Abstract
Background.
Depressive symptoms and cognitive outcomes are strongly interrelated. Despite that rates of depressive symptoms fluctuate during late life, little is known about the impact of long-term cumulative depressive symptom burden on cognitive decline and dementia in older adults. This study examines the association of nearly 20 years of cumulative depressive symptoms with cognitive outcomes in a cohort of older women.
Methods.
We assessed depressive symptoms in 7,240 women using the Geriatric Depression scale (GDS) at serial visits. We used a Poisson model with random slopes to estimate GDS trajectories for each participant from baseline to death or end of follow-up, and then characterized depressive symptom burden by quartile of the area under the curve. We assessed cognitive outcomes using repeated measures of the Mini-Mental State Examination (MMSE) and Trails B score over 20 years, Year-20 neuropsychological test battery, and adjudicated dementia and mild cognitive impairment (MCI).
Results.
Adjusting for potential confounders, compared with women in the lowest quartile of cumulative depressive symptoms burden, women in the highest quartile had 21% more MMSE errors over time (95% CI = 17%, 26%), 20% worse Trails B score over time (95% CI = 17%, 23%), worse scores on most of the Year-20 cognitive tests, and a twofold greater likelihood of developing dementia or MCI (95% CI = 1.48, 3.11).
Conclusions.
Long-term cumulative depressive symptom burden was associated with cognitive decline and risk of dementia or MCI. Older adults with a history of depression should be closely monitored for recurrent episodes or unresolved depressive symptoms as well as any cognitive deficits.
Key Words: Depression, Cognition, Alzheimer’s.
Depressive symptoms and cognitive impairment are separate clinical entities that are strongly interrelated (1), associated with poor quality of life (1,2), and both very common in old age. For example, as many as 25% of community-living older adults experience depressive symptoms (3) that continue to occur at high rates during late life (4). Additionally, the risk of cognitive impairment and dementia increases sharply after age 65 years, to more than 50% in the oldest old (aged 80 years and older) (5,6).
There are several potential mechanisms by which depressive symptoms processes could influence cognitive impairment and dementia-specific neuropathology. Some of the most prominent mechanisms include vascular disease and amyloid-β plaque formation (7). In line with the “vascular depression” hypothesis, there is strong evidence that vascular disease is a major mechanism underlying depression and cognitive outcomes (8–10). Behavioral risk factors such as smoking and physical inactivity are suggested to play a role in the mechanisms linking vascular disease, depression, and cognitive outcomes (7). In addition, cross-sectional and longitudinal results have suggested amyloid-β plaques formation and neurofibrillary tangles, which are major hallmarks of Alzheimer’s disease, as possible mechanisms linking depression to dementia and cognitive decline (11–13).
Importantly, rates of depression and depressive symptoms tend to fluctuate over time during late life (4,14), and the elderly adult may exhibit different trajectories of depressive symptoms compared with younger adults (15–18). Recent findings in a cohort of elderly women have shown approximately 20% had persistently high or increasing depressive symptoms over nearly 20 years (14). Given the fluctuating nature of depressive symptoms over time and their complex interrelationship and co-occurrence with cognitive outcomes and dementia; therefore characterizing the long-term course and chronicity of depressive symptoms may be more informative to our understanding of these relationships. Yet, and in addition to inconsistent findings in the literature, the vast majority of longitudinal studies of depressive symptoms and cognitive outcomes in older adults have examined single-time (usual baseline) measure of depressive symptoms (7,19–27).
In this study, we sought to capture the cumulative burden and chronicity of depressive symptom trajectories over nearly 20 years of follow-up. And then to investigate its association with cognitive decline and the risk for development of cognitive impairment and dementia in elderly women who were followed into their 9th and 10th decades of life. A better understanding of the relationship of depressive symptoms and cognitive function and dementia over the long term is vital, as it may provide important clinical implications for early intervention and prevention strategies.
Methods
Study Population
Participants were enrolled in the Study of Osteoporotic Fractures (SOF), an ongoing prospective cohort study of women aged 65 years and older originally recruited between September 1986 and October 1988. A total of 9,704 Study of Osteoporotic Fractures participants were recruited from population-based listings in four areas of the United States: Baltimore, Maryland; Minneapolis, Minnesota; Portland, Oregon; and Monongahela Valley, Pennsylvania. Women were excluded if they were unable to walk without help or had a bilateral hip replacement. Study participants have been followed for nearly 20 years. Every several years, biological and clinical data were collected from the participants who attended the clinics at the various sites. The study was approved by the appropriate committees on human research at each site. All participants provided written informed consent. Further details on the study design and recruitment have been published elsewhere (28). During the study, a total of 3,654 women died, and 622 women ended the study early. The median follow-up time duration was 12.2 years.
