Abstract
Age-related pseudocapillarization of the liver sinusoidal endothelium is associated with impaired lipid and drug metabolism and the development of disease. 2,5-Dimethoxy-4-iodoamphetamine is a serotonin receptor 2 agonist that has been shown to have beneficial effects on the liver sinusoidal endothelium in the setting of partial hepatectomy. Here, we have assessed whether 2,5-dimethoxy-4-iodoamphetamine influences ultrastructure of the sinusoidal endothelium in normal 7- and 24-month-old C57Bl6 mice. Following 48 hours of 2,5-dimethoxy-4-iodoamphetamine administration, we found that the liver endothelium in the young, but not in the old, mice had increased porosity compared with controls. This effect appeared to be modulated by increased fenestration size rather than a change in fenestration number. 2,5-Dimethoxy-4-iodoamphetamine is a useful manipulator of fenestration size in the young liver and could be harnessed in the search for therapeutic interventions for pseudocapillarization.
Key Words: Liver endothelium; Fenestrations; 2,5-Dimethoxy-4-iodoamphetamine.
The microcirculation of the liver has a unique morphology that facilitates the bi-directional exchange of substrates between hepatocytes and blood in the liver sinusoids (1–3). Liver sinusoidal endothelial cells (LSEC) are specialized endothelial cells that line the wall of the hepatic sinusoid. The cytoplasmic extensions of LSECs are very thin and perforated with fenestrations, which are nondiaphragmed, transcellular pores approximately 50–150nm in diameter. The fenestrations act as fundamental biologic ultrafilter, allowing diffusive and convective passage of substrates across cells without relying on endocytosis or other receptor-mediated mechanisms. They facilitate passive transfer of substances such as lipoproteins (4), parasites (5), pharmacological agents (6), and gene transfer vectors (7). In the LSEC, fenestrations are arranged in groups of between 10 and 100 fenestrations, termed “liver sieve plates,” reflecting their role as a filter or sieve (8). Fenestrations are essential for human health and loss of fenestrations in LSECs results in impaired lipid, drug, and insulin transfer (9–11).
Age-related pseudocapillarization is now well documented (12–14). This loss of endothelial fenestrations, endothelial thickening, and increased deposition of extracellular matrix with age has been shown to impact upon liver function, in particular leading to a reduction in the transfer of lipids and pharmaceutical agents (4,6,15). Through this mechanism, age-related pseudocapillarization is thought to contribute to the development of age-related diseases.
2,5-Dimethoxy-4-iodoamphetamine (DOI) is a 5-hydroxytryptamine (serotonin) receptor 2 agonist that has been shown to have beneficial effects on survival, liver regeneration, and LSEC morphology after partial hepatectomy (16). This survival benefit is thought to be mediated through LSECs. Exogenous DOI stimulates LSEC release of vascular endothelial growth factor (VEGF), which binds to the endothelial VEGF receptors leading to LSEC fenestration opening, improving hepatocyte nutrient access and platelet adhesion (17).
Here, we have treated 7- and 24-month-old C57Bl6 mice with DOI to investigate the effects of DOI on the LSEC in normal, untreated young and old mice to assess its therapeutic potential in age-related defenestration and pseudocapillarization.
Methods
Animals
Young mature adult (aged 7 mo, weight 28–36 g) and old adult (aged 24 mo, weight 32–40g) C57BL/6 male mice were obtained from in-house breeding colonies at the National Institute of Aging (Baltimore, MD). Mice were allowed ad libitum access to food and water throughout the study. Animal rooms were maintained at 20–22°C with 30%–70% relative humidity and a 12-hour light/dark cycle. All animal protocols were approved by the animal care and use committee of the National Institute on Aging.
Animal Procedures
Young and old mice were randomly allocated to either a control or a treatment group. For the treatment group, the serotonin agonist DOI (1mg/kg via intraperitoneal injection; Sigma-Aldrich) was injected twice daily for 48 hours before liver perfusion and the control group was injected with saline (dose-matched microliter) at the same time.
After 48 hours, the animals were anesthetized with an intraperitoneal injection of ketamine–xylazine and liver perfusion for scanning electron microscopy (SEM) studies was performed as described previously (18–20). Briefly, a midline incision was made in the abdomen, the portal vein cannulated and the inferior vena cava cut to allow outflow. Krebs–Henseleit buffer was briefly perfused through the liver vasculature at low pressure to wash blood out of the sinusoids. Following this, fixative for electron microscopy (2.5% glutaraldehyde/2% paraformaldehyde in 0.1M sodium cacodylate buffer) was introduced into liver until hard and pale. The liver was excised and samples were cut into 1-mm3 blocks.
