Abstract
Background.
Low lean mass is potentially clinically important in older persons, but criteria have not been empirically validated. As part of the FNIH (Foundation for the National Institutes of Health) Sarcopenia Project, this analysis sought to identify cutpoints in lean mass by dual-energy x-ray absorptiometry that discriminate the presence or absence of weakness (defined in a previous report in the series as grip strength <26kg in men and <16kg in women).
Methods.
In pooled cross-sectional data stratified by sex (7,582 men and 3,688 women), classification and regression tree (CART) analysis was used to derive cutpoints for appendicular lean body mass (ALM) that best discriminated the presence or absence of weakness. Mixed-effects logistic regression was used to quantify the strength of the association between lean mass category and weakness.
Results.
In primary analyses, CART models identified cutpoints for low lean mass (ALM <19.75kg in men and <15.02kg in women). Sensitivity analyses using ALM divided by body mass index (BMI: ALMBMI) identified a secondary definition (ALMBMI <0.789 in men and ALMBMI <0.512 in women). As expected, after accounting for study and age, low lean mass (compared with higher lean mass) was associated with weakness by both the primary (men, odds ratio [OR]: 6.9 [95% CI: 5.4, 8.9]; women, OR: 3.6 [95% CI: 2.9, 4.3]) and secondary definitions (men, OR: 4.3 [95% CI: 3.4, 5.5]; women, OR: 2.2 [95% CI: 1.8, 2.8]).
Conclusions.
ALM cutpoints derived from a large, diverse sample of older adults identified lean mass thresholds below which older adults had a higher likelihood of weakness.
Key Words: Muscle, Sarcopenia, Cutpoints.
Early efforts to create an operational definition of sarcopenia (including the creation of cutpoints) have relied on distributional definitions of lean mass (1), with sarcopenia defined as a value of appendicular lean mass (ALM)/height2 (derived from whole-body dual-energy x-ray absorptiometry [DXA]) below the young adult mean level of lean mass or was based on definitions that further account for body size or fatness (2–4). More recent efforts have added functional and/or strength measures to lean mass to define sarcopenia (5,6), but no approaches thus far have proposed and validated cutpoints and definitions based on discriminative and predictive ability using a data-driven approach from a variety of cohort studies.
The overarching goal of this set of concurrent reports from the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project was to determine preliminary data-driven criteria for clinically relevant weakness and low lean mass. The conceptual framework was based on a clinician making a “differential diagnosis” of mobility impairment, defined as slow gait speed. The clinician understands that there are many causes of slow walking, one of which is weakness. Similarly, low lean mass may be considered a potential contributing factor to the development of weakness (7). Data from multiple large cohort studies of aging were pooled for this effort (7). The first stage of analyses identified sex-specific cutpoints for weakness that discriminated slow participants (walking speed <0.8 m/s) from those who walked faster (8). In the second stage of the analyses, reported herein, we aimed to identify cutpoints in lean mass that discriminated those who were weak (grip strength <16kg in women or <26kg in men) from those who were stronger. The findings from this work were used to address subsequent goals of the Project, so it is important to consider these results within the context of all other articles in this series.
Methods
Participants
The cohort studies and the clinic visit used in this phase of the FNIH Sarcopenia Project analysis included: the Study of Osteoporotic Fractures (SOF), both the original cohort (study Visit 6) (9) and African American cohort (study Visit 1) (10); the Osteoporotic Fractures in Men Study (MrOS, baseline visit) (11); the Health, Aging and Body Composition Study (Health ABC, Year 6 Clinic Visit) (12); the Framingham Study Offspring Cohort (exam cycles 6 and 7, 1996–2001) (13) and Framingham Original cohort (exam cycle 22, 1992–1993) (14); the Boston Puerto Rican Health Study (BPRHS, baseline visit) (15); Rancho Bernardo (study Visit 7) (16); and several smaller clinical trials led by Dr. Anne Kenny at University of Connecticut (randomization visit for all studies) (17–22). To be included in these analyses, participants must have completed, at the time point identified above, the following measures: objectively measured height and weight; body mass index (BMI); ALM (sum of lean mass in the arms and legs), leg lean mass (LLM), and total fat by DXA; and grip strength. Of the 26,625 participants aged 65 years and older in the FNIH Consortium pooled data, 7,069 were ineligible because they were in studies that did not have DXA scans; 6,364 were not eligible for DXA scans within their study; 1,170 were eligible but missing DXA data; and an additional 752 were missing data for other covariates, yielding a final sample size of 11,270 (7,582 men and 3,688 women). Participants excluded due to missing data were older, slower, weaker, had lower BMI, and were more likely to be women than those included in the analyses.
