Abstract
Whether aging alone causes anemia is still controversial. In this study, we show that 28-month-old male C57BL/6 mice, maintained in a pathogen-free environment, had significantly lower hemoglobin, hematocrit, and erythrocyte counts than young mice. The anemic condition aggravated further from 28 to 30 months. Old mice displayed increased erythropoietic activity, evidenced by an increase in reticulocyte counts, serum erythropoietin, and splenic expression of erythropoietic genes. An increase in late-stage erythroid progenitors was detected in spleen but not in bone marrow of the old mice. However, old mice also had lower serum iron and transferrin saturation, as well as lower erythrocyte iron incorporation rate. Testosterone supplementation restored serum iron status in old mice to levels similar to that of young adults, further upregulated splenic expression of erythropoietic genes, increased splenic erythroid progenitors, and significantly improved the red cell index. In conclusion, we found that mice can become anemic at very old age without apparent illness. The endogenous compensatory erythropoietic activity was insufficient to normalize the red cell index in old mice, either due to impaired iron homeostasis, ineffective erythropoiesis, or other unknown factors. Testosterone supplementation normalized the iron status and further stimulated splenic erythropoietic activity; both may contribute to improve the anemic condition in the old mice.
Key Words: Anemia, Aging, Testosterone, Erythropoiesis, Iron bioavailability.
Anemia is a common chronic condition in older adults, and its prevalence increases from the sixth to the eighth decade of life (1–3). The occurrence of anemia has a negative influence on survival and function of older adults (4–6). The cause of anemia in the elderly adults is often not readily apparent or attributable to a single cause since older adults are often burdened with age-associated chronic conditions such as cancer and metabolic syndrome. About one third of the cases of anemia in the elderly adults are classified as “unexplained anemia” (1,7).
In this study, we characterized anemia in an experimental mouse model of aging. The National Institute of Aging colony of aged C57BL/6 mice contains inbred mice that have been maintained in a pathogen-free environment, which provides a preclinical model for studying anemia without confounding environmental influences. As we show in this study, hemoglobin (Hb) and hematocrit (Hct) are significantly lower in 28-month-old mice in comparison to 6-month-young adults. Furthermore, 28-month-old mice experience rapid decline in Hb levels and red cell counts. We determined whether anemia in these very old mice was due to reduced erythropoiesis. Accordingly, we characterized several markers of erythropoiesis, including reticulocyte count and erythropoietin (EPO) levels. We also measured serum iron, transferrin saturation, ferritin, reticulocyte Hb ratio, and direct iron incorporation into red cells.
Erythropoiesis in adult mice occurs primarily in bone marrow and spleen. In mice, bone marrow erythropoiesis is homeostatic, whereas stress erythropoiesis in anemic states often takes place in spleen (8). Spleens of both old and young mice serve as a major site of regenerative repopulation of hematopoietic progenitors under basal condition and after androgen treatment (9). Therefore, we analyzed the splenic expression of erythropoietic markers, including the master transcription factor GATA binding protein 1 (GATA-1) and its associated cofactors friend of GATA (FOG1), erythroid Krüppel-like factor (EKLF), and nuclear factor, erythroid-derived 2 (NF-E2), as well as EPO receptor and transferrin receptor. Finally, we used flow cytometry to measure the number of late-stage erythroid progenitors in both spleen and bone marrow as additional markers of changes in erythropoietic activity.
Previous studies in humans have shown a solid correlation between waning testosterone and anemia (10). Although the cause-and-result relationship is uncertain, androgens have been widely used to treat anemia of chronic disease including end-stage renal disease before the advent of recombinant EPO (11). To determine whether the anemia in these very old mice can be corrected by testosterone supplementation, we characterized the erythropoietic response of the old mice to testosterone supplementation and compared it to that of the younger adults. We characterized the response of red cell index, iron availability, splenic markers of erythropoiesis, and late-stage erythroid progenitors in spleen and bone marrow to testosterone or placebo administration.
Methods
Animals
Male C57BL/6 mice (6 and 28 months) were purchased from National Institute of Aging rodent longevity colony. All mice were housed in groups of 2–4 animals per cage assigned at birth, maintained in 12/12-hour light/dark cycle and 21°C room temperature, with free access to food and water. The protocol of animal use was approved by the IACUC of Boston University. Testosterone was administered by subcutaneous injections (50mg/kg, in 100 μL of sesame oil) twice per week for 3 weeks. The control animals received injections of 100-μL sesame oil.
Blood Collection and Analysis
After acclimation, 50 μL of blood was taken from facial vein to measure the baseline hematological index as described previously (12). One week later, the same mice were used for measurement of 58Fe incorporation into erythrocytes. Mice were allowed to recover for 3 weeks before the first injection of testosterone. Final blood was obtained by heart puncture. Complete blood counts were performed using an ADVIA 120 analyzer (Bayer, Tarrytown, NY). Serum iron and total iron binding capacity were determined using Iron/TIBC kit (ThermoDMA, Arlington, TX). Serum EPO and ferritin were measured using commercial assay kits (www.abcam.com).
