Abstract
Background.
Weakness is common and contributes to disability, but no consensus exists regarding a strength cutpoint to identify persons at high risk. This analysis, conducted as part of the Foundation for the National Institutes of Health Sarcopenia Project, sought to identify cutpoints that distinguish weakness associated with mobility impairment, defined as gait speed less than 0.8 m/s.
Methods.
In pooled cross-sectional data (9,897 men and 10,950 women), Classification and Regression Tree analysis was used to derive cutpoints for grip strength associated with mobility impairment.
Results.
In men, a grip strength of 26–32 kg was classified as “intermediate” and less than 26 kg as “weak”; 11% of men were intermediate and 5% were weak. Compared with men with normal strength, odds ratios for mobility impairment were 3.63 (95% CI: 3.01–4.38) and 7.62 (95% CI 6.13–9.49), respectively. In women, a grip strength of 16–20 kg was classified as “intermediate” and less than 16 kg as “weak”; 25% of women were intermediate and 18% were weak. Compared with women with normal strength, odds ratios for mobility impairment were 2.44 (95% CI 2.20–2.71) and 4.42 (95% CI 3.94–4.97), respectively. Weakness based on these cutpoints was associated with mobility impairment across subgroups based on age, body mass index, height, and disease status. Notably, in women, grip strength divided by body mass index provided better fit relative to grip strength alone, but fit was not sufficiently improved to merit different measures by gender and use of a more complex measure.
Conclusions.
Cutpoints for weakness derived from this large, diverse sample of older adults may be useful to identify populations who may benefit from interventions to improve muscle strength and function.
Key Words: Muscle, Sarcopenia, Grip strength, Physical function, Gait speed.
Muscle weakness is related to poor physical performance and incident mobility limitations among older adults (1–6). Weakness is considered a key element of frailty (7) and, increasingly, of sarcopenia (8,9). Although the association between weakness and functional limitations is strong, there is no consensus regarding a cutpoint for identification of risk for functional problems. In order to identify population subgroups in whom weakness is a potential contributor to functional limitations, it is necessary to determine what constitutes a clinically relevant degree of weakness.
This is the second in a series of reports from the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project, which pooled data from multiple studies to develop and evaluate clinically relevant criteria for weakness and low muscle mass (10). The purpose of the analysis presented here was to identify cutpoints that distinguish weakness (measured by grip strength) associated with mobility impairment (measured by gait speed) using cross-sectional data (ie, to maximize concurrent validity). This analysis builds on previous research on the association between strength and walking speed using a data-driven approach across multiple populations and an analytic technique (Classification and Regression Tree [CART] analysis) designed to optimize concurrent validity in the context of complex interactions. Findings were used to address subsequent Project goals reported separately (11–13).
Methods
Participants
Data available for this phase of the FNIH Sarcopenia Project analysis included: the Study of Osteoporotic Fractures, both the original cohort (study Visit 6) (14) and African American cohort (study Visit 1) (15); the Osteoporotic Fractures in Men Study (baseline visit) (16); the Health, Aging and Body Composition Study (Year 6 Clinic Visit) (17); the Framingham Study (both the Offspring cohort [exam cycles 6 and 7, 1996–2001] (18) and Original cohort [exam cycle 26, 1999–2001]) (19); the InCHIANTI Study (Aging in the Chianti Area, Year 3 visit) (20); the Boston Puerto Rican Health Study (baseline visit) (21); the Age, Gene/Environment Susceptibility-Reykjavik Study (baseline visit) (22); and four clinical trials from the University of Connecticut (UCONN, randomization visit for all studies) (23–26).
To be included in these analyses, participants were required to have height and weight, grip strength, and gait speed measured at a single time point: Of the 26,625 participants aged 65 and older in the pooled data, 1,403 were ineligible because they were in studies that did not collect the variables used in this analysis; 1,978 were not eligible for assessment of key measures within their study; and an additional 2,397 were excluded due to missing data, yielding a final sample size of 20,847 (9,897 men and 10,950 women). Participants excluded due to missing data were older, slower, weaker, had lower body mass index (BMI), higher rates of chronic conditions, and were more likely to be women.
Measures
Walking speed less than 0.8 m/s was selected as the primary outcome for the FNIH Sarcopenia Project because of its strong longitudinal associations with disability and mortality and because its use has been recommended by other experts (refs. (9) and (10)). Detailed descriptions of gait speed assessment are available elsewhere (10).
