Abstract
Background.
This analysis sought to determine the associations of the Foundation for the National Institutes of Health Sarcopenia Project criteria for weakness and low lean mass with likelihood for mobility impairment (gait speed ≤ 0.8 m/s) and mortality. Providing validity for these criteria is essential for research and clinical evaluation.
Methods.
Among 4,411 men and 1,869 women pooled from 6 cohort studies, 3-year likelihood for incident mobility impairment and mortality over 10 years were determined for individuals with weakness, low lean mass, and for those having both. Weakness was defined as low grip strength (<26kg men and <16kg women) and low grip strength-to-body mass index (BMI; kg/m2) ratio (<1.00 men and <0.56 women). Low lean mass (dual-energy x-ray absorptiometry) was categorized as low appendicular lean mass (ALM; <19.75kg men and <15.02kg women) and low ALM-to-BMI ratio (<0.789 men and <0.512 women).
Results.
Low grip strength (men: odds ratio [OR] = 2.31, 95% confidence interval [CI] = 1.34–3.99; women: OR = 1.99, 95% CI 1.23–3.21), low grip strength-to-BMI ratio (men: OR = 3.28, 95% CI 1.92–5.59; women: OR = 2.54, 95% CI 1.10–5.83) and low ALM-to-BMI ratio (men: OR = 1.58, 95% CI 1.12–2.25; women: OR = 1.81, 95% CI 1.14–2.87), but not low ALM, were associated with increased likelihood for incident mobility impairment. Weakness increased likelihood of mobility impairment regardless of low lean mass. Mortality risk patterns were inconsistent.
Conclusions.
These findings support our cut-points for low grip strength and low ALM-to-BMI ratio as candidate criteria for clinically relevant weakness and low lean mass. Further validation in other populations and for alternate relevant outcomes is needed.
Key Words: Muscle, Sarcopenia, Mobility, Impairment.
Rosenberg (1) first highlighted the functional significance of the ubiquitous age-related loss of muscle mass by naming it “sarcopenia,” and a surge of investigations into the functional consequences of low lean mass soon followed (2,3). It has become clear, however, that low lean mass, by itself, is a poor predictor of functional outcomes compared with low strength (4,5). Operational definitions of sarcopenia have evolved to include measures of strength and function, and several recommendations for clinical criteria have been published (6–9), yet there are currently no data-driven consensus criteria validated for their ability to predict relevant clinical outcomes. The Foundation for the National Institutes of Health (FNIH) Sarcopenia Project aimed to address this gap in knowledge, and in the first two phases of the project, we determined preliminary, evidence-based cut-points for both grip strength and grip strength standardized to body size as candidate indicators of clinically relevant weakness (10), and cut-points for both appendicular lean mass (ALM) and ALM standardized to body size as candidate criteria for clinically relevant low lean mass (11).
In the third phase, presented here, we sought to validate our candidate criteria by determining whether they predict future mobility impairment, defined as usual gait speed ≤0.8 m/s (12), over a 3-year follow-up among those without current mobility impairment. Testing this hypothesis is important to provide further evidence about whether to adopt these proposed criteria for research and clinical evaluation. In supplemental analyses, we examined all-cause mortality as a secondary clinically relevant outcome.