Assessment of Lean Mass
ALM, LLM, and total body fat (TBF) were assessed using DXA, on Hologic 4500 machines in MrOS, Rancho Bernardo, and Health ABC; on Hologic 2000 machines in SOF (both the original and African American cohorts); and Lunar Prodigy machines in Framingham (both the Original and Offspring cohorts), BPRHS, and the clinical trials at the University of Connecticut.
Assessment of Grip Strength and the Definition of Weakness
Maximum grip strength of either hand was measured by handheld dynamometers. In the first phase of analyses (reported in an accompanying article) (8), the cutpoint for grip strength as a discriminator of slowness (defined as a walking speed of ≤0.8 m/s) was identified using classification and regression tree (CART) analysis (23). Men with a grip strength less than 26kg and women with a grip strength less than 16kg were defined as “weak.” A secondary cutpoint for weakness based on grip strength standardized to body size (ie, the ratio of grip strength to BMI, weaknessBMI) was identified for men (men with a ratio <1.0 defined as weak) and women (women with a ratio of <0.56 defined as weak.) Those analyses to identify a cutpoint in grip strength included 20,847 participants, of whom 10,036 were also included in our analyses; 1,207 participants were included our analyses but not included in the grip strength analyses. Most of the exclusions from the grip strength analyses were due to missing data for walking speed; and most of the exclusions from the present analyses were due to missing values for body composition (ALM or body fat).
Statistical Analysis
LOESS plots were used to describe the overall shape of the relationship between lean mass (both ALM and ALMBMI) with grip strength and walking speed; Pearson correlation coefficients were calculated.
CART analysis was then performed to derive clinically meaningful cutpoints for lean mass as a discriminator of weakness. CART is particularly advantageous to this study because (i) the relationship between candidate predictors and weakness does not require specification, (ii) CART can identify complex multiway interactions between potentially important variables (eg, BMI, height), and (iii) predictors and cutpoints are selected to optimize discrimination of the outcome (weakness).
CART analysis was performed using the rpart procedure of R software (version 2.10.1), and cross-validation was used to “prune” less important splits to prevent overfitting and produce a more parsimonious tree. Cross-validation was performed by randomly partitioning the pooled data into 10 equally sized mutually exclusive data sets (ie, each set excluded 10% of the original pooled data). The tree was then applied to 10 subsamples that contained 90% of the data (ie, 10% of the data was left out of each subsample), and the prediction error from each subsample was calculated. The 10 prediction errors (error sum of squares) were used to calculate the empirical standard error of the prediction error. Following published guidelines (24), the tree was pruned to the most parsimonious tree that was within 1 SE of the tree with the smallest prediction error. This pruned tree contains the final set of lean mass cutpoints.
Several CART models were run. First, ALM and LLM were entered into a CART model as the only potential discriminators of weakness (defined as grip strength <26kg for men and <16kg for women). Then, body size variables (height, weight, BMI, TBF) were added to the model. Third, measures of lean mass standardized to body size (the ratio of ALM to each measure of body size) were added and included ALMheight, ALMweight, , ALMBMI, ALMTBF, LLMheight, LLMweight, , LLMBMI, and LLMTBF. Finally, we consider only ALMBMI and ALM as potential discriminators of weakness.
We report the prevalence for low lean mass by various subgroups in the cohorts (such as age, BMI, and history of disease) and the likelihood of prevalent weakness by low lean mass across these subgroups. We also report the likelihood of slowness and inability to rise from a chair by various weakness and low lean mass categories. These estimates were derived using mixed-effects logistic regression that included a random effect for cohort to account for the heterogeneity between studies.
Results
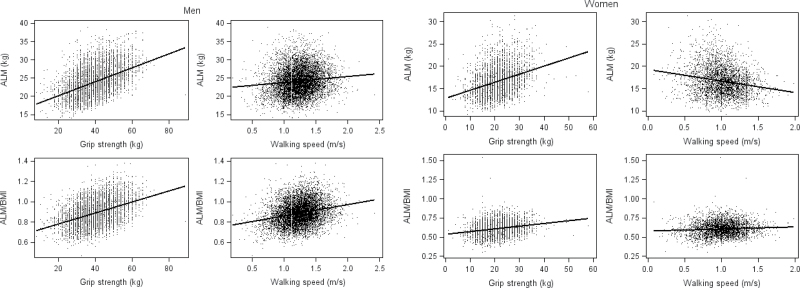
ALM was positively correlated with grip strength in men (r = .47, p < .001) and somewhat less strongly in women (r = .33, p < .001) (Figure 1). ALM was only modestly positively correlated with walking speed in men (r = .11, p < .001) and was inversely correlated with walking speed in women (r = −.20, p < .001). ALMBMI was positively correlated with grip strength (r = .42, p = <.001) and walking speed (r = .24, p < .001) in men as well as in women (grip strength, r = .22, p < .001 and walking speed, r = .07, p < .001). Body weight was strongly correlated with ALM in both men (r = .80, p < .001) and women (r = .81, p < .001).