RNA Isolation and Real-time PCR
Frozen whole spleen was pulverized, and about 25mg was taken for total RNA isolation using RNeasy Mini Kit (#74106; Qiagen). Real-time PCR was conducted on the ABI 7500 PCR system (Applied Biosystems) using SYBR Green qPCR master mix (#A6001; Promega) to measure messenger RNA expression levels, which were normalized to the expression level of hypoxanthine-guanine phosphoribosyl transferase. All PCR primers used were intron-spanning and designed using web-based software (www.roche-applied-science.com).
Measurement of 58Fe Incorporation into Red Cells
58Fe was obtained from Oakridge National Laboratory (www.ornl.gov). 58Fe/transferrin complex was prepared as described (12) and injected into tail vein at 10ng/g body weight. Blood (~50 μL) was drawn from facial vein after 12 hours, washed with saline, and red cells were digested for measurement of 58Fe/56Fe ratio using a multicollector-inductively coupled plasma mass spectrometer as described (12). The amount of iron incorporated into red cells was calculated using specific activity of 58Fe in steady-state serum iron pool size.
Detection of Erythrocyte Progenitors in Bone Marrow and Spleen Using Flow Cytometry
Cells were collected from bone marrow by flushing with a 26-gauge needle and from spleen by mechanical dissociation before passing through a 70-μm cell strainer. Single-cell suspensions were incubated with red blood cell (RBC) lysis buffer (Biolegend, San Diego, CA) to remove the mature erythrocytes. Cells were stained with a fluorescein isothiocyanate–conjugated antibody against CD71 (1 μg/mL) and a phycoerythrin-conjugated antibody against Ter119 (1 μg/mL; BD Biosciences, San Jose, CA). Dead cells were excluded using a live/dead cell vitality kit (Invitrogen, Grand Island, NY). Samples were analyzed on a LSR II Flow Cytometer (BD Biosciences), and the data were analyzed using Flow Jo software (Tree Star, Ashland, OR). Cells that stained positive for both CD71 and Ter119 were gated as late-stage erythroid progenitors (13).
Statistical Analyses
The results are presented as mean ± standard error of mean. For experiments that include more than two groups, comparison was conducted using one-way analysis of variance followed by Tukey’s test. Comparisons between control and testosterone-treated animals of the same age were performed using Student’s t test.
Results
Old Male Mice Are Anemic and Have Impaired Ability to Incorporate Iron Into Red Cells
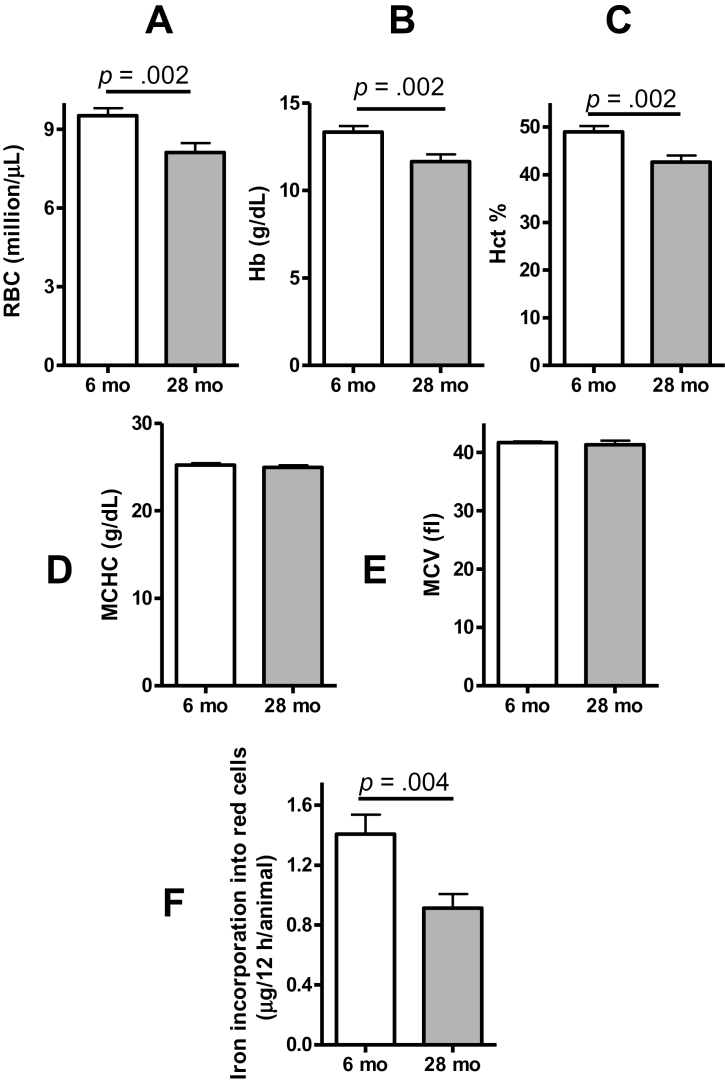
Compared with young adults (6 months), the old mice (28 months) had lower Hct, Hb, and RBC count (Figure 1A–C). The mean corpuscular volume (Figure 1D) and mean corpuscular Hb concentration (Figure 1E) did not differ significantly between the ages. Because iron incorporation into Hb is believed to be the rate-limiting factor for erythroid maturation (14), we determined whether anemia in the old mice was associated with reduced capacity for iron incorporation into red cells. The mice were administered a bolus of transferrin-bound 58Fe into the tail vein at a dose of 10ng/g body weight. Twelve hours later, the incorporation of 58Fe in red cells was measured by multicollector-inductively coupled plasma mass spectrometer. The amount of total iron incorporation was calculated based on Hct and blood volume estimated from body weight and serum iron concentration. As shown in Figure 1F, the iron incorporation into the erythrocytes was substantially lower in old mice than the young mice. These data suggest that limited iron utilization for Hb synthesis may contribute to the anemia in the old animals.