Methods
Participants
Recruitment criteria and description of the studies participating in the FNIH Sarcopenia Project have been previously described (10,11). Eligible participants for the current analyses included those from cohorts with concurrent information on gait speed, grip strength, and ALM at a single time point (baseline), baseline age ≥65 years, baseline gait speed >0.8 m/s, a follow-up gait speed measurement approximately 3 years after baseline, and follow-up information on survival. Of the 26,625 participants in the pooled data, 8,371 were ineligible because they were from studies without follow-up data; 10,052 did not have dual-energy x-ray absorptiometry scans or follow-up data; 960 were eligible but missing gait speed, grip strength, or ALM; and an additional 962 were missing follow-up data, yielding a final sample size of 6,280 (4,411 men and 1,869 women). Follow-up gait speed data were missing from 665 participants, leaving 5,615 (4,034 men and 1,581 women) eligible for analyses of incident mobility impairment. The final sample included participants from the Study of Osteoporotic Fractures original (study visit 6) (13) and African American cohorts (study visit 1; 14), the Osteoporotic Fractures in Men Study Sleep Study ancillary study (15–17), the Health, Aging and Body Composition study (year-6 clinic visit; 18), the Framingham Study Offspring cohort (exam cycles 6 and 7, 1996–2001; 19), and men from the Invecchiare in Chianti study (year-3 visit; 20).
Grip Strength
Maximum grip strength (kilograms) was measured using a handheld dynamometer using similar protocols across studies. Most studies used a Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL; 21). Grip strength less than 26kg for men and less than 16kg for women was considered weak (10). A secondary criteria for weakness based on grip strength-to-body mass index (BMI) ratio (weakBMI) was also determined, with weakness defined as weakBMI less than 1.00 in men and less than 0.56 in women (10).
Lean Mass
Whole-body dual-energy x-ray absorptiometry scans were obtained with either a Lunar or Hologic densitometer using similar protocols across studies. Our low lean mass criteria was defined as ALM less than 19.75kg for men and less than 15.02kg for women (11). A secondary criteria for low lean mass based on ALM-to-BMI ratio (ALMBMI) was identified, with low ALMBMI defined as less than 0.789 in men and less than 0.512 in women (11).
Gait Speed
Gait speed (meters per second) was assessed at baseline and after approximately 3 years of follow-up as speed over a 4-m course at the usual pace. For cohorts using a 6-m course, gait speed was converted to 4-m speeds using a published formula (22). Mobility impairment was defined as a gait speed ≤0.8 m/s (12).
Mortality
Date of death (any cause) for participants was ascertained according to protocols for each cohort. Survival time was calculated as the number of days from the baseline visit until death or up to 10 years of follow-up.
Other Variables
Information on baseline age (years) and BMI (weight in kilograms divided by height in meters squared, kg/m2) was available in all participants.
Statistical Analyses
To evaluate potential differences between participants included and excluded from the mobility impairment analysis, we compared baseline characteristics between those who did and did not have follow-up mobility impairment information up using analysis of variance or the Kruskal–Wallis test for continuous variables, and the chi-square test for categorical variables.
We planned a priori to pool data across all cohorts to evaluate the predictive validity of our candidate criteria for incident mobility impairment and mortality. For both outcomes, we calculated cohort-specific effect estimates (SAS version 9.2, SAS Institute Inc. Cary, NC), which were then combined to calculate pooled effect estimates using random-effects meta-analysis (23) using the Metafor package for R. The presence and extent of heterogeneity among studies were evaluated using the Q test (p < .10) and I 2 statistic.
Logistic regression was used to calculate the age-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for incident mobility impairment for our weakness (weak and weakBMI) and low lean mass (ALM and ALMBMI) criteria, separately for men and women. To determine whether combinations of weakness and low lean mass criteria predict incident mobility impairment, participants were further categorized into four groups: (a) not weak with normal lean mass, (b) not weak with low lean mass, (c) weak with normal lean mass, and (d) weak with low lean mass. ORs for incident mobility impairment were calculated for each group with the “not weak with normal lean mass” group as the referent. Analysis was done separately for each of the four different combinations of our weakness and low lean mass criteria: (a) weak + ALM; (b) weak + ALMBMI; (c) weakBMI + ALM and (d) weakBMI + ALMBMI.
For mortality, we used Cox proportional hazards regression to calculate the sex-specific age-adjusted mortality rate ratios and 95% CIs for the weakness and low lean mass criteria, and for their combinations. No violations of the proportional hazards assumption were detected (p < .05 for interactions of independent variables with time).