Figure 1.
Scatterplots and correlation of appendicular lean mass (ALM) or ALM/body mass index (BMI) versus grip strength or walking speed for men and women in the FNIH (Foundation for the National Institutes of Health) Sarcopenia Project.
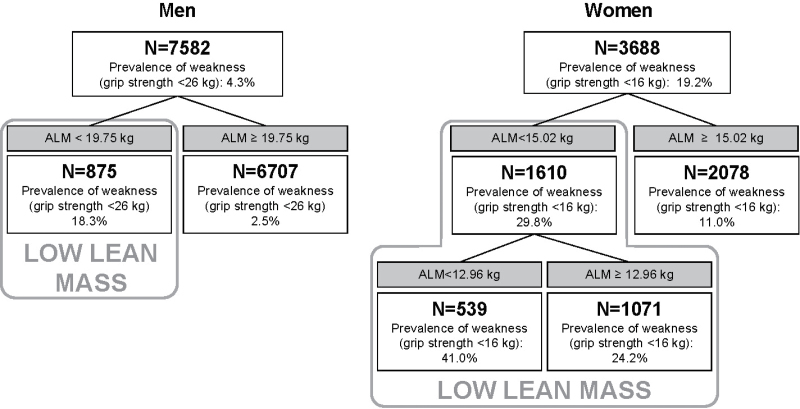
The CART models for the primary definition of weakness (grip strength <16kg in women and <26kg in men) demonstrated that ALM was the best discriminator of weakness, regardless of inclusion of body size variables (weight, TBF, height, height2 or BMI) or lean mass variables standardized to body size, or both. (Figure 2).
Figure 2.
Classification and regression tree models for measures of lean mass, body size, and lean mass standardized to body size discriminating weakness in older men and women in the FNIH (Foundation for the National Institutes of Health) Sarcopenia Project. Model included the following potential discriminators of weakness (grip strength <16kg in women and <26kg in men): ALM (appendicular lean mass), height, weight, height2, total body fat (TBF), BMI (body mass index), ALMheight (ALM/height), ALMweight (ALM/weight), (ALM/height2), ALMBMI (ALM/BMI), ALMTBF (ALM/TBF), LLMheight (LLM/height; LLM = leg lean mass), LLMweight (LLM/weight), (LLM/height2), LLMTBF (LLM/TBF), and ALMBMI (LLM/BMI).
In men, a single cutpoint for ALM was found. Men with an ALM less than 19.75kg were defined as having low lean mass; the prevalence of weakness was 18.3% in this group compared with only 2.5% for men with higher lean mass (ALM ≥ 19.75kg).
In women, two cutpoints for ALM were found: one at 15.02 kg and another at 12.09kg. For parsimony, and to propose a definition analogous to that for men, we defined two groups of women: those with low lean mass (ALM < 15.02kg) and higher lean mass (ALM ≥ 15.02kg). The prevalence of weakness for women with low lean mass was 29.8% compared with a prevalence of weakness of 11.0% for women with higher lean mass.
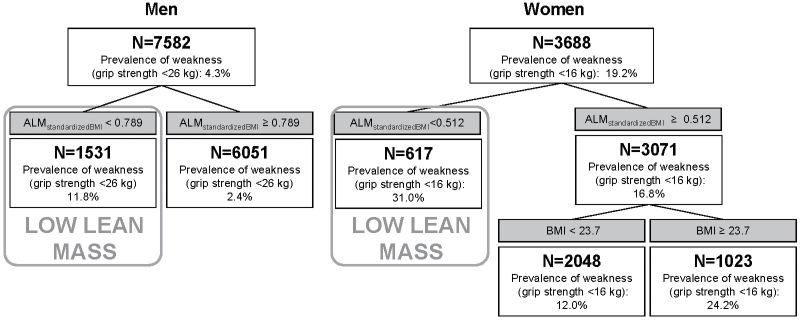
In secondary analyses, for men, when ALMBMI and BMI were the only potential discriminators of weakness, one cutpoint in ALMBMI was found. In these secondary analyses, we defined men with low lean mass as having a value of ALMBMI less than 0.789 (prevalence of weakness was 11.8%); men with an ALMBMI more than or equal to 0.789 had higher lean mass and a prevalence of weakness of 2.4% (Figure 3).
Figure 3.