Figure 1.
Twenty-eight-month-old male mice have lower baseline red blood cell (RBC) count (A), hemoglobin (Hb, B), hematocrit (Hct, C), and iron incorporation into RBCs (F) than 6-month-old mice. The mean corpuscular Hb concentration (MCHC, D) and mean corpuscular volume (MCV, E) did not differ significantly between the young and old mice. For iron incorporation studies, transferrin-bound 58Fe (10ng/g) was injected into the tail vein. Blood was drawn 12 h after injection. The ratio of 58Fe/56Fe was analyzed by multichannel collector inductively coupled plasma mass spectrometry, and the net incorporation of iron was calculated as described in Methods section. Results shown are mean ± standard error of mean, N = 18 for each group.
Testosterone Supplementation Increases Hb, Hct, and RBC Count
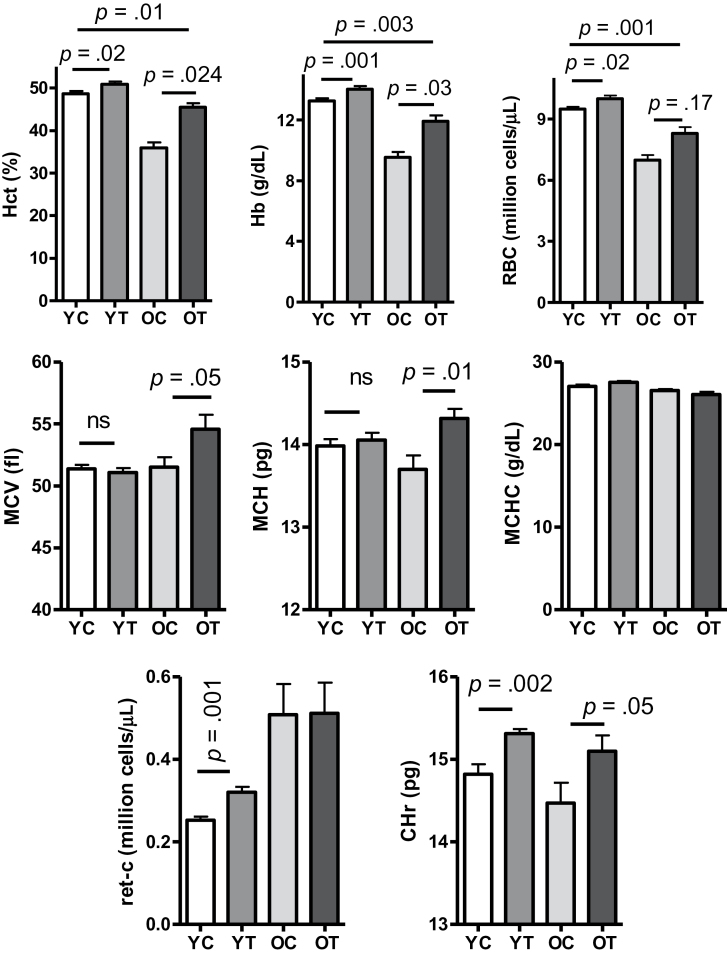
In randomized clinical trials, testosterone administration was shown to increase Hb and Hct in young and older men (15). However, the older men recruited for the first-generation testosterone trials were generally at an early stage of aging (61–75 year) and nonanemic (15). To establish an animal model that could facilitate studies of the mechanisms by which testosterone stimulates Hb and Hct in very old subjects, we treated the young and old mice (29 months by now) with either testosterone (50mg/kg, twice per week, subcutaneously) or vehicle for 3 weeks. Within the 2 months between the initial and the final blood test, Hb, Hct, and RBC counts did not change in vehicle-treated young mice but were substantially reduced in vehicle-treated old mice (Supplementary Figure s1). As shown in Figure 2 (upper panel), testosterone supplementation increased Hb, Hct, and RBC count in comparison to vehicle administration in both young and old mice. The changes were greater in old mice than the young mice (Supplementary Figure s2), although testosterone supplementation did not completely restore the red cell index to the level found in the untreated young animals (Figure 2, upper panel). Testosterone supplementation also increased mean corpuscular volume and mean corpuscular Hb in old mice but not in the young mice, without affecting mean corpuscular Hb concentration in either age group (Figure 2 and Supplementary Figure s2). Although mean corpuscular Hb was only marginally lower in old control animals than the young animals (p = .12), the significant increase after testosterone supplementation suggests a correction of defects in Hb synthesis (16). The reticulocyte Hb ratio increased equally in response to testosterone administration in both young and old mice (Figure 2, lower panel).