Grip strength was selected as the primary measure of strength for several reasons. It is clearly related to mobility outcomes (4,6,27) and is easy to use in both clinical and community settings. Standard protocols are available for use without a high level of investigator training, and similar protocols were used across Project studies. Conversely, measures of lower extremity strength were inconsistent across participating studies. Preliminary analysis suggested that grip strength explained a similar amount of variance in walking speed compared with knee extension strength (R 2 for grip strength = .01–.16, R 2 for knee extension strength = .04–.17).
Grip strength was measured by handheld dynamometer (28). A summary of study protocols is available in Supplementary Appendix Table 1). The majority of studies (11 out of 13 cohorts) utilized Jamar dynamometers. The maximum strength value in either hand was analyzed.
Statistical Analysis
Our approach relied on identifying a level of grip strength below which older persons are more likely to have a mobility impairment (gait speed < 0.8 m/s). Individual-level data from all cohorts were combined into a single, pooled data set. Scatterplots with overlaid locally weighted scatterplot smoothing (LOESS) curves were used to describe the shape of the relationship between grip strength and gait speed.
CART analysis was then performed as the primary method for deriving cutpoints for muscle strength (29). Briefly, CART recursively partitions study participants into mutually exclusive groups defined by predictor cutpoints within which participants have similar outcome probabilities. CART is virtually free of modeling assumptions, which provides several advantages in this context: (i) it optimizes concurrent validity by identifying predictors and cutpoints with the strongest relationship with the outcome based on the criterion of minimum error sum of squares; (ii) it does not require an a priori specified number of cutpoints; and (iii) it can identify complex interactions (ie, nonlinear interactions involving multiple variables) with other potentially important variables (eg, BMI, height). CART has been used previously to study the association between strength and walking speed (5,30).
CART analysis was performed using R version 2.10.1, and cross-validation was used to select the best-performing cutpoints. Internal cross-validation is intended to avoid overfitting and development of sample-specific cutpoints. Cross-validation was performed by randomly partitioning the pooled data into 10 equally sized mutually exclusive subsamples (ie, each sample excluded 10% of the original pooled data). The tree was then applied to the 10 subsamples each of which contained 90% of the data, and the error variance (called the prediction error, calculated using the error sum of squares) from each subsample was calculated. The 10 prediction errors were used to calculate the empirical standard error of the prediction error. Following published guidelines (31), the tree was pruned to the most parsimonious tree within one standard error of the tree with the smallest prediction error. This pruned tree contains the final set of strength cutpoints from pooled analysis.
Several sets of candidate predictors were included in CART models to identify the most appropriate model for the prediction of slow gait speed: maximum grip strength, body size indicators (BMI, height, weight), and the ratio of strength to body size (grip strength/height, grip strength/weight, grip strength/height2, and grip strength/BMI), because previous research suggests that the association between strength and mobility may differ across strata of BMI (4).
Sensitivity analysis examined the predictive power of recommended cutpoints across cohorts and by characteristics such as age, BMI, height, and comorbidities. Cutpoints were evaluated by predicting slow walking speed based on strength category in a mixed-effects logistic regression model including indicator functions for cutpoints as fixed effects and random intercepts accounting for heterogeneity between data sets. Additional sensitivity analysis examined the stability of CART-derived cutpoints using alternative gait speed outcomes (<0.6 m/s and continuous gait speed). Although additional analyses were planned based on inability to rise from a chair (an alternative physical performance measure), only 3.8% of men and 6.0% of women met this criterion, providing insufficient sample size to implement those analyses.