Classification and regression tree models for ALMBMI and BMI discriminating weakness in older men and women in the FNIH (Foundation for the National Institutes of Health) Sarcopenia Project. Model included ALM and ALMBMI (ALM/BMI) as potential discriminators of weakness (grip strength <16kg in women and <26kg in men). ALM = appendicular lean mass; BMI = body mass index.
In secondary analyses, for women, when ALMBMI and BMI were the only potential discriminators of weakness included in the CART model, one cutpoint in ALMBMI was found: 0.512 (Figure 3), and for those with ALMBMI more than or equal to 0.512, a second cutpoint was found for BMI (23.7kg/m2). In the secondary analyses, we defined women with low lean mass as having ALMBMI less than 0.512 (prevalence of weakness was 31.0%); women with ALMBMI more than or equal to 0.512 had a prevalence of weakness 16.8% (Figure 3). Of the women with ALMBMI more than or equal to 0.512, those who had a BMI less than 23.7kg/m2 had a prevalence of weakness of 12.0%, whereas those with a BMI of more than or equal to 23.7kg/m2 had a prevalence of weakness of 24.2%.
Men with low lean mass were more likely to be weak compared with those with higher lean mass, after accounting for age and study by both the primary definition (odds ratio [OR]: 6.9 [95% CI: 5.4, 8.9]) or secondary definition (OR: 4.3 [95% CI: 3.4, 5.5]) (Table 1). Similarly, women with low lean mass were also more likely to be weak compared with those with higher lean mass by the primary definition (OR: 3.6 [95% CI: 2.9, 4.3]) or secondary definition (OR: 2.2 [1.8, 2.8]), although the strength of the association was smaller in magnitude than it was for men. For most stratified analyses, the association between low lean mass and weakness was significant (p < .05), while the point estimates varied by strata (although the interaction by stratifying factors was not significant for any model).
Table 1.
Prevalence of Low Lean Mass (based on ALM or ALMBMI) and Likelihood of Weakness Across Various Subsamples in the FNIH Sarcopenia Project*
| N | Prevalence of Weakness | OR (95% CI) for Weakness | ||
|---|---|---|---|---|
| Low Lean Mass (based on ALM) | Low Lean Mass (based on ALMBMI) | |||
| Men | ||||
| Overall | 7,582 | 0.04 | 6.9 (5.4, 8.9) | 4.3 (3.4, 5.5) |
| Age | ||||
| 65–79 | 6,001 | 0.03 | 6.2 (4.4, 8.8) | 3.9 (2.8, 5.5) |
| 80 | 1,581 | 0.11 | 5.0 (3.5, 7.2) | 3.3 (2.4, 4.7) |
| BMI | ||||
| Normal weight/underweight | 2,198 | 0.07 | 6.2 (4.2, 9.1) | 7.5 (5.0, 11.1) |
| Overweight | 3,848 | 0.04 | 8.6 (5.8, 12.8) | 5.3 (3.7, 7.7) |
| Obese | 1,536 | 0.03 | 10.3 (3, 35.7) | 4.7 (2.3, 9.7) |
| Height | ||||
| Tertile 1 (<1.7080 m) | 2,524 | 0.09 | 4.4 (3.2, 6.0) | 2.8 (2.0, 3.9) |
| Tertile 2 (≥1.708 m, <1.765 m) | 2,514 | 0.03 | 3.6 (2.1, 6.4) | 1.3 (0.7, 2.4) |
| Tertile 3 (≥1.7650 m) | 2,544 | 0.01 | 13.7 (5.0, 37.7) | 5.6 (2, 15.9) |
| Cancer | ||||
| Yes | 1,791 | 0.03 | 6.8 (3.8, 12.1) | 5.8 (3.3, 10.2) |
| No | 5,578 | 0.05 | 7.8 (5.9, 10.4) | 4.4 (3.3, 5.7) |