Measures
Measurement of depressive symptoms
We evaluated depressive symptoms using the 15-item Geriatric Depression scale (GDS; range 0–15) with higher scores indicating more symptoms of depression. The GDS has been validated and widely used in population-based studies of the elderly (29). GDS was first administered to our study participants in Year 2 (1988–1990), which is the current study baseline, and then repeated at Years 6, 10, 15, and 20. Our study included 7,240 women who had at least two GDS measurements during nearly 20 study years. The mean number of GDS measurements was 3.4.
Estimating depressive symptom trajectories and cumulative burden
We used unadjusted Poisson models to estimate the age-dependent trajectories of depressive symptoms for each participant. The Poisson distribution is appropriate because GDS scores are right-skewed nonnegative integers. We modeled the association of increasing age using restricted cubic spline with three knots, placed at the 5th, 50th, and 95th percentiles of the age distribution of the repeated observations. The models also include a linear term in age as a participant-specific random effect along with a random intercept to account for within-subject correlation of the repeated depressive symptom scores. Then, in order to obtain an accurate measure of each participant’s trajectory of depressive symptom burden over the study, we obtained from the Poisson fitted model the best linear unbiased prediction of expected depressive symptom trajectory for each participant beginning at baseline and ending at death or end of follow-up. Calculating the best linear unbiased prediction summary measure of depressive symptom burden is important for participants with missing or highly variable depressive symptom scores. Then, for each participant, we calculated areas under the curve (AUCs) for the depressive symptom trajectory, as a measure of depressive symptom burden. Multiple AUCs were calculated for each participant, each beginning at age 67 and ending 3 years before the cognitive function measurements obtained at the 1st through 5th visits. For example, for a participant with cognitive function tests at age 72, 77, 82, 86, and 92, the AUCs would be evaluated from age 67 through ages 69, 74, 79, 83, and 89, respectively. We then compared quartile of the AUCs (quartile 1–4 = minimal to high depressive symptom burden), where quartile 1 has a mean of 1.4 (range 0.1–8.6), quartile 2 has a mean of 3.1 (range 0.12–17), quartile 3 has a mean of 6.2 (range 0.2–32), and quartile 4 has a mean of 15 (range 0.42–117).
Measurement of cognitive function
We evaluated the cognitive function using a modified Mini-Mental State Examination (MMSE) and Trails B that were administered at study baseline and repeated at subsequent clinic visits. The MMSE is a brief test of global cognitive function (30) with higher scores indicating better performance. The modified MMSE administered in our study (range 0–26) includes 5 items of the original 11 items, namely items for temporal orientation, registration, mental reversal, recall, and intersecting pentagons. The five items were chosen according to work by Magaziner and colleagues (31) and scored according to the scoring system developed by Teng. Trails B is a test of executive functioning during which participants were timed to connect letters and numbers (32) with higher scores indicating worse cognitive function.
At the Year-20 exam, an expanded neuropsychological test battery measuring various cognitive domains was administered to study participants. The battery included the California Verbal Learning Test, Digit Span, Modified Mini Mental State Exam (3MS), and category fluency and verbal fluency tests. California Verbal Learning Test is a verbal memory recall test with immediate and delayed memory (33). Higher California Verbal Learning Test scores indicate better function. The Digit Span is a test in which participants were presented with a list of numbers and then asked to repeat the list in the correct order in a forward and a backward fashion (34). The 3MS is a 100-point test of global cognitive function with higher scores indicating better function (35). Finally, for the category fluency and verbal fluency tests, participants were scored based on the number of words they could cite in 1 minute from a given category (ie, vegetables and words beginning with the letter “F”). Higher category fluency and verbal fluency scores indicate better function.
Dementia/mild cognitive impairment diagnosis
At Year-20 exam, dementia and mild cognitive impairment (MCI) diagnosis was determined using a two-stage process. First, participants were screened for cognitive impairment using the expanded neuropsychological test battery. Those who screened negative were considered cognitively normal. Those who screened positive were further clinically adjudicated by a panel of clinical experts. Criteria for a diagnosis of dementia were based on the Diagnostic and Statistical Manual of Mental Disorders (36) and that of MCI were based on a modified Peterson criteria (37). Further details on the dementia/MCI diagnosis have been published elsewhere (6).