Results
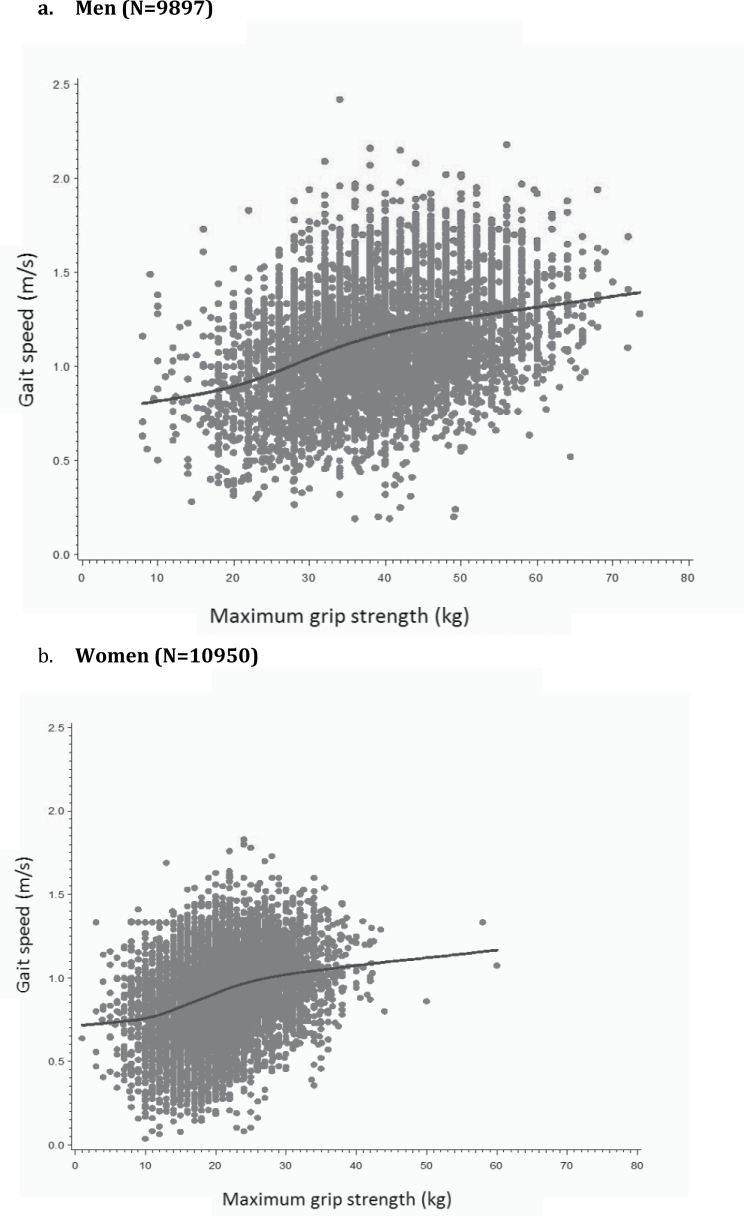
Sample descriptive characteristics are available in the online Supplementary Appendix and generally resemble characteristics of the parent studies described elsewhere (10). Figure 1 provides plots of the association between grip strength and gait speed in men and women. Visual inspection of the LOESS curves provided little evidence of a clear threshold effect of strength on continuous gait speed.
Figure 1.
Association of grip strength and gait speed in the FNIH Sarcopenia Project: scatterplot and smoothed locally weighted moving averages.
CART analysis predicting probability of slow walking (<0.8 m/s) yielded somewhat different results for men and women. In men, grip strength alone was the strongest predictor of slow walking (ie, resulted in the lowest relative error compared to other predictors), and CART analysis did not identify differences in grip strength cutpoints by BMI. In women, grip strength/BMI was the strongest predictor of slow walking. In order to further explore these gender effects, we evaluated cutpoints based on grip strength alone and grip strength/BMI in both men and women. We conducted logistic regression using alternative definitions of weakness and comparing both model fit statistics and the strength of the associations between weakness and slowness. Because the additional value of including BMI in the definition of weakness was unclear (ie, including BMI did not consistently improve model fit or result in stronger associations between weakness and slowness), we elected to use cutpoints based on grip strength alone as our primary analysis.
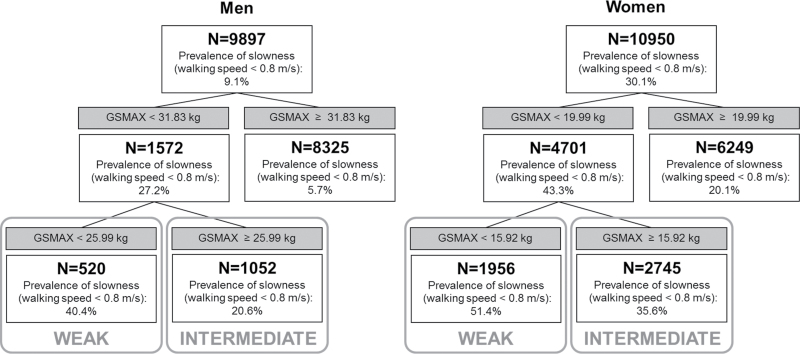
Figure 2 provides results for the primary definition. The first cutpoint identified in men was based on having grip strength equal to or above 31.83 kg versus below 31.83 kg. Within the low-strength group (<31.83 kg), a second cutpoint was identified at 25.99 kg. Among the weakest men (grip strength < 25.99 kg), 40.4% had slow gait speed, compared with 20.6% among men with intermediate strength (grip strength of 25.99–31.82), and 5.7% among men in the highest strength group (grip strength ≥ 31.83).
Figure 2.
Classification tree for gait speed <0.8 m/s in the FNIH Sarcopenia Project.