| CHF | ||||
| Yes | 373 | 0.10 | 9.2 (4.2, 19.8) | 4.7 (2.2, 10.0) |
| No | 6,248 | 0.04 | 7.6 (5.6, 10.3) | 4.7 (3.5, 6.2) |
| COPD | ||||
| Yes | 625 | 0.04 | 5.2 (2.2, 12.4) | 7.0 (2.8, 17.6) |
| No | 5,357 | 0.03 | 8.9 (6.2, 12.8) | 4.5 (3.2, 6.3) |
| Diabetes | ||||
| Yes | 759 | 0.07 | 5.1 (2.4, 10.8) | 2.7 (1.4, 5.1) |
| No | 6,809 | 0.04 | 7.3 (5.6, 9.5) | 4.5 (3.5, 5.9) |
| Women | ||||
| Overall | 3,688 | 0.19 | 3.6 (2.9, 4.3) | 2.2 (1.8, 2.8) |
| Age | ||||
| 65–79 | 2,633 | 0.15 | 3.5 (2.7, 4.5) | 2.4 (1.8, 3.1) |
| 80 | 1,055 | 0.29 | 2.9 (2.1, 4.0) | 2.0 (1.4, 2.9) |
| BMI | ||||
| Normal weight | 1,455 | 0.24 | 4.3 (3.0, 6.2) | 2.9 (1.8, 4.7) |
| Overweight | 1,299 | 0.17 | 3.2 (2.3, 4.5) | 3.0 (2.1, 4.3) |
| Obese | 934 | 0.14 | 3.6 (2.0.1, 6) | 3.3 (2.2, 5.1) |
| Height | ||||
| Tertile 1 (<1.560 m) | 1,229 | 0.28 | 3.3 (2.3, 4.6) | 1.3 (1.0, 1.8) |
| Tertile 2 (≥1.560 m, <1.610 m) | 1,225 | 0.18 | 2.4 (1.7, 3.5) | 1.8 (1.1, 2.8) |
| Tertile 3 (>1.610 m) | 1,234 | 0.11 | 2.5 (1.7, 3.8) | 2.4 (1.0, 5.7) |
| Cancer | ||||
| Yes | 241 | 0.26 | 5.3 (2.7, 10.6) | 1.8 (0.8, 3.9) |
| No | 2,508 | 0.17 | 3.8 (2.9, 5) | 2.4 (1.8, 3.1) |
| CHF | ||||
| Yes | 136 | 0.26 | 6.3 (2.7, 14.6) | 1.7 (0.7, 4.3) |
| No | 2,481 | 0.23 | 3.4 (2.7, 4.2) | 2.2 (1.7, 2.8) |
| COPD | ||||
| Yes | 196 | 0.20 | 5.6 (2.6, 12.2) | 2.5 (1.1, 5.9) |
| No | 1,356 | 0.22 | 3.5 (2.6, 4.6) | 2.5 (1.7, 3.6) |
| Diabetes | ||||
| Yes | 277 | 0.21 | 2.5 (1.2, 4.9) | 1.3 (0.6, 2.9) |
| No | 3,313 | 0.19 | 3.7 (3, 4.5) | 2.3 (1.9, 2.9) |
Notes: ALM = appendicular lean mass; BMI = body mass index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; FNIH = Foundation for the National Institutes of Health; OR = odds ratio.
*Weakness defined as grip strength <26kg in men and <16kg in women; low lean mass based on ALM defined as <19.75 in men and <15.02 in women; low lean mass based on ALMBMI defined as <0.789 in men and <0.512 in women. Medical conditions based on self-report of physician diagnosis. A total of 213 men and 939 women missing cancer information, 961 men and 1,071 women missing CHF information, 1,600 men and 2,136 women missing COPD information, and 14 men and 98 women missing diabetes status. No significant interactions were found in the stratified analyses.
In men, when weakness and low lean mass were considered jointly in the same model with slowness as the outcome, both factors were independently associated with prevalence of slowness (Table 2). Men who were weak (either by grip strength alone or by grip strength standardized to BMI) were about 3- to 4.5-fold more likely to be slow and 2.5- to 3.0-fold more likely to be unable to rise from a chair than men who were not weak. Men with low lean mass (either by ALM or ALMBMI) were about twice as likely to be slow compared with men with higher lean mass. Men with low lean mass by ALM, but not ALMBMI, were about 1.3- to 1.6-fold more likely to be unable to rise from a chair than men with higher lean mass.
Table 2.