Results
Among the 6,280 participants eligible for mobility impairment and mortality analyses, more women had low ALM compared to men (38% vs 9%), but the prevalence of low ALMBMI (15% vs 18%) was similar (Table 1). Weakness defined by weak and weakBMI was prevalent in 3% and 6% of men, respectively, and 11% of women for both definitions. The proportion of men and women with combined weakness and low lean mass was very low (≤7%) regardless of definition used. Baseline characteristics by participating cohort are listed in Supplementary Tables 1 and 2.
Table 1.
Baseline Characteristics* of FNIH Sarcopenia Project Participants Included in Longitudinal Analyses Including Pre-specified Categorizations of Baseline Weakness and Low Lean Mass
| Women | Men | |
|---|---|---|
| N | 1,869 | 4,411 |
| Age (y) | 76.5±4.5 | 74.0±5.5 |
| BMI (kg/m2) | 27.3±5.1 | 27.3±3.7 |
| <25 (%) | 34 | 27 |
| 25 to <30 (%) | 38 | 53 |
| ≥30 (%) | 26 | 20 |
| Gait speed (m/s) | 1.1±0.2 | 1.3±0.2 |
| Grip strength (kg) | 22.1±5.8 | 41.3±8.5 |
| Grip strength/BMI | 0.83±0.24 | 1.54±0.36 |
| ALM (kg) | 16.2±2.9 | 24.1±3.4 |
| ALM/BMI | 0.60±0.09 | 0.89±0.11 |
| Weak† (%) | 11 | 3 |
| WeakBMI ‡ (%) | 11 | 6 |
| Low ALM§ (%) | 38 | 9 |
| Low ALMBMI || (%) | 15 | 18 |
| Weak + ALM | ||
| Not weak, normal lean mass (%) | 58 | 89 |
| Not weak, low lean mass (%) | 31 | 8 |
| Weak, normal lean mass (%) | 4 | 2 |
| Weak, low lean mass (%) | 7 | 1 |
| Weak + ALMBMI | ||
| Not weak, normal lean mass (%) | 77 | 81 |
| Not weak, low lean mass (%) | 12 | 17 |
| Weak, normal lean mass (%) | 9 | 1 |
| Weak, low lean mass (%) | 2 | 1 |
| WeakBMI + ALM | ||
| Not weak, normal lean mass (%) | 55 | 86 |
| Not weak, low lean mass (%) | 34 | 8 |
| Weak, normal lean mass (%) | 7 | 5 |
| Weak, low lean mass (%) | 4 | 1 |
| WeakBMI + ALMBMI | ||
| Not weak, normal lean mass (%) | 79 | 80 |
| Not weak, low lean mass (%) | 10 | 14 |
| Weak, normal lean mass (%) | 7 | 2 |
| Weak, low lean mass (%) | 4 | 4 |
| Died over follow-up (%) | 18 | 19 |
| Mean follow-up time (y) | 7.4 | 8.4 |
Notes: ALM = appendicular lean mass; BMI = body mass index.
*Mean (±SD) unless otherwise noted.
†Weak: grip strength <26 men; <16 women.
‡WeakBMI: ratio of grip strength-to-body mass index <1.00 men; <0.56 women.
§ALM: appendicular lean mass <19.75 men; <15.02 women.
||ALMBMI: ratio of appendicular lean mass to body mass index <0.789 men; <0.512 women.
Incident Mobility Impairment
The 377 men (8.5%) excluded from the incident mobility impairment analysis due to missing follow-up mobility data were older, slower, weaker, and had lower BMI and lean mass than men who were included (Supplementary Table 3). The 288 (15%) excluded women were older, slower, and had lower lean mass. For the 5,615 participants included, 7% of men and 23% of women developed incident mobility impairment (Supplementary Table 4).
