (10) |
| DCIS | 4 (14) | — | 4 (100) | — |
| IDC | 1 (3) | — | — | 1 (100) |
| Total | 29 (100) | 22 (76) | 4 (14) | 2 (7) |
ALH, atypical lobular hyperplasia.
Comparison of Final Margin Status of DCIS/IDC in EB of Patients With MADH Versus DCIS/IDC on CNB
For patients diagnosed with MADH on CNB who had DCIS on follow-up EB, 11 (31%) patients had DCIS/IDC ≥ 2 mm from an inked margin, 17 (47%) had close margins with DCIS/IDC < 2 mm from an inked margin, and 8 (22%) of these patients had DCIS/IDC at an inked margin (Table 4). Separate specimen cavity margins were submitted with 3 (6%) of these EB specimens. Of control group patients with LGDCIS/IGDCIS on CNB who received a follow-up EB, 11 (50%) had DCIS/IDC ≥ 2 mm from an inked margin, 7 (32%) had close margins with DCIS/IDC < 2 mm from an inked margin, and 4 (18%) had DCIS/IDC present at an inked margin. Thirteen (59%) of these EB specimens were submitted with separate cavity margins.
TABLE 4.
Margin Status of EB Specimens (Accounting for Separately Submitted Cavity Margins) With DCIS and/or IDC From Patients With MADH Vs. LGDCIS/IGDCIS on CNB
| Margin Status | MADH, n (%) | LGDCIS/IGDCIS, n (%) |
|---|---|---|
| Positive, IDC/DCIS at inked margin | 8/36 (22) | 4/22 (18) |
| Close margins, IDC/DCIS ≤ 2 mm from margin | 17/36 (47) | 7/22 (32) |
| Clear margins, IDC/DCIS > 2 mm from margin | 11/36 (31) | 11/22 (50) |
Pathologic Features of MADH Core Biopsies That Predict DCIS or IDC on Excision
In order to determine whether any morphologic features of MADH on CNB are associated with finding DCIS or IDC on EB, we reviewed available CNB specimens from patients who had follow-up EB (Table 5). Of the 74 cases in our database, 42 CNBs (all of which were Johns Hopkins cases) were available for review (cases sent in consultation to our hospital are routinely returned after 1 month, so these cases were not available). MADH lesions involving an intermediate-sized duct on CNB were significantly associated with finding DCIS or IDC on EB (Fig. 3). Conversely, MADH involving columnar cell lesions and the presence of calcification were statistically more likely to be associated with benign pathology on EB (Fig. 4). Other factors, such as highest nuclear grade (grade 1 vs. grade 2) in the MADH and the presence or absence of an associated papillary lesion (Fig. 5) did not differ significantly between the 2 groups.
TABLE 5.
Correlation of Morphologic Characteristics of MADH Found on CNB With EB Specimen Pathology
| CNB Feature | EB Result
|
% Upgrade (DCIS/IDC on EB) | |
|---|---|---|---|
| DCIS ± IDC, n (%) | ADH, LN, or Benign, n (%) | ||
| No. cases | 19 | 23 | — |
| Grade 2 nuclei present | 4 (21) | 8 (35) | 33 |
| Mean largest focus (mm) | 2.6 | 2.4 | — |
| Mean number of foci | 3.1 | 2.2 | — |
| Columnar lesion | 6 (32)* | 16 (70)* | 27* |
| Papillary lesion | 4 (21) | 4 (17) | 50 |
| Involves intermediate-sized duct | 13 (68)* | 6 (26)* | 72* |
| Microscopic calcification | 11 (58)* | 21 (91)* | 34* |
| Necrosis | 0 (0) | 1 (4) | 0 |
| Apocrine change | 4 (21) | 1 (4) | 80 |
| Calcification on imaging | 13 (68) | 19 (83) | 41 |
| Mass lesion | 6 (32) | 5 (22) | 54 |
42 Specimens were available for review
The percentage upgrade indicates how frequently a lesion with the given feature on CNB is upgraded to DCIS or IDC on the EB.
P < 0.05 by the Fisher exact test.
LN indicates lobular neoplasia.