Covariates
Except for race and education, information on covariates was collected during baseline examination and at subsequent clinic visits. Participants self-reported their age, education, race (white vs African American), marital status, smoking status, current alcohol consumption (defined as number of glasses/week), and physical activity. Height and weight were measured, and body mass index was calculated as weight divided by height squared (in kg/m2). Participants self-reported whether they had ever been diagnosed by a physician with a variety of medical conditions including hypertension, heart attack, stroke, and diabetes. Finally, participants were asked about current use (within the past 30 days) of medications including antidepressants; reports of current use were checked by examining the labels of drugs. Coding of medications was conducted using computerized dictionary.
Statistical Analysis
We used linear mixed models with random intercepts and slopes to estimate the associations between quartile of depressive symptom burden (AUCs), as a time-dependent covariate, with all available repeated measures of the MMSE and Trails B scores. In all linear mixed models, age is modeled using a restricted cubic spline with three knots, placed at the 5th, 50th, and 95th percentiles of the age distribution of the repeated observations. To meet the normality assumption, we analyzed log-transformed errors on the MMSE and log-transformed Trails B scores. We then back-transformed estimates; because the AUCs are time-dependent, the back-transformed estimates are interpretable as the expected percent change in test scores when the AUC increases by 1 quartile between sequential visits. To reduce bias from increases in depressive symptom burden caused by, rather than resulting in, recent subclinical changes in cognitive function, we lagged the time-dependent AUCs by 3 years. In addition, to ensure that confounders were measured prior to the lagged AUCs, we obtained other time-dependent covariates from the previous visit. We also analyzed the association between quartiles of the lagged AUCs and Year-20 cognitive test scores with linear models and odds of MCI and dementia with logistic regression models (N = 1,293); this analysis also used covariates from the most recent visit before Year 20.
We first conducted unadjusted models. We then adjusted for a pre-specified list of potential confounders by first adjusting for sociodemographics (age, education, and marital status) and then additionally adjusting for health behaviors (smoking, alcohol use, body mass index, and exercise), comorbidities (hypertension, heart attack, stroke, diabetes, and use of antidepressants), and study site. Effects of the continuous covariates were flexibly modeled using three-knot restricted cubic splines.
There was substantial attrition from the Study of Osteoporotic Fractures over 20 years, primarily due to death, and secondarily due to dropout, which could induce bias in our estimates of the associations of cumulative depressive burden on cognitive function. Our maximum likelihood models would be expected to provide consistent estimates if the outcomes were missing at random, given the observed outcomes as well as the predictors included in the model. This assumption is made more plausible by our adjustment for the several important correlates of dropout, including age, living status, smoking, weight, exercise, and several comorbidities. In addition, we conducted sensitivity analyses using joint modeling of cognitive function and time to death or dropout (38). In these analyses, the mixed model for the repeated cognitive function measure was linked to the pooled logistic model for death/dropout by shared random intercepts and slopes. We also used joint modeling to assess potential bias from attrition in our estimates of the mean depressive burden trajectories.
Primary analyses were conducted using Stata version 12.1 software (Stata Corporation, College Station, TX). Sensitivity analyses using joint modeling were implemented using Proc Nlmixed in SAS version 9.2 (SAS Institute, Cary, NC).
Results
At baseline, the 7,240 women had a mean age of 73.3 years and a mean of 12.7 years of education (Table 1). Higher quartile of depressive symptom burden was associated with older age, less education, being unmarried, less alcohol consumption, less physical activity, and higher body mass index (p < .001 for all), at baseline. Higher quartile of depressive symptom burden was also associated with a baseline history of diabetes, hypertension, stroke, and myocardial infarction.
Table 1.