Figure 2.
Erythropoietic response to administration of either vehicle (C, 100 μL) or testosterone (T, 100 μL, 50mg/kg) weekly for 3 wk. Y, young adult; O, older mice. Results are shown as mean ± standard error of mean, N = 9 in each group. Data were analyzed by one-way analysis of variance followed by Tukey’s test. Hct, hematocrit; Hb, hemoglobin; RBC, red blood cell count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; ret-C, reticulocyte count; CHr, reticulocyte hemoglobin ratio; ns, nonsignificance.
Remarkably, the reticulocyte count in the old mice was about twofold greater than that in young animals, whether expressed as absolute count (million reticulocytes per microliter; Figure 2) or as a percentage of total number of blood cells (Supplementary Figure s3, left panel). Even after correction for the degree of anemia (15), the absolute reticulocyte count remained higher in old animals (Supplementary Figure s3, right panel). A similar increase in reticulocyte count with advanced age has been reported by others (17). Testosterone supplementation increased the relative reticulocyte count in young mice but not in the old mice (Figure 2, lower left panel and Supplementary Figure s3, left panel). However, after multiplication by Hb, testosterone-induced increase of reticulocyte production was found to be significant in both age groups (Supplementary Figure s3, right panel). Because juvenile erythrocytes are larger than the older ones (18), an increase in reticulocytes coupled with an increase in mean corpuscular volume suggests that testosterone supplementation was associated with increased proportion of juvenile erythrocytes in old mice.
Age and Testosterone Differentially Regulate Serum Iron, Transferrin Saturation, EPO, and Hepatic Hepcidin Expression
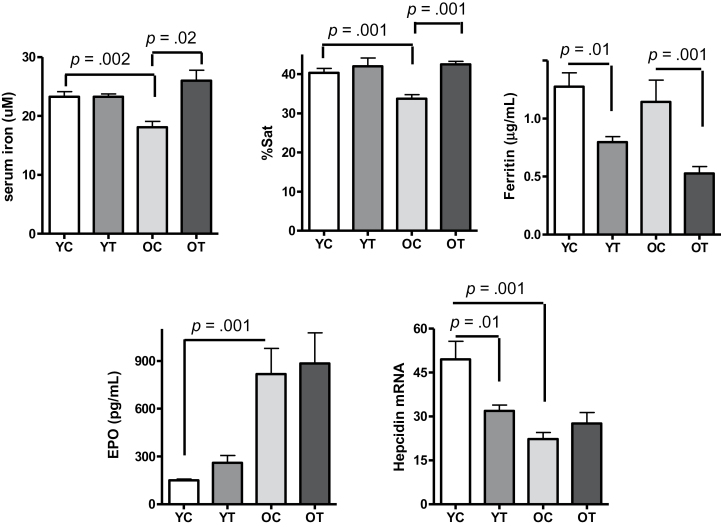
As shown in Figure 3, the lower Hb and Hct levels in old mice were associated with lower serum iron and lower transferrin saturation in comparison to the young mice. Total iron binding capacity was similar between the two age groups (Supplementary Figure s4). Testosterone supplementation restored serum iron and transferrin saturation in old mice to levels found in the young mice (Figure 3) and increased total iron binding capacity in both age groups with a greater change found in old mice (Supplementary Figure s4). Serum ferritin concentration did not differ significantly between vehicle-treated young and old mice and was similarly reduced by testosterone supplementation in both age groups (Figure 3).
Figure 3.
Twenty-eight-month-old mice have lower serum iron concentration and lower transferrin saturation than 6-month-old mice. Testosterone supplementation increased serum iron and transferrin saturation in the old mice without a significant effect on the young mice but decreased serum ferritin similarly in both young and old animals. Baseline serum erythropoietin (EPO) was higher, and hepatic hepcidin messenger RNA (mRNA) expression level was lower in the old mice than that in the young mice. Testosterone did not affect serum EPO levels at either age but reduced hepatic hepcidin mRNA expression levels in the young mice. Results are shown as mean ± standard error of mean, N = 9 for each group. Data were analyzed by one-way analysis of variance, followed by Tukey’s test. Y, young adult; C, vehicle; T, testosterone; O, older mice.
Because hepcidin is an important regulator of iron homeostasis and is known to regulate both dietary iron intake and endogenous iron recycled from senescent erythrocytes (19), we measured the effect of testosterone supplementation on hepatic hepcidin messenger RNA expression. As shown in Figure 3 (lower right panel), baseline hepcidin messenger RNA expression was significantly lower in old mice than the young mice. Testosterone supplementation reduced hepcidin messenger RNA expression in young mice as expected (12) but had no effect on hepcidin expression in the old mice.