Likelihood of Slowness* or Inability to Complete Chair Stands† (OR, 95% CI)‡, by Weakness and low Lean Mass in the FNIH Sarcopenia Project§
| Men | Women | |||
|---|---|---|---|---|
| Model 1 | Weak | Low lean mass (ALM) | Weak | Low lean mass (ALM) |
| Slowness | 3.04 (2.11, 4.38) | 1.56 (1.16, 2.09) | 2.21 (1.72, 2.83) | 0.74 (0.59, 0.92) |
| Inability to complete chair stands | 2.46 (1.62, 3.73) | 1.32 (0.94, 1.85) | 2.05 (1.37, 3.08) | 0.49 (0.36, 0.69) |
| Model 2 | Weak | Low lean mass (ALMBMI) | Weak | Low lean mass (ALMBMI) |
| Slowness | 2.91 (2.02, 4.17) | 2.12 (1.66, 2.71) | 1.96 (1.53, 2.51) | 1.55 (1.20, 2.02) |
| Inability to complete chair stands | 2.35 (1.56, 3.55) | 1.60 (1.21, 2.12) | 1.62 (1.09, 2.40) | 1.26 (0.86, 1.83) |
| Model 3 | WeakBMI | Low lean mass (ALM) | WeakBMI | Low lean mass (ALM) |
| Slowness | 4.24 (3.20, 5.62) | 1.71 (1.28, 2.28) | 2.91 (2.31, 3.66) | 0.86 (0.69, 1.07) |
| Inability to complete chair stands | 2.90 (2.10, 4.01) | 1.39 (0.99, 1.94) | 2.40 (1.67, 3.46) | 0.54 (0.39, 0.75) |
| Model 4 | WeakBMI | Low lean mass (ALMBMI) | WeakBMI | Low lean mass (ALMBMI) |
| Slowness | 3.55 (2.64, 4.79) | 1.71 (1.32, 2.22) | 2.77 (2.18, 3.51) | 1.27 (0.97, 1.66) |
| Inability to complete chair stands | 2.63 (1.86, 3.70) | 1.37 (1.02, 1.84) | 2.31 (1.58, 3.39) | 1.04 (0.70, 1.55) |
Notes: ALM = appendicular lean mass; BMI = body mass index; FNIH = Foundation for the National Institutes of Health; OR = odds ratio.
*Slowness is walking speed ≤0.8 m/s. For men, N = 337 (4.7%); 7,113 men included in slowness models. For women, N = 673 (22.8%); 2,950 women included in slowness models.
†Inability to complete five repeated chair stands. For men, N = 250 (3.5%); 7,095 men included in chair stand models (487 men were missing data for chair stands ability). For women, N = 198 (6.7%); 2,971 women included in chair stand models (717 women were missing data for chair stands ability).
‡Both weakness and low lean mass included in the same model adjusted for age and study. Slowness models include study as a random effect. Chair stands models include study as a covariate since the mixed-effects logistic regression models with study as a random effect did not converge.
§Weak defined as grip strength <26kg in men and <16kg in women; Weak BMI defined as grip strength/BMI as <1.001 in men; <0.56 in women; low lean mass based on ALM defined as <19.75kg in men and <15.02kg in women; low lean mass based on ALMBMI defined as <0.789 in men and <0.512 in women.
In women, when weakness and low lean mass were considered jointly in the same model with slowness as the outcome, only weakness was consistently associated with slowness. Women who were weak (either by grip strength lone or by grip strength standardized to BMI) were 2- to 3-fold more likely to be slow than women who were not weak. One the other hand, and in contrast to results for men, women with low lean mass based on ALM had a somewhat lower likelihood of being slow or unable to rise from a chair compared with women with higher lean mass. Women with low lean mass (based on ALMBMI) had about a 50% increased likelihood of slowness after adjustment for weakness based on grip strength alone. However, when the association between ALMBMI and slowness was adjusted for weakness standardized to BMI, the association was not significant. ALMBMI was not significantly associated with inability to rise from a chair for women.
When we compare the prevalence of low lean mass by our definition based on ALM with the prevalence of low lean mass by Baumgartner, overall percent agreement was 83.0% for men and 75.4% in women (Table 3). When we compare the prevalence of low lean mass by our definition based on ALMBMI with the prevalence of low lean mass by Baumgartner, the overall percent agreement is 69.1% in men and 70.7% in women.
Table 3.
Cross-Classification of Low Lean Mass by Baumgartner, ALM, and ALMBMI*
| Baumgartner criterion | ALM criterion | ALMBMI criterion | ||
|---|---|---|---|---|
| Men | Low lean mass | High lean mass | Low lean mass | High lean mass |
| Low lean mass | 5,510 (72.7%) | 1,197 (15.8%) | 4,657 (61.4%) | 1,390 (18.3%) |
| High lean mass | 97 (1.3%) | 778 (10.3%) | 950 (12.5%) | 585 (7.7%) |
| Women | Low lean mass | High lean mass | Low lean mass | High lean mass |
| Low lean mass | 2,050 (55.6%) | 28 (0.8%) | 2,462 (66.8%) | 615 (16.7%) |
| High lean mass | 879 (23.8%) | 731 (19.8%) | 467 (12.7% | 144 (3.9%) |
Notes: ALM = appendicular lean mass; BMI = body mass index.
*Low lean mass based on ALM criterion: <19.75kg in men, <15.02kg in women. Low lean mass based on ALMBMI criterion: <0.789 in men, <0.512 in women. Baumgartner criteria: ALM/height2 = 7.26kg/m2 in men and 5.45kg/m2 in women.