The first cutpoint identified in women was based on having grip strength equal to or above 19.99 kg versus below 19.99 kg. Within the low-strength group (<19.99 kg), a second cutpoint was identified at 15.92 kg. Among the weakest women (grip strength < 15.92 kg), 51.4% of women had slow gait speed, compared with 35.6% among women with intermediate strength (grip strength of 15.92–19.98 kg), and 20.1% among women in the highest strength group (grip strength ≥ 19.99 kg).
Based on these results, we defined three strength categories for both men and women (normal strength, intermediate, and weak). Table 1 provides the prevalence of strength categories in the full sample, accounting for heterogeneity across studies, and provides the relative odds of slow walking across strength groups. The majority of men were classified as normal strength (84%), 11% were classified as intermediate, and 5% as weak. Men in the intermediate and weak groups, respectively, had 3.6 (odds ratio [OR] = 3.63, 95% CI: 3.01–4.38) and 7.6 (OR = 7.62, 95% CI: 6.13–9.49) times greater odds of having slow gait speed relative to men in the normal strength group. Over half of women were classified as normal strength (57%), 25% were classified as intermediate and 18% as weak. Women in the intermediate and weak groups, respectively, had 2.4 (OR = 2.44, 95% CI: 2.20–2.71) and 4.4 (OR = 4.42, 95% CI: 3.94–4.97) times the odds of slow walking relative to women in the normal strength group. Test statistics (sensitivity, specificity, positive predictive value) are provided in the Supplementary Appendix.
Table 1.
Sensitivity Analysis: Prevalence of Categories of Strength and Likelihood of Slowness Across Subsamples in the FNIH Sarcopenia Project
| N | Prevalence of Strength Category, N (%) |
Likelihood of Slowness (walking speed <0.8 m/s), OR (95% CI)* |
||||||
|---|---|---|---|---|---|---|---|---|
| Normal Strength | Intermediate | Weak | Normal Strength | Intermediate | Weak | p for Interaction† | ||
| Men | ≥32.0 kg | 26–31.9 kg | <26 kg | <26 kg | 26–31.9 kg | ≥32.0 kg | ||
| All | 9,897 | 8,312 (84.0) | 1,065 (10.8) | 520 (5.3) | 1.0 (referent) | 3.63 (3.01, 4.38) | 7.62 (6.13,9.49) | |
| Age | ||||||||
| 65–79 | 7,599 | 6,801 (89.5) | 566 (7.5) | 232 (3.1) | 1.0 (referent) | 4.45 (3.40, 5.82) | 7.42 (5.28,10.43) | <.001 |
| 80 | 2,298 | 1,511 (65.8) | 499 (21.7) | 288 (12.5) | 1.0 (referent) | 1.74 (1.34, 2.27) | 4.17 (3.10,5.61) | |
| Body mass index | z | |||||||
| Underweight | 40 | 26 (65.0) | 8 (20.0) | 6 (15.0) | 1.0 (referent) | 3.30 (0.55, 19.65) | 2.75 (0.37,20.40) | .275 |
| Normal weight | 2,814 | 2,257 (80.2) | 360 (12.8) | 197 (7.0) | 1.0 (referent) | 4.04 (2.89, 5.65) | 10.17 (7.05,14.66) | |
| Overweight | 5,000 | 4,260 (85.2) | 507 (10.1) | 233 (4.7) | 1.0 (referent) | 3.55 (2.67, 4.73) | 8.10 (5.80,11.31) | |
| Obese | 2,043 | 1,769 (86.6) | 190 (9.3) | 84 (4.1) | 1.0 (referent) | 3.60 (2.43, 5.34) | 5.95 (3.54,9.98) | |
| Height (m) | .035 | |||||||
| 1.308 ≤ Tertile 1 < 1.559 | 3,285 | 2,434 (74.1) | 543 (16.5) | 308 (9.4) | 1.0 (referent) | 3.08 (2.35, 4.05) | 6.69 (4.93,9.09) | |
| 1.559 ≤ Tertile 2 < 1.611 | 3,294 | 2,826 (85.8) | 324 (9.8) | 144 (4.4) | 1.0 (referent) | 3.16 (2.23, 4.48) | 5.57 (3.69,8.40) | |
| 1.611 ≤ Tertile 3 < 1.826 | 3,318 | 3,052 (92.0) | 198 (6.0) | 68 (2.1) | 1.0 (referent) | 4.11 (2.75, 6.14) | 10.24 (5.98,17.54) | |
| Cancer: Yes | 2,087 | 1,773 (85.0) | 229 (11.0) | 85 (4.1) | 1.0 (referent) | 5.25 (3.47, 7.96) | 10.67 (6.20,18.36) | .081 |