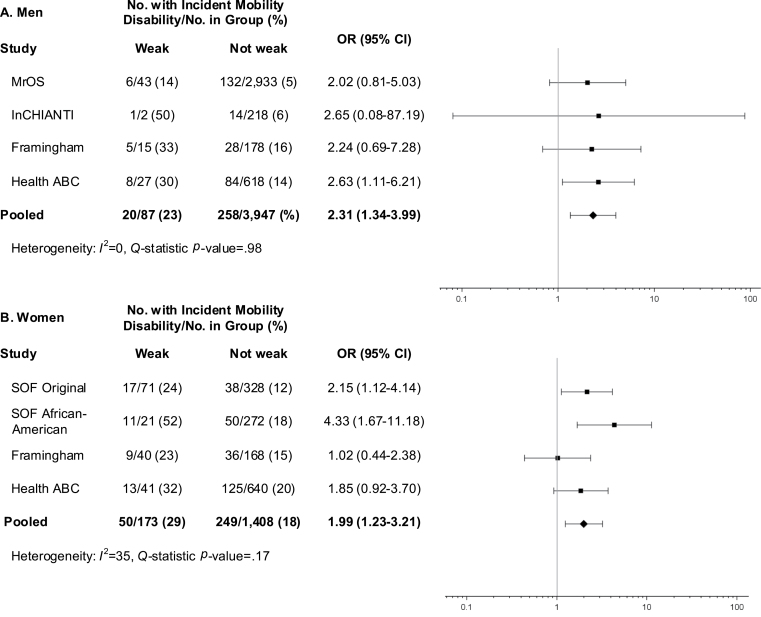
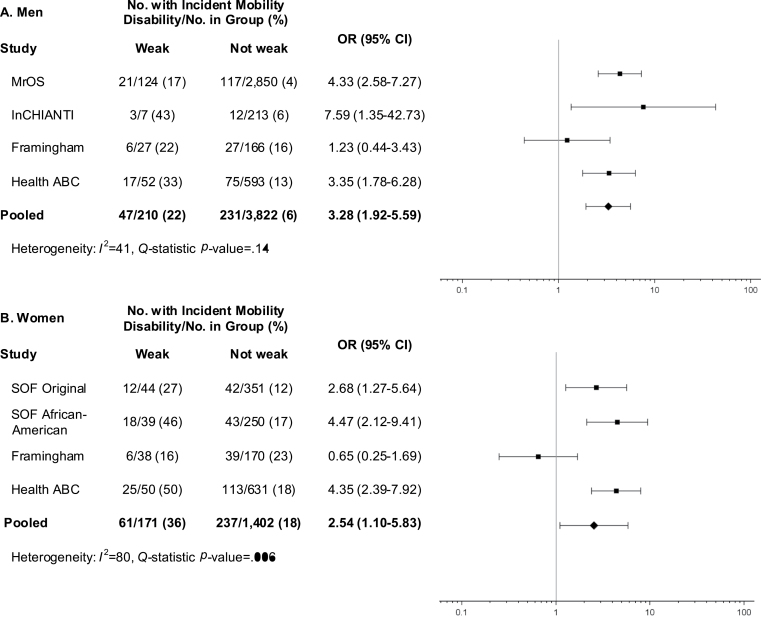
Weak (Figure 1) was associated with a twofold higher odds of mobility impairment for men (OR = 2.31, 95% CI 1.34–3.99) and women (OR = 1.99, 95% CI 1.23–3.21). WeakBMI (Figure 2) was more strongly associated with incident mobility impairment in both men (OR = 3.28, 95% CI 1.92–5.59) and women (OR = 2.54, 95% CI 1.10–5.83), though there was evidence of heterogeneity among women (Q test: p < .01; I 2 = 80%).
Figure 1.
Age-adjusted odds ratios (OR) for incident mobility impairment (gait speed ≤ 0.8 m/s) after approximately 3 years of follow-up for proposed low grip strength criteria (weak) among men (A) and women (B) in the FNIH Sarcopenia Project.