Consistent with prior studies showing a rise in serum EPO in anemic older humans (3,20) and animals (21), we found that serum EPO levels were strikingly higher in old mice as compared with the young mice (Figure 3, lower left panel). Testosterone supplementation did not affect serum EPO concentration in either age group (Figure 3). This is consistent with a recent study that found no correlation between serum EPO and the hematopoietic effect of testosterone (22).
Studies in humans have shown that serum EPO levels rise with age before the onset of anemia (23). We measured EPO concentration in sera collected from young (5–6 months), middle (15–16 months), early-old (23–24 months), and late-old (28–29 months) ages. Serum EPO levels did not differ between the young- and middle-age groups (Supplementary Figure s5) but were nearly twofold higher in the 23–24-month group than the young group (Supplementary Figure s5). The 28–29-month very old mice had substantially higher EPO levels than all other age groups (Supplementary Figure s5).
Age and Testosterone Differentially Regulate Splenic Expression of Erythropoietic Markers
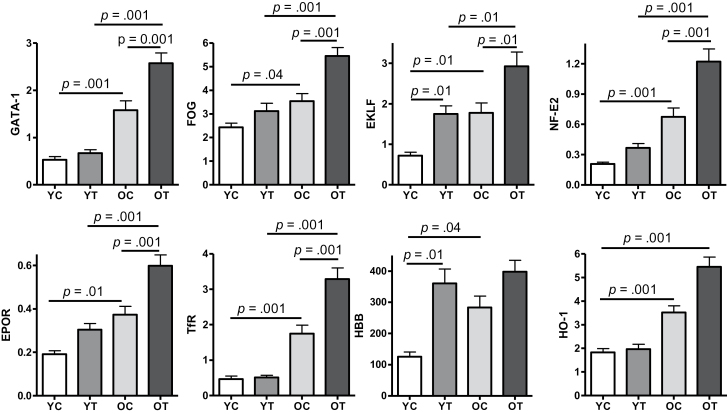
Spleen is the primary site of anemia-related stress erythro poiesis known to be regulated by androgens (8,9). Thus, we analyzed splenic expression of selected erythropoietic markers, including GATA-1 and its associated transcription factors FOG1, EKLF, and NF-E2. We found that expression level of GATA-1, the master transcription factor that regulates erythropoiesis at multiple stages from erythroid lineage commitment to red cell maturation (24–26), was increased in spleen of old mice compared with the young mice (Figure 4). The expression levels of FOG1, which binds GATA-1 to ensure erythroid lineage commitment (27), and EKLF, the transcription factor that co-operates with GATA-1 to activate β-globin transcription (28), were higher in old mice than the young mice. NF-E2, the transcription factor that plays an important role in globin gene expression (29), was also expressed at a higher level in old mice than the young mice. As GATA-1 is known to be in control of FOG1, EKLF, and NF-E2, and GATA-1 itself can be activated by anemia (30), the increased expression of these erythropoietic transcription factors in old mice might be reflective of a compensatory response to the anemic condition in these animals. Consistently, we also found increased splenic expression of receptors for EPO and transferrin in old mice than the young mice, suggesting an increase of splenic erythroid progenitor cells. In addition, expression of β-globin, the adult Hb β-subunit and also a direct target of GATA-1/EKLF transcription complex (28), was increased in old mice (Figure 4). Finally, splenic expression of heme oxygenase, the enzyme that extracts iron from degraded Hb after red cell phagocytosis (31), was also increased in old mice (Figure 4). Taken together, these results suggest that mechanisms for increasing erythropoiesis were activated in old mice but were insufficient to reverse the age-related anemia.
Figure 4.
Age and testosterone differentially increase splenic messenger RNA expression of key erythropoietic transcription factors and selected erythroid-related markers. Results are shown as mean ± standard error of mean, N = 9 for each group. Data were analyzed by one-way analysis of variance followed by Tukey’s test. GATA-1, GATA binding protein 1; FOG, friend of GATA; EKLF, erythroid Krüppel-like factor; NF-E2, nuclear factor, erythroid-derived 2; EPOR, erythropoietin receptor; TfR, transferrin receptor; HBB, hemoglobin β-subunit; HO-1, heme oxygenase 1; Y, young adult; O, older mice; C, vehicle; T, testosterone.
As shown in Figure 4, testosterone supplementation significantly upregulated the expression of erythropoietic transcription factors, GATA-1, FOG1, EKLF, and NF-E2; the increase in expression levels of these transcription factors was generally more pronounced in old mice than the young mice. Testosterone supplementation also further increased the expression of EPO and transferrin receptors. These results together support the hypothesis that testosterone supplementation enhances splenic erythropoiesis in these anemic old mice.