| Cancer: No | 7,340 | 6,228 (84.9) | 747 (10.2) | 365 (5.0) | 1.0 (referent) | 3.39 (2.71, 4.25) | 7.08 (5.45,9.19) | |
| CHF: Yes | 536 | 398 (74.3) | 84 (15.7) | 54 (10.1) | 1.0 (referent) | 4.10 (2.29, 7.34) | 5.31 (2.67,10.58) | .277 |
| CHF: No | 8,365 | 7,158 (85.6) | 807 (9.7) | 400 (4.8) | 1.0 (referent) | 3.65 (2.96, 4.50) | 8.04 (6.29,10.27) | |
| COPD: Yes | 703 | 591 (84.1) | 82 (11.7) | 30 (4.3) | 1.0 (referent) | 2.21 (1.02, 4.77) | 9.23 (3.74,22.82) | .663 |
| COPD: No | 7,445 | 6,412 (86.1) | 681 (9.2) | 352 (4.7) | 1.0 (referent) | 3.90 (3.11, 4.89) | 7.44 (5.72,9.67) | |
| Diabetes: Yes | 1,157 | 908 (78.5) | 154 (13.3) | 95 (8.2) | 1.0 (referent) | 2.70 (1.75, 4.17) | 6.72 (4.08,11.05) | .348 |
| Diabetes: No | 8,708 | 7,389 (84.9) | 903 (10.4) | 416 (4.8) | 1.0 (referent) | 3.88 (3.15, 4.78) | 7.54 (5.89,9.66) | |
| Women | ≥20 kg | 16–19.9 kg | <16 kg | <16 kg | 16–19.9 kg | ≥20 kg | ||
| All | 10,950 | 6,249 (57.1) | 2,736 (25.0) | 1,965 (18.0) | 1.0 (referent) | 2.44 (2.20, 2.71) | 4.42 (3.94,4.97) | |
| Age | ||||||||
| 65–79 | 6,772 | 4,523 (66.8) | 1,417 (20.9) | 832 (12.3) | 1.0 (referent) | 2.27 (1.95, 2.63) | 3.70 (3.11,4.40) | .323 |
| 80 | 4,178 | 1,726 (41.3) | 1,319 (31.6) | 1,133 (27.1) | 1.0 (referent) | 1.97 (1.69, 2.29) | 3.49 (2.96,4.12) | |
| Body mass index | ||||||||
| Underweight | 210 | 80 (38.1) | 63 (30.0) | 67 (31.9) | 1.0 (referent) | 2.83 (1.26, 6.35) | 4.6 (2.11,10.02) | .497 |
| Normal weight‡ | 3,926 | 2,032 (51.8) | 1,035 (26.4) | 859 (21.9) | 1.0 (referent) | 2.45 (2.05, 2.94) | 4.80 (4.01,5.75) | |
| Overweight | 4,104 | 2,427 (59.1) | 1,017 (24.8) | 660 (16.1) | 1.0 (referent) | 2.71 (2.28, 3.21) | 4.74 (3.89,5.77) | |
| Obese | 2,710 | 1,710 (63.1) | 621 (22.9) | 379 (14.0) | 1.0 (referent) | 2.38 (1.95, 2.90) | 4.41 (3.45,5.64) | |
| Height (m) | ||||||||
| 1.470 ≤ Tertile 1 < 1.710 | 3,635 | 1,545 (42.5) | 1,107 (30.5) | 983 (27.0) | 1.0 (referent) | 2.08 (1.74, 2.48) | 3.80 (3.15,4.58) | .116 |
| 1.710 ≤ Tertile 2 < 1.767 | 3,652 | 2,157 (59.1) | 926 (25.4) | 569 (15.6) | 1.0 (referent) | 2.46 (2.05, 2.94) | 4.56 (3.69,5.64) | |
| 1.767 ≤ Tertile 3 < 1.989 | 3,663 | 2,547 (69.5) | 703 (19.2) | 413 (11.3) | 1.0 (referent) | 2.56 (2.11, 3.10) | 4.09 (3.26,5.13) | |
| Cancer: Yes | 591 | 394 (66.7) | 134 (22.7) | 63 (10.7) | 1.0 (referent) | 3.16 (2.06, 4.83) | 4.51 (2.52,8.06) | .303 |
| Cancer: No | 4,716 | 3,381 (71.7) | 832 (17.6) | 503 (10.7) | 1.0 (referent) | 2.78 (2.34, 3.30) | 3.54 (2.86,4.38) | |
| CHF: Yes | 448 | 204 (45.5) | 132 (29.5) | 112 (25.0) | 1.0 (referent) | 2.42 (1.50, 3.90) | 4.92 (2.86,8.48) | .944 |
| CHF: No | 9,394 | 5,236 (55.7) | 2,409 (25.6) | 1,749 (18.6) | 1.0 (referent) | 2.52 (2.25, 2.81) | 4.49 (3.97,5.08) | |
| COPD: Yes | 649 | 322 (49.6) | 197 (30.4) | 130 (20.0) | 1.0 (referent) | 3.08 (2.08, 4.55) | 5.49 (3.46,8.73) | .221 |
| COPD: No‡ | 8,165 | 4,480 (54.9) | 2,142 (26.2) | 1,543 (18.9) | 1.0 (referent) | 2.26 (2.02, 2.53) | 3.92 (3.47,4.43) | |
| Diabetes: Yes | 789 | 476 (60.3) | 182 (23.1) | 131 (16.6) | 1.0 (referent) | 2.81 (1.94, 4.06) | 2.32 (1.53,3.51) | .095 |
| Diabetes: No | 10,003 | 5,691 (56.9) | 2,518 (25.2) | 1,794 (17.9) | 1.0 (referent) | 2.44 (2.19, 2.72) | 4.64 (4.11,5.25) | |
Notes: CHF = Congestive heart failure; COPD = chronic obstructive pulmonary disease.*Odds ratio (OR) from logistic regression predicting probability of gait speed <0.8 m/s with random effect for study.
† p for interaction between strength groups and row characteristic in predicting walking speed.