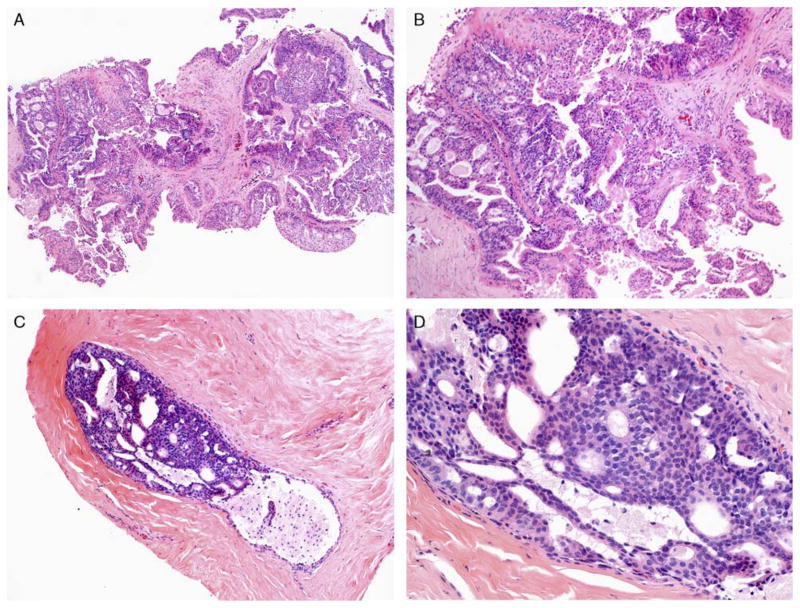
FIGURE 3.
A and B, MADH involving a papillary lesion on CNB (H&E) and (C and D) a separate case of MADH involving an intermediate-sized duct on CNB (H&E). Both are low-grade lesions that are < 3 mm in size. Patients with MADH involving an intermediate-sized duct were statistically more likely to have DCIS on follow-up EB (P < 0.01). H&E indicates hematoxylin and eosin.
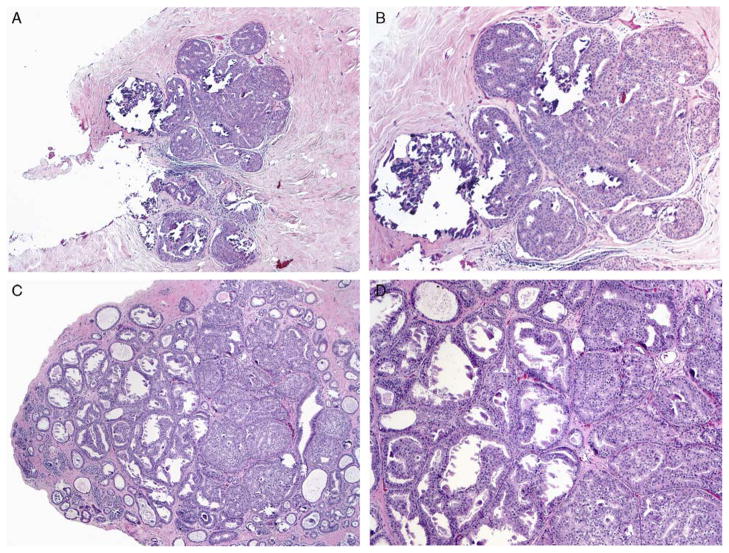
FIGURE 4.
A–D, MADH involving columnar cell lesions with calcification on CNB (H&E). The lesion is < 3 mm and consists of low-grade, monotonous cells. Follow-up EB in such cases were statistically more likely to show ADH or benign findings compared with DCIS (P < 0.05). H&E indicates hematoxylin and eosin.