Age and Testosterone Differentially Regulate Splenic Production of Late-stage Erythroid Progenitors
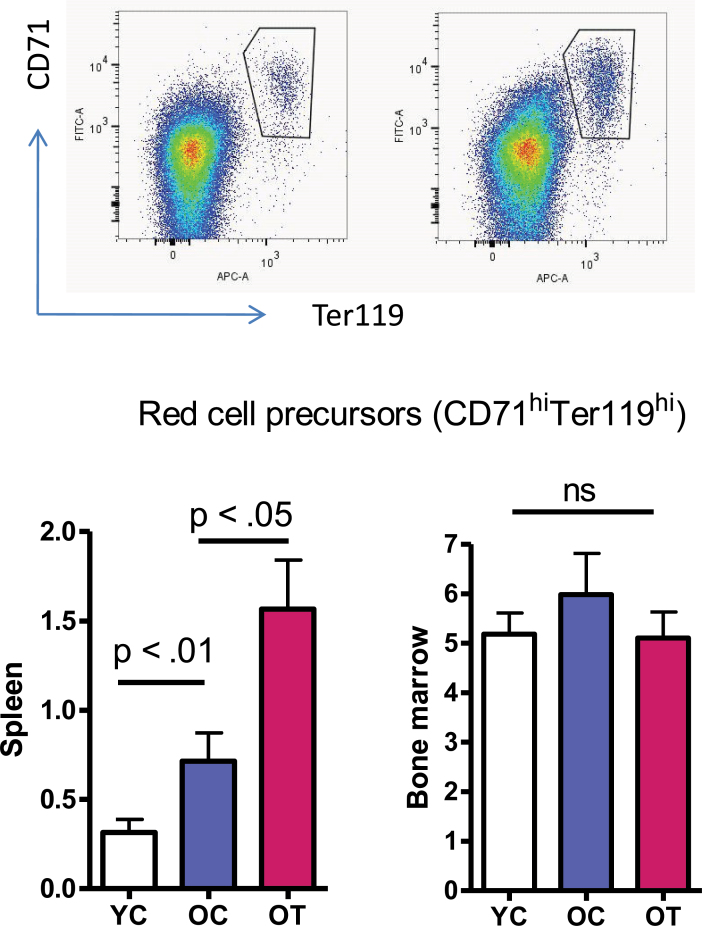
To further test the hypothesis that age and testosterone each has a distinct effect on stress erythropoiesis in the anemic old mice, we measured the effect of each on the proportion of late-stage erythroid progenitors in spleen and bone marrow. As shown in Figure 5, a greater percentage of late-stage erythroid progenitors (stained positive for both CD71 and Ter119) (32) was detected in bone marrow than in spleen. However, the percentage of bone marrow late-stage erythroid progenitors did not differ among age groups with or without testosterone supplementation. This is consistent with previous studies showing that bone marrow erythropoiesis is homeostatic and does not decline with aging (33). In contrast, although erythroid progenitors account for only about 0.3% of cells in spleen of young animals, much less than the 5% found in bone marrow, the percentage was nearly double in old mice, and further increased twofold after testosterone supplementation (Figure 5, left panel). This finding is in agreement with previous studies showing that murine spleen can be the primary site for stress erythropoiesis with androgen administration (9,34) and also in line with the increase of splenic erythropoietic gene expression found in this study (Figure 4).
Figure 5.
Age and testosterone differentially increase the percentage of late-stage erythroid progenitors (Cd71+Ter119+) in the spleen but not in the bone marrow. Upper panel: representative cell distribution patterns in spleen (left) and bone marrow (right) after costaining with anti-CD71 and anti-Ter119. Cells stained positive for both CD71 and Ter119 were gated as late-stage erythroid progenitors. Lower panel: the percentage of late-stage erythroid progenitors in spleen (left) and bone marrow (right). Results are mean ± standard error of mean, N = 7 for each group. Data were analyzed by one-way analysis of variance followed by Tukey’s test. Y, young adults; O, older mice; C, vehicle; T, testosterone; ns, nonsignificance.
Effect of Age and Testosterone Supplementation on Inflammatory Cytokine Expression
Anemia in the elderly population has been postulated to be a reflection of increased expression of proinflammatory cytokines (5). As shown in Supplementary Figure s6, liver expression of tumor necrosis factor α was higher in old anemic mice than in the young mice. However, liver expression of interleukin 6 was moderately lower in old mice than in the young mice. In contrast, splenic tumor necrosis factor α expression did not differ between the two age groups, and interleukin 6 expression was modestly lower in old mice. Testosterone supplementation had no effect on expression of tumor necrosis factor α or interleukin 6 in either liver or spleen.
Discussion
There is considerable debate whether anemia in older individuals is a result of aging or a consequence of comorbid conditions often associated with aging. The studies in older humans are often confounded by variations in genetic background, lifestyle, and other chronic medical conditions that cluster together. As an experimental model of anemia of aging, we studied a genetically homogeneous mouse population maintained on a standardized diet and living environment. The old mice used in this study were within the age range of 40%–25%-survival window (www.nia.nih.gov/aged-rodent-colonies-handbook). Even when maintained under pristine laboratory conditions, the old mice displayed clear evidence of anemia with lower Hb, Hct, and RBC counts than young mice. Furthermore, the vehicle-treated old mice displayed rapid declines in red cell index over the 2-month period of observation. Some studies have suggested that anemia in old mice can be related to social stress as singly housed mice or the dominant male in a group-housed setting do not become anemic (35,36). Because the mice used in this study were group housed, we cannot compare our findings with those prior observations. However, mice are social animals, in our opinion, findings from mice that aged in a group-housed setting (without overt social stress) are more reflective of real-life aging than those kept in a single-housed setting.