Distribution of the Sample Baseline Characteristics by Quartile of Depressive Symptom Burden, Study of Osteoporotic Fractures, 1988–2008 (N = 7,240)
| Covariates | Overall | Quartiles of Depressive Symptoms Burden | p Value | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| N = 7,240 | n = 1,810 | n = 1,810 | n = 1,810 | n = 1,810 | ||
| Age, y, mean (SD) | 73.3 (4.9) | 71.2 (3.5) | 73.0 (4.4) | 74.1 (5.2) | 74.9 (5.3) | <.001 |
| Education, y, mean (SD) | 12.7 (2.8) | 13.1 (2.7) | 13.0 (2.7) | 12.6 (2.8) | 12.2 (2.8) | <.001 |
| Marital status, % married | 46.8 | 50.6 | 47.7 | 45.8 | 42.9 | <.001 |
| Alcohol use, glasses/wk, mean (SD) | 1.8 (4.0) | 2.2 (4.1) | 1.8 (3.5) | 1.6 (3.4) | 1.5 (3.5) | <.001 |
| Smoking, % current | 6.3 | 5.6 | 5.6 | 6.3 | 7.7 | .45 |
| Physical activity, kcal, mean (SD) | 622 (688) | 730 (778) | 642 (682) | 605 (657) | 510 (603) | <.001 |
| Body mass index, kg/m2, mean (SD) | 26.3 (4.6) | 25.9 (4.4) | 26.2 (4.5) | 26.5 (4.6) | 26.7 (4.9) | <.001 |
| Diabetes, % yes | 6.4 | 5.3 | 5.6 | 6.2 | 8.6 | <.001 |
| Hypertension, % yes | 40.0 | 37.0 | 36.1 | 42.6 | 44.2 | .002 |
| Stroke, % yes | 3.1 | 2.1 | 2.4 | 4.0 | 3.9 | <.001 |
| Myocardial infarction, % yes | 6.1 | 4.2 | 5.0 | 6.8 | 8.5 | <.001 |
| Use of antidepressants, % yes | 3.3 | 2.8 | 2.2 | 3.0 | 5.2 | <.001 |
Results from linear mixed models of the associations between quartiles of cumulative depressive symptom burden and cognitive decline (MMSE errors) over the 20 years of study are presented in Table 2. Women in the higher quartile of cumulative depressive symptom burden had greater increase in average number of MMSE errors over time. In unadjusted models, compared with women in the lowest quartile of depressive symptom burden, women in quartile 2 had 16% more errors over time (95% CI = 14%, 19%), women in quartile 3 had 36% more errors (95% CI = 33%, 39%), and women in quartile 4 had 68% more errors (95% CI = 64%, 73%). After adjustment for demographics, health behaviors, comorbidities, and study site, corresponding percent increases in MMSE errors over time were 5% in quartile 2 (95% CI = 2%, 8%), 12% in quartile 3 (95% CI = 9%, 16%), and 21% in quartile 4 (95% CI = 17%, 26%), as compared with women in the lowest quartile of depressive symptom burden.
Table 2.
Differences in MMSE Errors and Trails B Scores Between Quartile of Long-term Depressive Symptom Burden, From Longitudinal Linear Mixed Models, Study of Osteoporotic Fractures, 1988–2008 (N = 7,240)
| Quartiles of Depressive Symptoms Burden | MMSE Errors | Trails B | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Fully Adjusted* | Unadjusted | Fully Adjusted* | |||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Quartile 1 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | 16% | 14%, 19% | 5% | 2%, 8% | 14% | 13%, 15% | 4% | 3%, 6% |
| Quartile 3 | 36% | 33%, 39% | 12% | 9%, 16% | 31% | 29%, 33% | 10% | 8%, 12% |
| Quartile 4 | 68% | 64%, 73% | 21% | 17%, 26% | 58% | 55%, 61% | 20% | 17%, 23% |
Notes: Results are shown as percentage between-quartile difference in average MMSE errors or Trails B scores. MMSE, Mini-Mental State Examination.
*Models are adjusted for demographics (age, education, and marital status), health behaviors, and comorbidities (smoking, alcohol use, body mass index, exercise, hypertension, heart attack, stroke, diabetes, and use of antidepressants), and study site.
Similarly, higher quartile of depressive symptom burden was associated with worse performance on Trails B over time (Table 2). In unadjusted models, compared with women in the lowest quartile of depressive symptom burden, women in quartile 2 had 14% higher Trails B scores over time (95% CI = 13%, 15%), women in quartile 3 had 31% higher Trails B scores over time (95% CI = 29%, 33%), and women in quartile 4 had 58% higher Trails B scores over time (95% CI = 55%, 61%). After adjustment for demographics, health behaviors, comorbidities, and study site, corresponding percent increases in Trails B scores over time were 4% in quartile 2 (95% CI = 3%, 6%), 10% in quartile 3 (95% CI = 8%, 12%), and 20% in quartile 4 (95% CI = 17%, 23%), as compared with women in the lowest quartile of depressive symptom burden.