Figure 2.
Age-adjusted odds ratios (OR) for incident mobility impairment (gait speed ≤ 0.8 m/s) after approximately 3 years of follow-up for proposed low grip strength-to-BMI ratio criteria (weakBMI) among men (A) and women (B) in the FNIH Sarcopenia Project.
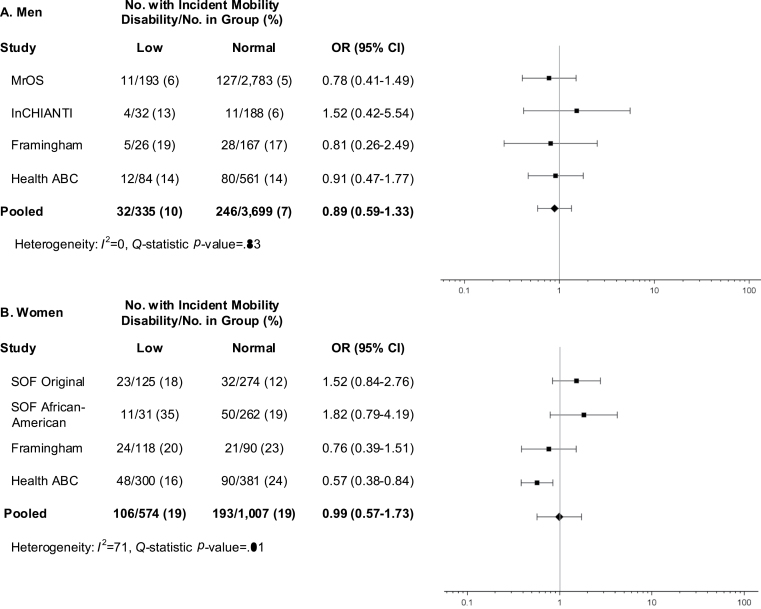
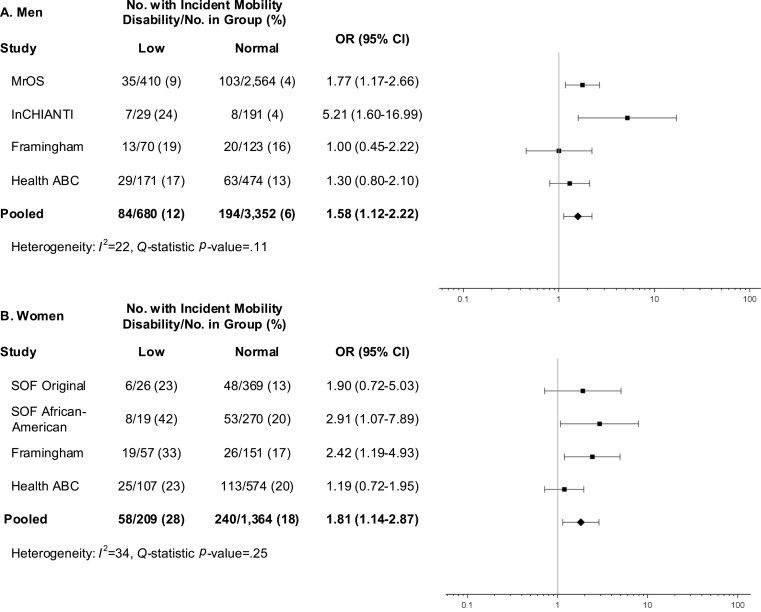
Low lean mass defined by ALMBMI (Figure 3) was associated with higher odds of incident mobility impairment for both men (OR = 1.58, 95% CI = 1.12–2.22) and women (OR = 1.81, 95% CI 1.14–2.87), yet the ALM criterion was not (Figure 4).
Figure 3.
Age-adjusted odds ratios (OR) for incident mobility impairment (gait speed ≤ 0.8 m/s) after approximately 3 years of follow-up for proposed low appendicular lean mass criteria (ALM) among men (A) and women (B) in the FNIH Sarcopenia Project.