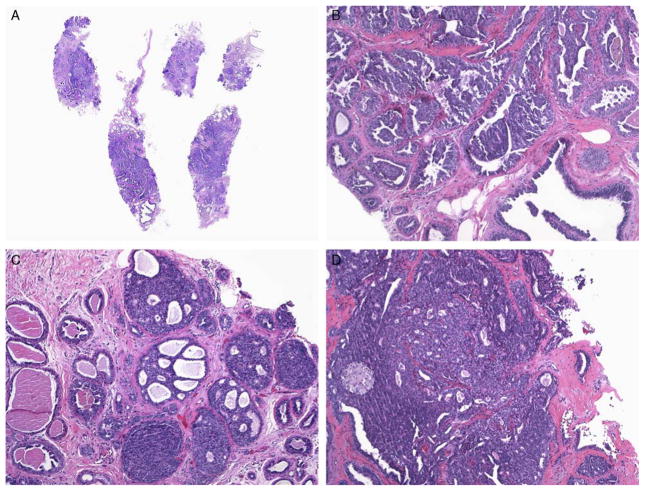
FIGURE 5.
A–D, MADH involving a sclerosed papilloma on CNB (H&E). Multiple cores are involved by a low-grade lesion, but each focus is < 3 mm. This follow-up EB demonstrated benign findings even though multiple fragments were involved on the CNB. H&E indicates hematoxylin and eosin.
Radiologic Features of MADH Core Biopsies That Predict DCIS or IDC on Excision
To determine whether any radiologic findings could be associated with DCIS or IDC on follow-up EB, the radiology and associated records were reviewed by an experienced, dedicated breast radiologist (N.K.). Factors that were investigated included the number of core biopsies, whether vacuum assistance was used, the level of suspicion for cancer, the indication for biopsy (mass and/or calcification), the needle gauge used, and the amount of residual lesion present after biopsy (Fig. 6). The presence of DCIS or IDC on follow-up EB was significantly associated with the amount of residual lesion present after CNB (Fig. 6D). Abundant residual lesion was significantly associated with DCIS/IDC on EB (P < 0.05 vs. all other amounts of residual lesion; Fisher exact test); conversely, the absence of any residual lesion was significantly associated with the absence of DCIS/IDC on EB (P < 0.001 vs. any amount of residual lesion; Fisher exact test). In addition, a high level of suspicion on imaging was significantly associated with the presence of DCIS/IDC on EB when compared with a low level of suspicion; however, the number of patients with a low level of suspicion on imaging was very small (n = 2). All other variables, including the number of core biopsies taken and the use of vacuum assistance (data not shown) did not statistically differ between the groups.
FIGURE 6.
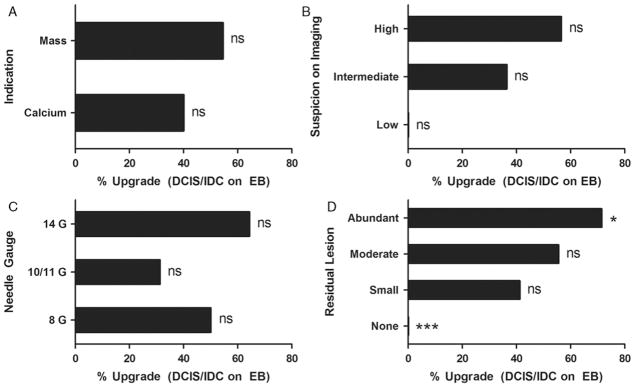
A, MADH Upgrade rates to DCIS/IDC on EB by clinical indication for CNB. B, Suspicion for cancer on imaging. C, Needle gauge used. D, Residual lesion on imaging after CNB. Each group was statistically compared with all other groups combined using the Fisher exact test (ns, not significant; *P < 0.05; ***, P < 0.001).
DISCUSSION
The existence of lesions that are difficult to classify as either ADH or DCIS is well recognized.16–18 However, our study is, to our knowledge, the first to address the clinical implications of the diagnosis of borderline ADH/LGDCIS lesions identified on breast needle core biopsy. Despite the fact that the majority of pathologists outside our institution who reviewed these cases (11 of 16 cases with available diagnoses, or 69%) regarded them as DCIS, we have favored a conservative approach to these lesions (ie, diagnosis as marked atypical duct hyperplasia, which triggers a conservative excision) for 3 main reasons. First, if the lesion proves to be a small sample of an otherwise well-developed DCIS in the excision specimen, the patient is still most likely well served by breast-conserving therapy, which begins with conservative excision. Second, avoiding a diagnosis of DCIS for a borderline lesion diminishes the chances of overtreatment of a localized lesion that should be curable by conservative excision. Third, even if the core biopsy is interpreted as a limited focus of LGDCIS and there is no cancer in the excision specimen, we believe that the patient is likely adequately treated by conservative excision, given that multiple studies using detailed pathologic examination have shown that patients with small foci of LGDCIS excised with wide margins have an excellent prognosis without the need for XRT.6,7,19,20 While our study is relatively small and the follow-up is limited, our study provides data supporting all of these assertions.