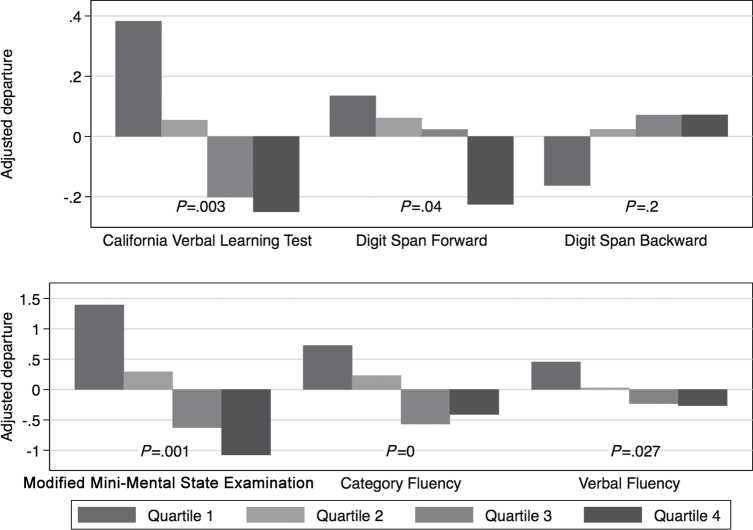
Results from linear regression models of the associations between quartiles of depressive symptom burden and Year-20 cognitive test scores included a total of 1,293 participants who completed Year-20 exam (Figure 1). We summarized Year-20 cognitive test scores as adjusted differences between the mean cognitive function score within each quartile of cumulative depressive symptom burden and the overall mean score. From fully adjusted linear regression models, we found that higher quartile of long-term depressive symptom burden was associated with worse Year-20 cognitive function, on most cognitive tests including delayed California Verbal Learning Test, forward Digit Span test, the 3MS, and verbal fluency tests (p < .05). Long-term depressive symptom burden was not associated with Year-20 backward Digit Span test.
Figure 1.
Differences in mean scores on Year-20 cognitive outcomes for each quartile of depressive symptom burden using linear regression models, relative to the overall mean, Study of Osteoporotic Fractures, 1998–2008 (N = 1,293). Results were adjusted for demographics (age, education, and marital status), health behaviors, and comorbidities (smoking, alcohol, body mass index, exercise, hypertension, heart attack, stroke, diabetes, and antidepressants), and study site.
Results from logistic regression models of the associations between quartiles of depressive symptom burden and odds of dementia/MCI after 20 years included a total of 1,293 participants who completed Year-20 exam (Table 3). Of the 1,293 participants, a total of 510 participants were diagnosed with dementia (16.5%) or MCI (23%). Higher quartile of long-term depressive symptom burden was associated with greater odds of developing dementia/MCI. In unadjusted logistic regression models, as compared with quartile 1, the odds ratio was 1.80 for quartile 2 (95% CI = 1.29, 2.53), 2.31 for quartile 3 (95% CI = 1.65, 3.22), and 3.17 for quartile 4 (95% CI = 2.26, 4.44). After adjusting for sociodemographic factors, health behaviors, comorbidities, and study site, corresponding odds ratios were 1.51 (1.06, 2.15), 1.77 (1.24, 2.54), and 2.14 (1.48, 3.11), respectively.
Table 3.
Associations Between Quartile of Long-term Depressive Symptom Burden and Odds of Dementia/Mild Cognitive Impairment, From Logistic Regression Models, Study of Osteoporotic Fractures, 1988–2008 (N = 1,293)
| Quartiles of Depressive | Number of Cases | Unadjusted | Fully Adjusted* | ||
|---|---|---|---|---|---|
| Symptom Burden | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Quartile 1 (N = 324) | 83 | Ref | Ref | Ref | Ref |
| Quartile 2 (N = 323) | 123 | 1.80 | 1.29, 2.53 | 1.51 | 1.06, 2.15 |
| Quartile 3 (N = 324) | 142 | 2.31 | 1.65, 3.22 | 1.77 | 1.24, 2.54 |
| Quartile 4 (N = 323) | 163 | 3.17 | 2.26, 4.44 | 2.14 | 1.48, 3.11 |
Note. *Models are adjusted for demographics (age, education, and marital status), health behaviors, and comorbidities (smoking, alcohol use, body mass index, exercise, hypertension, heart attack, stroke, diabetes, and use of antidepressants), and study site.