Discussion
The goal of these analyses, using pooled data from several large cohort studies of older men and women, was to identity cutpoints in values of low lean mass that discriminated those who were weak from those who were not weak. Primary analyses resulted in a definition of low lean mass as ALM less than 19.75kg in men and less than 15.02kg in women. Sensitivity analyses suggested an alternative definition using a value of ALM standardized to BMI (ALMBMI). Men with a value of ALMBMI less than 0.789 and women with a value of ALMBMI less than 0.512 were considered to have low lean mass by this secondary definition.
While our analyses were not designed to define or evaluate “sarcopenic obesity,” our results suggest that body size and potentially fatness influence the association between lean mass and weakness. This finding is similar to another report (4) that found that low lean mass based on the Baumgartner (1) criteria using ALM/height2 was less strongly related to physical disability than a measure of low lean mass that was adjusted for height and body fat mass (2). On the other hand, measures of obesity were not selected by the CART models as primary discriminators of weakness in older people.
The associations between our definitions of low lean mass and concurrent weakness are quite strong as would be expected by our analytical methodology. Men who had low lean mass by the primary definition based on ALM alone were about 7 times more likely to be weak (grip strength <26kg) than men with higher lean mass. In women, the association between low lean mass and weakness was somewhat lower but still substantial, as women with low lean mass by the primary definition based on ALM alone were about 4 times more likely to be weak (grip strength <16kg) than women with higher lean mass. However, the analysis technique employed requires caution when interpreting these data. The strong associations between our proposed definitions of low lean mass and weakness are not surprising, because of the analysis method (CART) derived the cutpoints to maximize the association between lean mass and weakness. We expressed the association between our lean mass cutpoints and weakness as ORs to further ease the clinical interpretation of our results. To understand the clinical implications of the proposed definitions of low lean mass described here, further analyses must be completed, including those of longitudinal data that would establish the predictive validity of our cutpoints and the independent association of these cutpoints with clinical outcomes thought to be related to weakness and low lean mass.
The association between low lean mass and slowness was independent of weakness in men. Men who had low lean mass (by either definition) were about 2–4.5 times more likely to be slow when compared with men with higher lean mass after accounting for weakness. On the other hand, in women, the association between low lean mass and slowness was inconsistent across definitions and was not consistently independent of concurrent weakness. These sex differences could be due to a number of factors. Men and women have drastically different body types and body composition, and women have more disability than men. It is possible that the association between body size, lean mass, weakness, and slowness truly differs between men and women. Another possibility is that differences between single-sex cohorts in the pooled data set (such as SOF and MrOS, for example) explain the discrepant results.
Our results are not directly comparable to other proposed definitions of low lean mass or sarcopenia, because these other definitions have accounted for body size in a different manner than our analyses. For example, the Baumgartner definition (1) (which has been included in consensus statements about sarcopenia) divides ALM by height2 to quantify lean mass and then defines individuals as “sarcopenic” if their value is at least 2 SDs below a young normal mean value. This equates to 7.26kg/m2 for men and 5.45kg/m2 for women. We found modest agreement between our definitions and the Baumgartner definition of low lean mass; thus, we conclude that our cutpoints and other sarcopenia definitions, as discussed in an accompanying report from this project (25), differentially classify individuals. We could not compare our definition against others that used non-DXA methods to determine lean mass (such as bioelectrical impedance analysis [BIA]) (3), because BIA was not available in most participating cohorts.
There are some limitations of these analyses. First, CART, by definition, partitions data into groups, even when the underlying relationship between the predictor and outcome is linear. Second, our CART models did not account for differences between studies included in the model, although we did account for study in subsequent logistic models. Third, while we accounted for cohort study a random effect in the logistic models, we did not specifically correct or adjust values to account for differences across cohorts. For example, the cohorts used various makes and models of DXA machines. There are no methods aside from in vivo cross-calibration studies that can accurately compare soft tissue estimates across DXA machines (26). As the machines included in this project were located throughout the world, such a study was not feasible. Additionally, unlike T-scores for bone mineral density (BMD) in the diagnosis of osteoporosis (which are used in part to account for machine differences in BMD estimates), there are no population-based values for all the DXA manufacturers for all the soft tissue compartments that would allow calculation for T or Z scores. Finally, no statistical model alone can identify a disease state. Thus, further work is necessary to understand the biological implications of these results.
We intend for the cutpoints in ALM and ALMBMI to be used within the context of the larger analysis project we describe in which we are trying to identify those older persons who are slow and weak whose impairments are likely attributable to lower levels of lean mass. We have shown that there is a level of ALM and ALMBMI below which strength is lower. In another report in this series, the independent predictive validity of these lean mass cutpoints for future mobility impairment is evaluated. Given the discordant literature that suggests that lean mass may not be independently predictive of functional decline once strength is known (27), these next analyses will be imperative.