Several important findings have emerged from this study. First, the anemic old mice included in this study displayed evidence of increased—not decreased—activation of erythropoiesis as indicated by significantly higher expression of splenic EPO and transferrin receptors, and increased percentage of late-stage erythroid progenitors in comparison to young mice. The circulating levels of EPO and the expression of key erythropoietic transcription factors were both higher in old mice than the young mice. The old mice also showed higher reticulocyte counts after correction for anemia, which serves as another marker for increased erythropoiesis (16). However, these compensatory erythropoietic mechanisms were insufficient to maintain normal Hb and RBC counts, suggesting that anemia in these old mice may be due to either defective steps in erythroid development such as iron incorporation for Hb synthesis or increased peripheral destruction of immature erythroid precursors as implied by previous studies (17).
Second, several lines of evidence suggest that iron availability for erythropoiesis may be limited in old mice. Serum iron and transferrin saturation was lower in old mice than the young mice. Mean reticulocyte Hb concentration, a marker of iron availability for Hb synthesis, was reduced in old mice. Furthermore, direct measurements of transferrin-bound iron incorporation into erythrocytes demonstrated a reduced rate of iron incorporation in old mice compared with the young mice. Considered together, these data support the proposal that constrained iron availability may contribute to anemia detected in the old mice.
Hepcidin has been recognized as an important regulator of iron homeostasis that blocks iron release into circulation by multiple mechanisms and reduces iron bioavailability. Surprisingly, hepatic hepcidin expression was lower in old mice than in the young mice. It is not completely clear whether this reflects a compensatory mechanism for an anemic state or other age-related mechanisms may contribute to downregulate hepcidin expression. Regardless, the observed changes of hepcidin do not explain the alterations in markers of iron status found in the old mice. Indeed, others have also suggested that hepcidin may not be a key player in age-related anemia (37).
Aging is known to blunt the responsiveness to pro-erythropoietic stimuli, such as hypoxia or EPO administration (21,38). However, we found that testosterone supplementation increased Hb and Hcts in both young and old mice with even a greater increase in the old mice, although the hormone supplementation did not completely restore the red cell index to the level found in untreated young animals. Testosterone supplementation upregulated the expression of several important transcription factors for splenic erythropoiesis, including GATA-1, FOG1, EKLF, and NF-E2; the increments in the expression levels of these transcription factors were generally greater in old mice than in the young mice. Testosterone supplementation also induced greater increments in EPO and transferrin receptors in old mice and raised the percentage of late-stage erythroid progenitors in spleen, providing a direct evidence of increased splenic erythropoiesis. In addition, testosterone supplementation restored serum iron and transferrin saturation in old mice to those found in the young mice and increased reticulocyte Hb ratio in both young and old animals, indicating improved iron availability for erythropoiesis. Hepcidin does not appear to play an important role in mediating these effects in the anemic old mice. Alternative mechanism remains to be identified to interpret the elevation of serum iron and transferrin saturation in the old animals after testosterone supplementation.
In summary, very old mice near a 25%-survival time window may serve as a useful preclinical model for studying the mechanism by which testosterone improves red cell index under the anemic condition in late life. In this experimental model, anemia is associated with reduced serum iron, decreased iron incorporation into erythrocytes, and increased (ineffective) splenic erythropoiesis. Our data demonstrated that anemia in these old mice is significantly improved by testosterone supplementation. Further studies are needed to characterize the functional impact of such late-age anemia compared with that caused by anemia at an earlier life. In addition, as this study was conducted on mice from an inbred homogenous genetic background, key findings should also be tested in additional animal species and strains with mixed genetic backgrounds in order to better characterize late-life anemia, the mechanism of disease development and its treatment using androgen therapy.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported in part by National Institute on Aging (9R01AG037193-06 and R21AG037859).
Acknowledgments
We thank Dr. Michael Yee for the mouse sera from different age groups, Ms. Siu Wong and Mr. Wentao Liang for technical assistance, and Dr. Jerzy Blusztajn of Woods Hole Oceanographic Institution for the multichannel collector inductively coupled plasma mass spectrometry analysis.