Figure 4.
Age-adjusted odds ratios (OR) for incident mobility impairment (gait speed ≤ 0.8 m/s) after approximately 3 years of follow-up for proposed low appendicular lean mass-to-BMI ratio criteria (ALMBMI) among men (A) and women (B) in the FNIH Sarcopenia Project.
Men who were weak with normal lean mass had two to nearly five times the odds of incident mobility impairment than those who were not weak with normal lean mass (Supplementary Figures 1–4). This group also tended to have greater odds of mobility impairment than weak men with low lean mass, with the exception of the weak + ALMBMI combination (Supplementary Figure 2), where weak men with low lean mass had the greatest odds of mobility impairment, though there was some evidence of heterogeneity across cohorts for the not weak with low lean mass group (Q test: p = .08, I 2 = 51%). Among men who were not weak, low lean mass defined by either criterion was not associated with incident mobility impairment. When weakness and low lean mass criteria were combined in women, there was significant heterogeneity among cohorts for the combinations of weak +ALM, weakBMI + ALM, and weakBMI + ALMBMI (Q test: all p < .10, all I 2 > 52%). For the weak + ALMBMI combination (Supplementary Figure 2), there were higher odds in all three risk groups relative to the not weak with “normal lean mass group,” with the highest odds in the “weak with low lean mass” group.
Mortality
Over 10 years of follow-up, 18% of women and 19% of men died (Supplementary Table 1). Weak and weakBMI criteria were associated with 63%–74% higher mortality rates among men, whereas in women, only weak was associated with a 48% higher rate (Supplementary Figures 5–6). Among men, ALM was associated with 37% greater morality rate, while ALMBMI was not associated with mortality (Supplementary Figures 7–8), though there was significant heterogeneity for this criterion (Q test: p = .02, I 2 = 75%). Neither ALM nor ALMBMI were associated with mortality among women. For all combinations of weakness and low lean mass criteria among men, mortality rates were consistently highest among those with weakness, though estimates were statistically significant only for men with normal lean mass (Supplementary Figures 9–12). Among men who were not weak, ALM was associated with 33%–41% higher mortality rate compared to normal lean mass, yet there was evidence of significant heterogeneity for ALMBMI. For combinations of weakness and low lean mass among women, those who were weak tended to have increased mortality rates, though mortality rate ratio CIs exclude the null value only for those who were weak with low lean mass for the weak + ALM combination (Supplementary Figure 9), and those who were weak with normal lean mass for the weak + ALMBMI combination (Supplementary Figures 10).
Discussion
Our results suggest that our criteria for low-absolute grip strength (weak) and low BMI-standardized grip strength (weakBMI) both strongly predict incident mobility impairment after 3 years among nonimpaired older men and women, although in women there was evidence of heterogeneity among cohorts for the association with weakBMI. Low BMI-standardized ALM (ALMBMI) was not as strongly associated with incident mobility as weakness, but it did significantly predict incident mobility impairment. When we explored whether the likelihood for mobility impairment varied across groups defined by combinations of our weakness and low lean mass criteria, there was no clear performance difference among the different combinations among the men. Yet among women, the weak + ALMBMI combination was the only one for which there was no evidence of heterogeneity among cohorts in the women. Based on our overall findings, we recommend weak and ALMBMI as potential criteria for clinically relevant weakness and low lean mass, respectively, among older men and women.