‡ORs reflect model without random effect (required for model convergence).
Table 1 also provides the results of sensitivity analysis across stratification variables. As expected, the prevalence of weakness varied across age, BMI, height, and diseases status. However, in most cases, the excess prevalence of slowness associated with weakness was similar across groups. For example, the prevalence of weakness was higher among women aged 80 and older (27%) than among women aged 65–74 (12%). However, in both age groups, weakness was associated with an excess 3.5–3.7 times the odds of slow walking relative to normal strength. Notably, this was also true for BMI. Underweight women were the most likely to be weak (32%), whereas obese women were the least likely to be weak (14%). However, weakness was associated with an excess 4.4–4.8 times the odds of slow walking across all BMI groups.
The only statistically significant interactions occurred in men, for age and height. The association between weakness and slow walking was stronger in men in the 65–79 age group (OR = 7.42, 95% CI: 5.28–10.43) than in men aged 80 and older (OR = 4.17, 95% CI: 3.10–5.61). The association between weakness and slow walking was strongest in men in the tallest height tertile (OR = 10.24, 95% CI: 5.98–17.54) compared with the lowest tertile (OR = 6.69, 95% CI: 4.93–9.09).
Additional analysis (Supplementary Appendix Table 4) examined the prevalence of strength categories and low mobility across cohorts. Although the prevalence of weakness and mobility limitations differed across cohorts, the relationship between weakness and mobility limitation was generally similar. Relationships were less consistent in the clinical trials and in the Boston Puerto Rican Health Study.
Sensitivity analysis also considered the stability of grip strength cutpoints based on alternative gait speed outcomes (results not shown). In men, use of gait speed less than 0.6 m/s produced results similar to the main analysis (<26kg), identifying a weak group with grip strength less than 25.90kg. Use of a continuous gait speed outcome resulted in selection of a slightly higher cutpoint for weakness (<27.94kg). In women, both alternative specifications yielded slightly higher cutpoints for grip strength than the cutpoint found in the main analysis (<16 kg). Models using gait speed less than 0.6 m/s as the outcome identified a weak group with grip strength less than 17.78kg, and models using a continuous gait speed outcome identified a weak group with grip strength less than 19.99kg, similar to the “intermediate” group in the main analysis.
Results for an alternative definition utilizing grip strength/BMI are reported in the Supplementary Appendix and briefly summarized here. Using a definition of weakness based on grip strength alone, 14% of obese women were classified as weak, and weakness in obese women was associated with 4.4 times the odds of slow walking relative to normal strength (OR = 4.41, 95% CI: 3.45–5.64). Alternatively, using a definition based on grip strength divided by BMI (see Supplementary Appendix Table 5), 33% of obese women were classified as weak, and weakness using this definition was associated with 4.8 times the odds of slow walking relative to normal strength (OR = 4.84, 95% CI: 3.94–5.95). In contrast, underweight women were more likely to be classified as weak when using a definition based on grip strength alone (32%) than when using a definition based on grip strength divided by BMI (5%).