Sample Preparation
After fixation, blocks were prepared for SEM by submerging in 2% osmium tetroxide, dehydration in an ethanol gradient followed by drying with hexamethyldisilazane. Finally, six blocks per animals were mounted on stubs and sputter-coated with platinum. SEM was performed on a JEOL 6340 SEM by an operator blinded to age and treatment status of the animal. At least 10 scanning electron micrographs were taken at 15,000× magnification from each animal from randomly selected areas of the sinusoid. Fenestration number and diameter were measured, together with total area assessed, using Image J (NIH). These data were used to calculate porosity (the percentage of the LSEC surface area covered with fenestrations). Results are presented as mean ± standard error and comparisons were performed using analysis of variance (SigmaPlot version 11; Systat Software).
Results
DOI treatment was tolerated well in the animals and no apparent adverse effects were observed. Five animals began treatment in each group and all animals are included in data presented here (except for one old control that died during the course of the study due to unknown reasons).
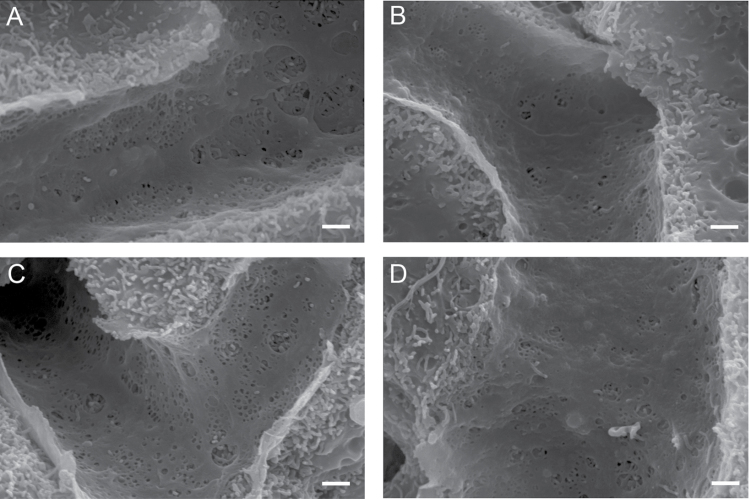
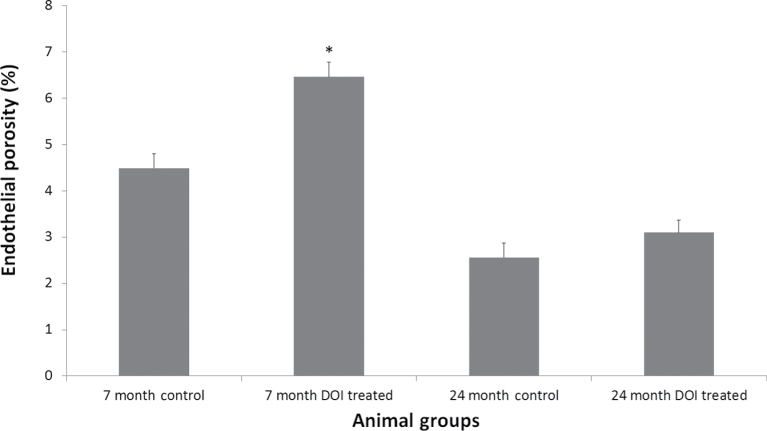
SEM of the livers revealed intact sinusoids and unremarkable liver architecture in all animal groups. The LSEC of the untreated young animals had numerous fenestrations, and as expected, the untreated 24-month-old mice showed marked age-related defenestration (porosity: 7-mo control: 4.488% ± 0.320%; 24-mo-old control: 2.562% ± 0.308%, p < .001; Figures 1 and 2).
Figure 1.
Scanning electron micrographs of the intact sinusoid and the fenestrated endothelium. DOI induced changes in fenestration size in young, but not in old, animals. (A) 7-mo untreated control, (B) 24-mo untreated control, (C) 7-mo DOI treated, and (D) 24-mo DOI treated. DOI = 2,5-dimethoxy-4-iodoamphetamine.
Figure 2.
Quantification of liver endothelial porosity. DOI treatment increased porosity in the young animals (*p < .001) but had no effect in the old animals. DOI = 2,5-dimethoxy-4-iodoamphetamine.