First, the majority of patients who proved to have DCIS or IDC on their excision after a core biopsy showing MADH were able to be successfully managed by breast-conserving therapy, with only 25% (9 of 36) undergoing and 19.5% (7 of 36) requiring mastectomy. In limited follow-up, these patients did not have an excess of in-breast recurrences, and none developed metastases. Second, we did find evidence that some of our patients given the diagnosis of LGDCIS/IGDCIS were potentially overtreated. In our control group, 13% of patients with LGDCIS/IGDCIS diagnosed on CNB elected to undergo mastectomy as their initial curative procedure. Of these 6 patients, 3 had disease thought to be confined to 1 quadrant on preoperative imaging and proven to be so on pathologic review of their mastectomy specimen, indicating that conservative excision would likely have been possible and curative. One wonders whether the patients who elected to undergo mastectomy overreacted to their diagnosis and wished to eliminate any chance of “recurrent breast cancer.” Along these lines, there is a growing body of published data indicating that patient preference is a major factor leading to mastectomy21,22 and that the incidence of mastectomy may be increasing. Moreover, of the 6 patients who had a negative excision biopsy after a diagnosis of LGDCIS on core biopsy, 2 (33%) underwent additional XRT. Given published data suggesting the absence of necessity for XRT for widely excised LGDCIS, one can argue that these 2 patients were overtreated. The potential for overtreatment was also highlighted by our solitary case of MADH, which “recurred” as LGDCIS 3 years later. On radiologic and pathologic review, this “recurrence” was at the same site of the prior excision in which MADH extended to the specimen margin. Parenthetically, we usually recommend reexcision for MADH that extends to surgical margins because of the difficulty in excluding DCIS, but in this particular case immediate reexcision was not performed. We suspect that this “recurrent” lesion represented residual MADH left behind from the original surgery, although clearly this was a borderline lesion that some pathologists would regard as DCIS. Even if the “recurrence” were regarded as LGDCIS, as the patient had not received XRT after the initial EB, she would still have been eligible for breast-conserving therapy with adjuvant XRT. The localized, curable nature of the process was emphasized by the absence of any lesion in the subsequent bilateral mastectomy, which again seems to have been unnecessary.
Our study showed that almost half of patients with MADH on needle core biopsy had neither DCIS nor IDC in their EB. Aside from the single case mentioned above, all 37 of these patients on whom we have follow-up information had no evidence of recurrence without further adjuvant XRT (mean follow-up of 4.5 y; range, 2 to 159 mo). These data would seem to support the idea that borderline ADH/DCIS lesions, if essentially confined to a core biopsy and absent from the subsequent surgical excision specimen, can safely be managed by conservative excision without adjuvant XRT. We caution, however, that our data are at this point incomplete. It is known that patients with LGDCIS may recur decades after their initial diagnosis.2 Hence, it will be important to further closely follow these patients over time to be sure that there is not a tendency toward late recurrences at the site of excision. However, on the basis of the available follow-up data, the outcome appears to be excellent. As others have pointed out and as mentioned above, even if the disease were to recur in the patients, the fact they did not receive XRT at the time of their first excision would make them still eligible to undergo excision and XRT (ie, standard breast-conserving therapy) for the recurrence. Such an option would not be typically available to patients who have recurred after receiving XRT after their first excision.