Discussion
The purpose of this analysis was to identify cutpoints that distinguish weakness associated with poor mobility performance using cross-sectional data. The cutpoints developed here and carried forward in additional analysis as part of the FNIH Sarcopenia Project define grip strength less than 26 kg in men and less than 16 kg in women as “weak.” These cutpoints classified 5% of men and 18% of women as weak, and weakness based on this definition was associated with more than 7 times the odds of slow walking in men and more than 4 times the odds of slow walking in women relative to normal strength. Cutpoints performed well across a range of subgroups defined by anthropometric characteristics (BMI, height) and the presence of chronic conditions. In addition to identifying a weak group, models identified a group with detectable, although less severe weakness in both men and women (termed “intermediate”). This intermediate level of weakness (<32 kg in men and <20 kg in women) was associated with 3.6 times the odds of slow walking in men and 2.4 times the odds of slow walking in women relative to normal strength.
The analytic approach used here focused on maximizing concurrent validity between strength and mobility impairment. This approach resulted in a relatively conservative definition of weakness, based both on test statistics and on comparison to existing definitions. Our aim was to maximize confidence that the criterion was detecting a clinically relevant degree of weakness. In men who were not slow (ie, walking speed ≥ 0.8 m/s), only 3% were weak, whereas 23% of men who were slow were weak. In women who are not slow, 13% were weak, compared with 31% of women who are slow. Thus, the definition of weakness proposed here identifies a subgroup of older persons with a higher probability of combined weakness and slowness than expected in the older population.
The European Working Group on Sarcopenia in Older Persons defined weakness based on a grip strength less than 30 kg in men and less than 20 kg in women (9). This definition corresponds most closely to the cutpoints we identified to classify the intermediate level of weakness. The cutpoints identified here as “weak” are lower (<26 kg in men and <16 kg in women) and resulted in a smaller proportion of the population being classified as weak (13).
Importantly, the role of BMI differed by gender. In models including all potential variations on grip strength and body size (grip strength, body size, and grip strength/body size ratios), grip strength alone was the best predictor for men, whereas grip strength/BMI was the best predictor for women. After carrying forward both definitions for additional analysis and comparing the strength of the relationships between weakness and slowness, as well as model fit statistics, neither definition performed consistently better than the other. In the absence of clear evidence that including BMI in the definition of weakness added to our ability to predict mobility disability, we elected to use the simpler indicator of weakness unadjusted for BMI.
Although the two different definitions of weakness had similar relationships with slow walking (as indicated by the strength of the relationship and the model fit statistics), they characterized different subgroups of the population as weak—a group with limited ability to generate strength and a group unable to generate sufficient strength relative to BMI. Because the a priori focus of this analysis was on identifying a subgroup of older adults in whom weakness might be due to low lean mass, we selected the definition based on strength unadjusted for BMI. However, another group may exist in whom weakness is due to low muscle quality.
It remains unclear why BMI would be more important for women than for men. This may be an artifact of the samples used in this analysis. Men and women were drawn from different study populations (MrOs in men, SOF in women, different UCONN clinical trials available for each gender). It may also reflect sex differences in body composition or the association between strength and mobility. Strength declines more rapidly in men at older ages (32), and the association between strength and physical function appears stronger in men than in women (5,33).
A central finding of this analysis is the lack of a clear threshold effect in the association between grip strength and gait speed. Previous research has reported nonlinearities in the relationship between strength and mobility (1,3), suggesting that there may a level of strength below which mobility becomes more difficult. However, we did not find evidence of a threshold in the relationship between grip strength and gait speed. Although grip strength is well correlated with lower extremity strength and is associated with mobility outcomes, it may not detect subtle differences in the association between lower extremity strength and function. However, preliminary analysis of knee extension strength also did not demonstrate nonlinearities in the association of strength and gait speed. It is possible that other measures of function, such ability to rise from a chair, would exhibit threshold effects that we were not able to observe using gait speed as an outcome. However, we were unable to examine inability to rise from a chair as a mobility outcome, because of low prevalence in this sample. The populations included in the FNIH Sarcopenia Project were all drawn from community-dwelling samples of older adults and may be underrepresentative of older populations with mobility limitations and weakness. Notably, however, some other studies have also been unable to identify thresholds in the association between strength and functional performance (34). Importantly, strength is one of many factors that influence gait speed, including balance, vision, cognition, and muscle power.