The conceptual framework we used for this larger project—that there is a clinical syndrome that includes walking speed, strength, and lean mass that identifies individuals at risk for disability—is similar to the framework described by other consensus groups that have addressed this broad topic of “sarcopenia.” However, there is not clear consensus regarding which part or parts of this clinical syndrome constitute “sarcopenia” and which should be designated by some other distinction such as “mobility impairment with clinically relevant weakness and low lean mass.” To avoid confusion, we prefer terminology that precisely describes the results. For example, we have identified values of low lean mass that discriminate weakness in older adults.
In summary, we have identified cutpoints in lean mass that discriminate those who are weak from those who are stronger; secondary analyses suggest that adjustment for body size may influence the cutpoints selected. Different values were found in men and women, given the sex differences in body size. Future analyses must evaluate the independent predictive validity of these cutpoints.
Funding
Support for the conference and the consortium was provided by the National Institute on Aging (1U13AG041583 and P30 AG024827), the Food and Drug Administration, and through a grant from the Foundation of the NIH, supported by funds from Abbott Nutrition, Amgen, Eli Lilly, Merck, Novartis, and The Dairy Research Institute.
Acknowledgments
Additional acknowledgements for each contributing cohort and members of the FNIH Sarcopenia Project can be found in an online supplement.
References
- 1. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- 2. Newman AB, Kupelian V, Visser M, et al. ; Health ABC Study Investigators. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51(11):1602–1609. 10.1046/j.1532-5415.2003.51534.x [DOI] [PubMed] [Google Scholar]
- 3. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–421. 10.1093/aje/kwh058 [DOI] [PubMed] [Google Scholar]
- 4. Delmonico MJ, Harris TB, Lee JS, et al. ; Health, Aging and Body Composition Study. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55(5):769–774. 10.1111/j.1532-5415.2007.01140.x [DOI] [PubMed] [Google Scholar]
- 5. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Studenski SA, Peters KW, Alley DE, et al. The FNIH Sarcopenia Project: rationale and study description. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. [DOI] [PMC free article] [PubMed]
- 8. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. [DOI] [PMC free article] [PubMed]
- 9. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773 [DOI] [PubMed] [Google Scholar]
- 10. Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–2108. 10.1001/jama.293.17.2102 [DOI] [PubMed] [Google Scholar]
- 11. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 12. Newman AB, Haggerty CL, Goodpaster B, et al. ; Health Aging And Body Composition Research Group. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(3):323–330. 10.1046/j.1532-5415.2003.51105.x [DOI] [PubMed] [Google Scholar]
- 13. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–290 [DOI] [PubMed] [Google Scholar]
- 14. Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. 10.2105/AJPH.41.3.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. 10.1186/1471-2458-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25(3):226–231. 10.1016/S0749-3797(03)00197-1 [DOI] [PubMed] [Google Scholar]
- 17. Hutchins-Wiese HL, Kleppinger A, Annis K, et al. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging. 2013;17:76–80 [DOI] [PubMed] [Google Scholar]
- 18. Judge JO, Kleppinger A, Kenny A, Smith JA, Biskup B, Marcella G. Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporos Int. 2005;16(9):1096–1108. 10.1007/s00198-004-1816-x [DOI] [PubMed] [Google Scholar]
- 19. Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–M272. 10.1093/gerona/56.5.M266 [DOI] [PubMed] [Google Scholar]
- 20. Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc. 2003;51(12):1762–1767. 10.1046/j.1532-5415.2003.51561.x [DOI] [PubMed] [Google Scholar]
- 21. Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58(9):1707–1714. 10.1111/j.1532-5415.2010.03019.x [DOI] [PubMed] [Google Scholar]
- 22. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290(8):1042–1048. 10.1001/jama.290.8.1042 [DOI] [PubMed] [Google Scholar]
- 23. Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984 [Google Scholar]
- 24. Maindonald J, Braun WJ. Data Analysis and Graphics Using R: An Example-Based Approach (Cambridge Series in Statistical and Probabilistic Mathematics). 3rd ed. Cambridge UniversityPress; 2010 [Google Scholar]
- 25. Dam TL, Studenski SA, Peters KW. et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. [DOI] [PMC free article] [PubMed]
- 26. Blake GM, Fogelman I. Technical principles of dual energy x-ray absorptiometry. Semin Nucl Med. 1997;27(3):210–228 [DOI] [PubMed] [Google Scholar]
- 27. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35(1): 51–65. 10.1093/epirev/mxs006 [DOI] [PubMed] [Google Scholar]