References
- 1. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268 [DOI] [PubMed] [Google Scholar]
- 2. Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel KV, Longo DL, Ershler WB, Yu B, Semba RD, Ferrucci L, Guralnik JM. Haemoglobin concentration and the risk of death in older adults: differences by race/ethnicity in the NHANES III follow-up. Br J Haematol. 2009;145:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaves PH, Ashar B, Guralnik JM, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc. 2002;50:1257–1264 [DOI] [PubMed] [Google Scholar]
- 5. Balducci L. Anemia, fatigue and aging. Transfus Clin Biol. 2010;17:375–381 [DOI] [PubMed] [Google Scholar]
- 6. Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115:104–110 [DOI] [PubMed] [Google Scholar]
- 7. Penninx BW, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724 [DOI] [PubMed] [Google Scholar]
- 8. Millot S, Andrieu V, Letteron P, et al. Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a mouse model of generalized inflammation. Blood. 2010;116:6072–6081 [DOI] [PubMed] [Google Scholar]
- 9. Saitoh T, Morimoto K, Kumagai T, Tsuboi I, Aikawa S, Horie T. Comparison of erythropoietic response to androgen in young and old senescence accelerated mice. Mech Ageing Dev. 1999;109:125–139 [DOI] [PubMed] [Google Scholar]
- 10. Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166:1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shahidi NT. Androgens and erythropoiesis. N Engl J Med. 1973;289:72–80 [DOI] [PubMed] [Google Scholar]
- 12. Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12:280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci USA. 2009;106:17413–17418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25 [PubMed] [Google Scholar]
- 15. Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adamson JW, Longo DN. Anemia and Polycythemia. New York, NY: McGraw-Hill; 2001. [Google Scholar]
- 17. Magnani M, Rossi L, Stocchi V, Cucchiarini L, Piacentini G, Fornaini G. Effect of age on some properties of mice erythrocytes. Mech Ageing Dev. 1988;42:37–47 [DOI] [PubMed] [Google Scholar]
- 18. Shiga T, Maeda N, Kon K. Erythrocyte rheology. Crit Rev Oncol Hematol. 1990;10:9–48 [DOI] [PubMed] [Google Scholar]
- 19. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Powers JS, Krantz SB, Collins JC, et al. Erythropoietin response to anemia as a function of age. J Am Geriatr Soc. 1991;39:30–32 [DOI] [PubMed] [Google Scholar]
- 21. Benderro GF, LaManna JC. Kidney EPO expression during chronic hypoxia in aged mice. Adv Exp Med Biol. 2013;765:9–14 [DOI] [PubMed] [Google Scholar]
- 22. Maggio M, Snyder PJ, Ceda GP, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1:24–28 [DOI] [PubMed] [Google Scholar]
- 23. Ershler WB, Sheng S, McKelvey J, et al. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53:1360–1365 [DOI] [PubMed] [Google Scholar]
- 24. Lowry JA, Mackay JP. GATA-1: one protein, many partners. Int J Biochem Cell Biol. 2006;38:6–11 [DOI] [PubMed] [Google Scholar]
- 25. Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life. 2009;61:800–830 [DOI] [PubMed] [Google Scholar]
- 26. Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002;21:3368–3376 [DOI] [PubMed] [Google Scholar]
- 27. Mancini E, Sanjuan-Pla A, Luciani L, et al. FOG-1 and GATA-1 act sequentially to specify definitive megakaryocytic and erythroid progenitors. EMBO J. 2012;31:351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cao A, Moi P. Regulation of the globin genes. Pediatr Res. 2002;51:415–421 [DOI] [PubMed] [Google Scholar]
- 29. Shivdasani RA, Orkin SH. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc Natl Acad Sci USA. 1995;92:8690–8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Capron C, Lacout C, Lécluse Y, et al. LYL-1 deficiency induces a stress erythropoiesis. Exp Hematol. 2011;39:629–642 [DOI] [PubMed] [Google Scholar]
- 31. Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koulnis M, Pop R, Porpiglia E, Shearstone JR, Hidalgo D, Socolovsky M. Identification and analysis of mouse erythroid progenitors using the CD71/TER119 flow-cytometric assay. J Vis Exp. 2011; Aug 5;(54). pii 2809. 10.3791/2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schofield R, Dexter TM, Lord BI, Testa NG. Comparison of haemopoiesis in young and old mice. Mech Ageing Dev. 1986;34:1–12 [DOI] [PubMed] [Google Scholar]
- 34. Rothman IK, Zanjani ED, Gordon AS, Silber R. Nucleoside deaminase: an enzymatic marker for stress erythropoiesis in the mouse. J Clin Invest. 1970;49:2051–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Page DL, Glenner GG. Social interaction and wounding in the genesis of “spontaneous” murine amyloidosis. Am J Pathol. 1972;67:555–567 [PMC free article] [PubMed] [Google Scholar]
- 36. Williams LH, Udupa KB, Lipshitz DA. Evaluation of the effect of age on hematopoiesis in the C57BL/6 mouse. Exp Hematol. 1986;14:827–832 [PubMed] [Google Scholar]
- 37. Lee P, Gelbart T, Waalen J, Beutler E. The anemia of ageing is not associated with increased plasma hepcidin levels. Blood Cells Mol Dis. 2008;41:252–254 [DOI] [PubMed] [Google Scholar]
- 38. Udupa KB, Lipschitz DA. Erythropoiesis in the aged mouse: I. Response to stimulation in vivo. J Lab Clin Med. 1984;103:574–580 [PubMed] [Google Scholar]