One important potential drawback to our approach comes from closer examination of the final margin status of patients who had an excision after a core biopsy diagnosis of MADH compared with those who underwent excision after core biopsy showing LGDCIS/IGDCIS. A slightly higher percentage of patients with MADH on core biopsy (22%) had positive final EB margins compared with patients with LGDCIS/IGDCIS on core biopsy (18%) (P = not significant). Moreover, the number of close margins was greater in the MADH (47%) group compared with that in the LGDCIS/IGDCIS group (32%), although this again did not reach statistical significance. At first, these data seem counterintuitive, as one would expect a more fully developed lesion on a CNB (LGDCIS/IGDCIS) to be more extensive in the EB and thus more likely associated with positive margins than EB of ADH on CNB. One possible explanation for these results is that a surgeon may tend to be more aggressive when excising a lesion diagnosed as DCIS on core biopsy relative to their excision for a lesion diagnosed as MADH. One can argue that a diagnosis of MADH may make a surgeon excise less widely, resulting in a greater likelihood of a close and inadequate margin if the lesion proves to be DCIS. In fact, at our institution, our surgeons routinely take extra cavity margins, which effectively diminishes the chance of false-positive margins in the setting of a core biopsy showing cancer23; however, they have rarely taken extra cavity margins for a lesion diagnosed as ADH. This proved to be the case in our study, as 59% of excisions for DCIS included extra margins, whereas only 6% of excisions for MADH did. Although further data are needed to support this explanation, we now generally suggest to our surgeons that a diagnosis of MADH on CNB (particularly if the lesion involves intermediate-sized ducts or if abundant lesion remains on imaging) be regarded as one that may well represent an incomplete sample of DCIS and encourage a complete but conservative excision along the lines as would be performed for diagnosis of LGDCIS.
Finally, we were also able to identify radiologic and pathologic features on the core biopsy specimens, which help predict whether or not the MADH found on CNB would or would not prove to be part of a cancer (DCIS or IDC) on the excision specimen. On radiologic grounds, a greater amount of residual lesion predicted that the EB would show cancer, which is logical as limited size of the lesion on CNB was the major factor precluding a diagnosis of DCIS and leading to the diagnosis of MADH. The needle gauge, number of core biopsies taken, and usage of vacuum assistance are individual factors that could potentially affect the amount of residual lesion present and therefore the rate of DCIS/IDC found on EB. However, no significant association could be found between these individual factors and the upgrade rate (Fig. 6). On pathologic grounds, MADH involving an intermediate-sized duct favored the lesion to represent DCIS or IDC, whereas MADH involving a columnar lesion or calcification favored the lesion to represent either ADH or benign findings on the EB. These findings make sense when one considers some of the basic distinguishing features (besides size) between ADH and LGDCIS. ADH tends to be a lobulocentric process, whereas DCIS extends out of the lobules to involve intermediate-sized ducts. Hence, the fact that an atypical intraductal lesion on core biopsy involves an intermediate-sized duct logically suggests that it represents a limited sample of an LGDCIS. In contrast, columnar cell lesions, which are almost always biopsied because of their associated calcification, represent dilated lobules that expand and are replaced by cells that have a cytology (and genetic features) identical to that of LGDCIS.24 Although LGDCIS and well-differentiated invasive carcinoma often arise from columnar cell lesions, determining that the MADH involves a columnar cell lesion on core biopsy provides evidence that at least some of the lesion is in a lobule. As these lesions are typically densely calcified, the radiologist can usually easily target the lesion, increasing the likelihood that much of the lesion will be present in the core biopsy specimen and hence is less likely to be present in the excision specimen. Hence, the identification of MADH in a calcified columnar cell lesion logically predicts that the excision is less likely to harbor cancer. This finding has significant implications, given that the dilated acini of a columnar cell lesion may have a diameter that approaches that of intermediate-sized ducts, although columnar cell lesions are usually readily distinguished from intermediate-sized ducts by the dense calcification and clustering of dilated acini in the former. On the basis of these data, we tend to be even more conservative when we encounter MADH involving a columnar cell lesion on core biopsy, recognizing that the MADH is at least in the core biopsy material confined to a lobule and thus at this point better classified as MADH rather than LGDCIS.