We used CART to identify weakness cutpoints, but it is important to recognize limitations in this approach. First, CART, by definition, partitions data into groups, even when the underlying relationship between the predictor and outcome is linear. Second, our CART models did not account for differences between studies included in the model, although we accounted for study in subsequent logistic models. Finally, no statistical model alone can identify a disease state, thus further work is necessary to understand the physiological implications of these results.
The definition of weakness proposed here relies on grip strength measurement. Although grip strength was measured using a variety of protocols, the proposed strength cutpoints performed well across cohorts. Unfortunately, data are unavailable from the present studies to directly compare measurement across protocols. By using the maximum measured grip strength from either hand, we sought to minimize variation based on these factors, but variation due to dynamometer used, hand position, and other factors may still exist (28). Future research should consider standardized protocols to facilitate comparison (28).
The analysis used a data-driven approach in a large and diverse pooled data set to identify weakness cutpoints associated with mobility impairment defined by gait speed. This is an important first step in identifying populations that may benefit from interventions to improve strength and muscle function. Results from this analysis highlight the utility of an absolute measure of weakness across population groups but also point to the need to consider strength relative to body size, particularly in women. Results also cast doubt on the existence of a strong threshold effect in the association between strength and gait speed, suggesting that increases in strength may have positive effects on physical function across the spectrum of strength observed in community-dwelling older adults. Despite lack of strong evidence of threshold effects, criteria for the identification of weakness may help identify populations that would experience the greatest benefit from interventions to improve strength and help clinicians identify patients at risk of weakness-associated mobility limitations.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
Funding support for the conference and the work of the consortium was provided by the National Institute on Aging (1U13AG041583 and P30 AG024827), the Food and Drug Administration, and through grants from the Foundation for the NIH, made possible by funding from Abbott Nutrition, Amgen, Eli Lilly, Merck, Novartis, and The Dairy Research Institute. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Additional acknowledgements for each contributing cohort and members of the FNIH Sarcopenia Project can be found in an online supplement.
References
- 1. Ferrucci L, Guralnik JM, Buchner D, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M275–M285 [DOI] [PubMed] [Google Scholar]
- 2. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457 [DOI] [PubMed] [Google Scholar]
- 4. Sallinen J, Stenholm S, Rantanen T, Heliövaara M, Sainio P, Koskinen S. Hand-grip strength cutpoints to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58:1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95:1851–1860 [DOI] [PubMed] [Google Scholar]
- 7. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266 [DOI] [PubMed] [Google Scholar]
- 8. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale and study description. J Gerontol A Biol Sci Med Sci. 2014;5:547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cawthon PM, Peters KW, Shardell MD, et al. Cut-points for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;5:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McLean RR, Peters KW, Shardell MD, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project. J Gerontol A Biol Sci Med Sci. 2014;5:576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dam TL, Peters KW, Fragala M, et al. An evidence-based comparison of candidate criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;5:584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773 [DOI] [PubMed] [Google Scholar]
- 15. Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–2108 [DOI] [PubMed] [Google Scholar]
- 16. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585 [DOI] [PubMed] [Google Scholar]
- 17. Newman AB, Haggerty CL, Goodpaster B, et al. ; Health Aging And Body Composition Research Group. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330 [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290 [DOI] [PubMed] [Google Scholar]
- 19. Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 21. Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutchins-Wiese HL, Kleppinger A, Annis K, et al. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging. 2013;17:76–80 [DOI] [PubMed] [Google Scholar]
- 24. Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58:1707–1714 [DOI] [PubMed] [Google Scholar]
- 25. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042–1048 [DOI] [PubMed] [Google Scholar]
- 26. Judge JO, Kleppinger A, Kenny A, Smith JA, Biskup B, Marcella G. Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporos Int. 2005;16:1096–1108 [DOI] [PubMed] [Google Scholar]
- 27. Giampaoli S, Ferrucci L, Cecchi F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–288 [DOI] [PubMed] [Google Scholar]
- 28. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429 [DOI] [PubMed] [Google Scholar]
- 29. Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and Regression Trees. Belmont, CA: Wadsworth International Group; 1984 [Google Scholar]
- 30. Tilson JK, Sullivan KJ, Cen SY, et al. ; Locomotor Experience Applied Post Stroke (LEAPS) Investigative Team. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maindonald J, Braun WJ. Data Analysis and Graphics Using R: An Example-Based Approach. 3rd ed. Cambridge: Cambridge University Press; 2010 [Google Scholar]
- 32. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 33. Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386 [DOI] [PubMed] [Google Scholar]
- 34. Marsh AP, Miller ME, Saikin AM, et al. Lower extremity strength and power are associated with 400-meter walk time in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61:1186–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]