In the sensitivity analyses using joint modeling of cognitive function and death or dropout using shared random effects models, the estimated associations of AUC quartile on MMSE errors and Trails B were virtually unchanged in the longitudinal analysis. The mean GDS trajectory was also little affected, with the two curves only diverging slightly after age 80; by age 90, the joint model estimate was 3.7 points, compared with 3.2 points for the simpler estimate.
Discussion
In this ongoing large cohort of elderly women followed into their 9th and 10th decade of life, we found that long-term cumulative depressive symptom burden over nearly 20 years was strongly and independently associated with worse cognitive functioning, greater cognitive decline, and higher odds of developing dementia/MCI.
Our findings suggest that higher depressive symptom burden was associated with worse performance on all Year-20 cognitive outcomes except for the backward Digit Span test. Higher depressive symptom burden was also associated with greater cognitive decline, and the magnitude of the associations was somewhat similar for the test of global cognition (MMSE) and the test of executive function (Trails B). We found that 16.5% of our participants at Year 20 were diagnosed with dementia and 23% were diagnosed with MCI. The prevalence of dementia and MCI is lower than what we would expect at this age, potentially due to being an educated and healthy community-dwelling cohort. Our findings are consistent with previous longitudinal studies in which higher baseline depressive symptoms were associated with greater cognitive decline and with twofold to fivefold increase risk of dementia (20–25,39,40). For example, results among 949 participants of the Framingham Heart Study showed that higher depressive symptoms at baseline were associated with increased risk of dementia and Alzheimer’s disease over 17 years of follow-up (40). Although the Framingham study has a long follow-up time, it is limited, like most previous studies, with only a single measure of depressive symptoms. However, our findings are inconsistent with those of other longitudinal studies, which found no association between depression and dementia (26,27). As such, our study builds on previous work by examining the influence of the cumulative burden of repeated measures of depressive symptoms over a long follow-up period on cognitive outcomes.
Our findings showed that participants in different quartiles of depressive symptom burden differ on many characteristics including behavioral risk factors and comorbidities. For example, those with higher quartile of depressive symptom burden were less likely to exercise and more likely to have comorbidities than those with lower quartile of depressive symptom burden. Compared with the unadjusted results, adjusting for these covariates in our multivariate models attenuated the magnitude of the associations between depressive symptom burden and cognitive decline and dementia/MCI. Therefore, our results suggest that behavioral risk factors and comorbidities play an important mechanistic role linking depressive symptoms and cognitive outcomes in our study population.
Strengths of our study include a large cohort of participants who were followed over nearly 20 years and for whom we have repeated measures of depressive symptoms and cognitive outcomes. This rich study design enabled us to use an advanced method to better capture the cumulative burden and chronicity of estimates of long-term depressive symptom trajectories, which are imperfectly reflected in widely spaced, generally noisy, and frequently missing measurements. Finally, we attempted to address potential reverse causality by lagging the depressive symptom burden measure. However, our study has some limitations. First, our study sample was comprised of women only and thus our findings may not be generalizable to men. Second, our sample was mostly white, and thus, our findings may not be applicable to other racial/ethnic groups. Finally, while we adjusted for a number of potential confounders, we may not rule out the possibility of residual confounding.
In light of the demographic expansion of the older adult population in the United States and because the oldest old constitute the fastest growing segment with high incidence of depressive symptoms and cognitive impairment, it is important to capture their long-term associations. One of the advantages of our study compared with the previous literature is that we had the opportunity to demonstrate the important role of long-term cumulative depressive symptom burden on shaping cognitive outcomes. Our study is the first to provide evidence that long-term trajectories of depressive symptoms over nearly 20 years are independently associated with cognitive outcomes among a cohort of older women, including the oldest old. Given that chronicity and cumulative exposure of long-term depressive symptoms matter, older adults with a history of depression should be closely monitored for recurrent episodes or unresolved depressive symptoms. Careful monitoring and early intervention for the prevention and treatment of depressive symptoms may help delay cognitive impairment and the development of dementia in old age.