In summary, our study provides to our knowledge the first data regarding the clinical management of borderline ADH/DCIS lesions on needle core biopsy. Although we recognize that the data are necessarily incomplete because of the long clinical follow-up needed to fully understand the clinical behavior of these lesions, our study does provide preliminary data that justify a conservative approach: that is, diagnose as MADH and treat by conservative excision.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Ma XJ, Salunga R, Tuggle JT, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders ME, Schuyler PA, Dupont WD, et al. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 3.Page DL, Dupont WD, Rogers LW, et al. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 5.Marshall LM, Hunter DJ, Connolly JL, et al. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997;6:297–301. [PubMed] [Google Scholar]
- 6.Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1455–1461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein MJ, Lagios MD. Should all patients undergoing breast conserving therapy for DCIS receive radiation therapy? No. One size does not fit all: an argument against the routine use of radiation therapy for all patients with ductal carcinoma in situ of the breast who elect breast conservation. J Surg Oncol. 2007;95:605–609. doi: 10.1002/jso.20708. [DOI] [PubMed] [Google Scholar]
- 8.Di Saverio S, Catena F, Santini D, et al. 259 Patients with DCIS of the breast applying USC/Van Nuys prognostic index: a retrospective review with long term follow up. Breast Cancer Res Treat. 2008;109:405–416. doi: 10.1007/s10549-007-9668-7. [DOI] [PubMed] [Google Scholar]
- 9.Buchholz TA, Haffty BG, Harris JR. Should all patients undergoing breast conserving therapy for DCIS receive radiation therapy? Yes. Radiation therapy, an important component of breast conserving treatment for patients with ductal carcinoma in situ of the breast. J Surg Oncol. 2007;95:610–613. doi: 10.1002/jso.20711. [DOI] [PubMed] [Google Scholar]
- 10.Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031–1036. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 11.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Johnson NB, Collins LC. Update on percutaneous needle biopsy of nonmalignant breast lesions. Adv Anat Pathol. 2009;16:183–195. doi: 10.1097/PAP.0b013e3181a9d33e. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs TW, Connolly JL, Schnitt SJ. Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol. 2002;26:1095–1110. doi: 10.1097/00000478-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Corben AD, Edelweiss M, Brogi E. Challenges in the interpretation of breast core biopsies. Breast J. 2010;16(suppl 1):S5–S9. doi: 10.1111/j.1524-4741.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 15.Bilous M. Breast core needle biopsy: issues and controversies. Mod Pathol. 2010;23(suppl 2):S36–S45. doi: 10.1038/modpathol.2010.34. [DOI] [PubMed] [Google Scholar]
- 16.Ely KA, Carter BA, Jensen RA, et al. Core biopsy of the breast with atypical ductal hyperplasia: a probabilistic approach to reporting. Am J Surg Pathol. 2001;25:1017–1021. doi: 10.1097/00000478-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ellis IO, Humphreys S, Michell M, et al. Best Practice No 179. Guidelines for breast needle core biopsy handling and reporting in breast screening assessment. J Clin Pathol. 2004;57:897–902. doi: 10.1136/jcp.2003.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AH, Denley HE, Pinder SE, et al. Excision biopsy findings of patients with breast needle core biopsies reported as suspicious of malignancy (B4) or lesion of uncertain malignant potential (B3) Histopathology. 2003;42:331–336. doi: 10.1046/j.1365-2559.2003.01582.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanders ME, Simpson JF. Can we know what to do when DCIS is diagnosed? Oncology (Huntingt) 2011;25:852–856. [PubMed] [Google Scholar]
- 20.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adkisson CD, Bagaria SP, Parker AS, et al. Which eligible breast conservation patients choose mastectomy in the setting of newly diagnosed breast cancer? Ann Surg Oncol. 2012;19:1129–1136. doi: 10.1245/s10434-011-2080-x. [DOI] [PubMed] [Google Scholar]
- 22.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16:2682–2690. doi: 10.1245/s10434-009-0635-x. [DOI] [PubMed] [Google Scholar]
- 23.Cao D, Lin C, Woo SH, et al. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005;29:1625–1632. doi: 10.1097/01.pas.0000180448.08203.70. [DOI] [PubMed] [Google Scholar]
- 24.Schnitt SJ, Vincent-Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol. 2003;10:113–124. doi: 10.1097/00125480-200305000-00001. [DOI] [PubMed] [Google Scholar]