Those classified as weak had significantly greater odds of mobility impairment compared to those who were not weak, yet among the weak, the odds were similar for those with and without low lean mass. This finding suggests that among those without mobility problems, weakness is likely the key to identifying individuals at risk for future mobility impairment. Our results are consistent with the literature in that muscle strength is a stronger predictor of mobility problems than lean mass (5,24,25). The observed higher risk for mobility impairment in the group that was weak but had normal lean mass also highlights the important contributions of nonmass factors for muscle function, such as inter- and intramuscular fat infiltration (26,27), excitation-contraction coupling (28), and functions of the central nervous system and neuromuscular junction (29). Yet while weakness is clearly clinically important, there may be a specific subgroup among the weak for whom low muscle mass is an important cause of their weakness. It is important for clinicians to be able to identify these patients because they are the most likely to benefit from therapies that increase muscle mass. Our cut-points may be useful for finding these individuals who are both weak and have low lean mass. The low numbers of individuals who were classified as having both weakness and low lean mass in our analyses (≤8% of women and ≤4% of men), was due, in part, to the fact that our studies were composed of generally healthy, nondisabled older adults. Future research is needed to determine whether improvements in lean mass among those who are weak translate into improved mobility.
Two recent prospective studies evaluated the validity of the European Working Group on Sarcopenia in Older People sarcopenia criteria (7) for predicting mortality in older adults: Arango-Lopera and colleagues (30) examined 345 residents of Mexico City, who were aged 70 years or older, over 3 years of follow-up, and Landi and colleagues (31) followed 364 men and women, who were aged 80–85 years and living in the Sirente area of Italy, for 7 years. In both cohorts, sarcopenia, defined by the European Working Group on Sarcopenia in Older People as slow gait speed or low grip strength combined with low lean mass, was associated with a greater than twofold higher risk for all-cause mortality. Because the European Working Group on Sarcopenia in Older People criteria combines both weakness and low lean mass in a single classification of sarcopenia, these prior studies could not determine which of these two elements may be most important for predicting mortality. Similar to incident mobility impairment, our all-cause mortality results support weakness over low lean mass as the predominant predictor of clinical outcomes. Both of our weakness criteria predicted mortality among men, although in women only weakness by absolute grip strength was associated with mortality. Conversely, low lean mass was not a consistent predictor of mortality in either men or women. Combining weakness and low lean mass criteria resulted in no consistent patterns of associations for the four groups, though results suggested that those classified as weak had the highest mortality rates regardless of their lean mass status. Our mortality results were not fully consistent with the mobility impairment results perhaps because death is farther down the causal pathway from strength and lean mass than mobility impairment. Additional studies are needed to examine the relation of our criteria with other, more proximal relevant clinical geriatric outcomes (eg, self-reported disability, active vs disabled life expectancy, falls).
This study has some important limitations. First, mobility impairment analyses included only those who were not impaired at baseline. Thus, further investigation of the ability of our criteria to predict relevant clinical outcomes within more impaired populations is needed. Nevertheless, our criteria may identify subgroups of older adults that may be targeted for prevention of mobility problems. Second, results may have been influenced by differences among the pooled cohorts, including the varying follow-up times for gait speed assessments. In our meta-analyses, however, we did assess the heterogeneity across cohorts for associations of weakness and low lean mass criteria with mobility impairment and mortality, and we selected our recommended criteria only from among those without statistically significant evidence for heterogeneity. Third, over 26,000 individuals were included in the Sarcopenia Project, yet only 6,280 were included in the current analysis, limiting generalizability. Fourth, it is important to note that there are many other factors apart from muscle strength and mass that contribute to incident mobility impairment in older adults, which we did not account for in our analyses. In particular, several co-morbid conditions can influence the amount of strength required to ambulate (32). Nevertheless, in this initial phase of the Sarcopenia Project, our goal was to establish the predictive validity of our criteria for the general population of community-dwelling older adults. Future work should assess the performance of these preliminary criteria within relevant disease states as well as other specific populations (eg, institutionalized older adults, racial/ethnic groups). Finally, the methods used to derive our cut-points for weakness and low lean mass do not provide estimates of precision nor do they evaluate any underlying biologic relation among strength, lean mass, and mobility (10,11). Thus, our proposed criteria should be interpreted as preliminary recommendations.