Funding
This work was supported by the National Institute of Mental Health (R01 MH086498); National Institute on Aging (K24 AG031155); and Alzheimer’s Association (IIRG-08-88872). Dr. Z.A.H. is supported by the American Heart Association/American Stroke Association/American Brain Foundation Lawrence M. Brass, M.D. Stroke Research Postdoctoral Fellowship. The Study of Osteoporotic Fractures was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging (Public Health Service grants: 2 R01 AG027574-22A1, R01 AG005407, R01AG027576-22, 2 R01 AG005394-22A1, AG05407, AG05394, AR35582, AR35583, AR35584, AG026720, R01 AG18037, and R01 AG028144-01A1).
References
- 1. Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265 [DOI] [PubMed] [Google Scholar]
- 2. McKenna MT, Michaud CM, Murray CJ, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28:415–423 [DOI] [PubMed] [Google Scholar]
- 3. Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clin Geriatr Med. 1992;8:235–251 [PubMed] [Google Scholar]
- 4. Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67:489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343 [DOI] [PubMed] [Google Scholar]
- 6. Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003;51:1178–1180 [DOI] [PubMed] [Google Scholar]
- 9. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922 [DOI] [PubMed] [Google Scholar]
- 10. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970 [DOI] [PubMed] [Google Scholar]
- 11. Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–167 [DOI] [PubMed] [Google Scholar]
- 12. Metti AL, Cauley JA, Newman AB, et al. Plasma beta amyloid level and depression in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Byers AL, Vittinghoff E, Lui LY, et al. Twenty-year depressive trajectories among older women. Arch Gen Psychiatry. 2012;69:1073–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stek ML, Vinkers DJ, Gussekloo J, van der Mast RC, Beekman AT, Westendorp RG. Natural history of depression in the oldest old: population-based prospective study. Br J Psychiatry. 2006;188:65–69 [DOI] [PubMed] [Google Scholar]
- 16. Andreescu C, Chang CC, Mulsant BH, Ganguli M. Twelve-year depressive symptom trajectories and their predictors in a community sample of older adults. Int Psychogeriatr. 2008;20:221–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cui X, Lyness JM, Tang W, Tu X, Conwell Y. Outcomes and predictors of late-life depression trajectories in older primary care patients. Am J Geriatr Psychiatry. 2008;16:406–415 [DOI] [PubMed] [Google Scholar]
- 18. Kuo SY, Lin KM, Chen CY, Chuang YL, Chen WJ. Depression trajectories and obesity among the elderly in Taiwan. Psychol Med. 2011;41:1665–1676 [DOI] [PubMed] [Google Scholar]
- 19. Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–279 [DOI] [PubMed] [Google Scholar]
- 21. Buntinx F, Kester A, Bergers J, Knottnerus JA. Is depression in elderly people followed by dementia? A retrospective cohort study based in general practice. Age Ageing. 1996;25:231–233 [DOI] [PubMed] [Google Scholar]
- 22. Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182 [DOI] [PubMed] [Google Scholar]
- 23. Geerlings MI, Schoevers RA, Beekman AT, et al. Depression and risk of cognitive decline and Alzheimer’s disease. Results of two prospective community-based studies in The Netherlands. Br J Psychiatry. 2000;176:568–575 [DOI] [PubMed] [Google Scholar]
- 24. Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370 [DOI] [PubMed] [Google Scholar]
- 25. Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–430 [DOI] [PubMed] [Google Scholar]
- 26. Becker JT, Chang YF, Lopez OL, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. 2009;17:653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453 [DOI] [PubMed] [Google Scholar]
- 28. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773 [DOI] [PubMed] [Google Scholar]
- 29. Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157:449–454 [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 31. Magaziner J, Bassett SS, Hebel JR. Predicting performance on the Mini-Mental State Examination. Use of age- and education-specific equations. J Am Geriatr Soc. 1987;35:996–1000 [DOI] [PubMed] [Google Scholar]
- 32. Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Battery: Theory and Clinical Interpretation. Tuscon, AZ: Neuropsychology Press; 1985 [Google Scholar]
- 33. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT-II). 2nd ed San Antonio, TX: Psychological Corporation; 2000 [Google Scholar]
- 34. Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation; 1997 [Google Scholar]
- 35. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318 [PubMed] [Google Scholar]
- 36. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 37. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992 [DOI] [PubMed] [Google Scholar]
- 38. Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000;1:465–480 [DOI] [PubMed] [Google Scholar]
- 39. Gatz JL, Tyas SL, St John P, Montgomery P. Do depressive symptoms predict Alzheimer’s disease and dementia? J Gerontol A Biol Sci Med Sci. 2005;60:744–747 [DOI] [PubMed] [Google Scholar]
- 40. Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]