Our study has several strengths. This is the largest endeavor to date to develop data-driven criteria for clinically relevant weakness and low lean mass. Our analyses included a large number of men and women from six diverse, community-based cohorts with information on objective measures of lean mass, strength, and mobility, as well as longitudinal information on incident mobility impairment and mortality, two clinically important aging outcomes. We analyzed pooled, individual-level data, which has advantages over aggregating results from individual studies, including assessment of individual-level missing data, standardized statistical analysis, and consistent adjustment for confounders (33).
Conclusion
Among older adults without mobility impairment, our criteria for clinically relevant weakness and low lean mass predict incident mobility impairment over 3 years of follow-up. Our analyses suggest that grip strength (<26kg in men and <16kg in women) and ALM standardized to BMI (<0.789 in men and <0.512 in women) are preliminary candidate criteria for weakness and low lean mass, respectively. These cut-points must be evaluated in additional studies to determine whether they identify the groups of older adults with mobility problems that are likely to benefit from interventions designed to maintain or improve mobility.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
Funding support for the conference and the work of the consortium was provided by the National Institute on Aging (1U13AG041583 and P30 AG024827), the Food and Drug Administration and through grants from the Foundation for the National Institutes of Health, made possible by funding from Abbott Nutrition, Amgen, Eli Lilly, Merck, Novartis, and The Dairy Research Institute.
Acknowledgments
Additional acknowledgments for each contributing cohort and members of the FNIH Sarcopenia Project can be found in an online supplementary material.
References
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763 [DOI] [PubMed] [Google Scholar]
- 3. Melton LJ, 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630 [PubMed] [Google Scholar]
- 4. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860 [DOI] [PubMed] [Google Scholar]
- 5. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 6. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. :10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. :10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. :10.1016/j.clnu.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 9. Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. :10.1016/j.jamda.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;5:559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cawthon PM, Peters KW, Shardell MD, et al. Cut-points for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;5:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale and study description. J Gerontol A Biol Sci Med Sci. 2014;5:547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773 [DOI] [PubMed] [Google Scholar]
- 14. Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–2108 [DOI] [PubMed] [Google Scholar]
- 15. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585 [DOI] [PubMed] [Google Scholar]
- 16. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials. 2005;26:557–568 [DOI] [PubMed] [Google Scholar]
- 17. Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56:1665–1673. :10.1111/j.1532-5415.2008.01846.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koster A, Visser M, Simonsick EM, et al. Association between fitness and changes in body composition and muscle strength. J Am Geriatr Soc. 2010;58:219–226. :10.1111/j.1532-5415.2009.02681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290 [DOI] [PubMed] [Google Scholar]
- 20. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 21. Härkönen R, Piirtomaa M, Alaranta H. Grip strength and hand position of the dynamometer in 204 Finnish adults. J Hand Surg Br. 1993;18:129–132 [DOI] [PubMed] [Google Scholar]
- 22. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231 [DOI] [PubMed] [Google Scholar]
- 23. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Orlando, FL: Academic Press; 1985 [Google Scholar]
- 24. Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609 [DOI] [PubMed] [Google Scholar]
- 25. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. :10.1093/gerona/glr010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985). 2001;90:2157–2165 [DOI] [PubMed] [Google Scholar]
- 27. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904 [DOI] [PubMed] [Google Scholar]
- 28. Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging. 2000;4:162–164 [PubMed] [Google Scholar]
- 29. Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25 [DOI] [PubMed] [Google Scholar]
- 30. Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, Pérez-Zepeda MU, Cesari M. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging. 2013;17:259–262. :10.1007/s12603-012-0434-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203–209. :10.1093/ageing/afs194 [DOI] [PubMed] [Google Scholar]
- 32. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263 [DOI] [PubMed] [Google Scholar]
- 33. Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. :10.1136/bmj.c221 [DOI] [PubMed] [Google Scholar]