Treatment with DOI induced a significant increase in LSEC porosity in the 7-month-old animals (4.488% ± 0.320% vs 6.464% ± 0.312%, p < .001); however, this positive effect of DOI was not maintained in the 24-month-old animals (2.562% ± 0.308% vs 3.098% ± 0.269%, not significant; Figures 1 and 2).
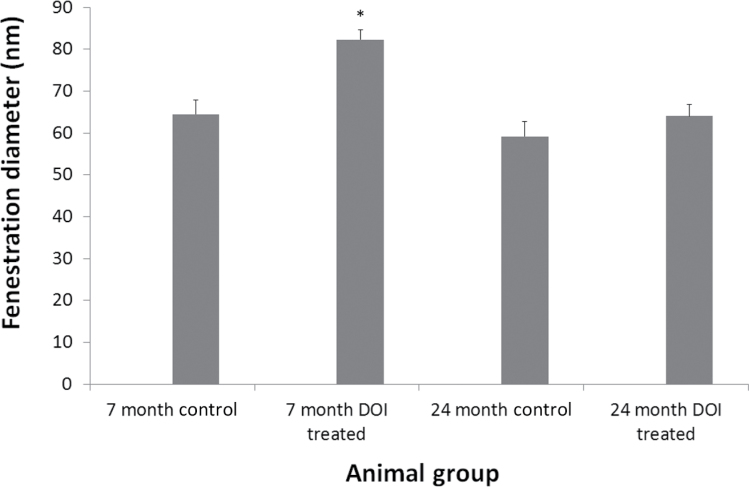
Next, we determined that fenestration diameter was increased by approximately 30% by DOI treatment in the 7-month-old animals from 64.5±3.3 to 82.3±2.4nm (Figure 3). Fenestration diameter was the same in the young and old control groups 64.5±3.3 and 59.2±3.5nm, respectively. DOI treatment had no effect on fenestration diameter in the 24-month-old animals (64.0±2.8nm).
Figure 3.
Fenestration diameter of the liver sinusoidal endothelium with age and DOI treatment. DOI induced an increase in fenestration diameter in the 7-mo-old animals (*p < .001), but not in the 24-mo-old animals. No change in fenestration diameter was seen with age. DOI = 2,5-dimethoxy-4-iodoamphetamine.
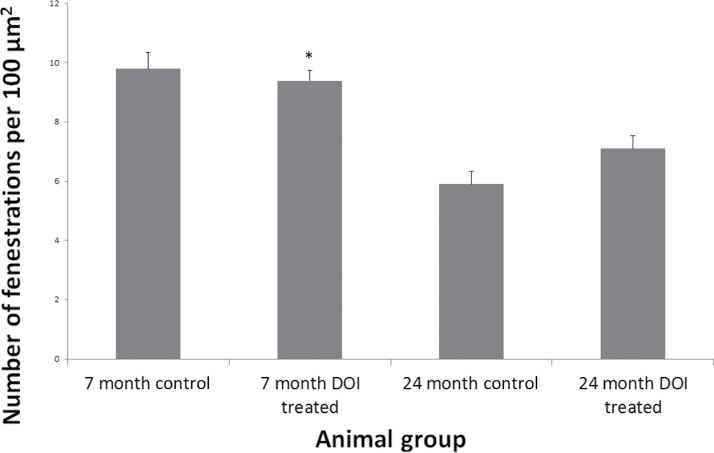
Fenestration density was also assessed in all groups (Figure 4). Fenestration number per square micrometer fell significantly with age (9.8±0.6 vs 5.9±0.4, p < .001). No significant increase in fenestration number was seen with DOI treatment in either age groups (7 mo: 9.8±0.6 vs 9.4±0.3 fenestrations/µm2, 24 mo: 5.9±0.4 vs 7.1±0.4 fenestrations/µm2).
Figure 4.
The density of fenestrations in the LSEC is unchanged by DOI treatment. The number of fenestrations per square micrometer was quantified in young and old control and treated mice. DOI = 2,5-dimethoxy-4-iodoamphetamine; LSEC = liver sinusoidal endothelial cells.
Discussion
DOI has been shown to confer a survival benefit in animals undergoing partial hepatectomy in young and old animals through its effects on fenestrations (17). Our study confirms age-related defenestration of the liver sinusoidal endothelium and shows that DOI treatment for 48 hours induces a significant increase in LSEC porosity in young but not old animals. DOI treatment was not associated with any structural hepatocellular changes and had no obvious detrimental effects on animal health.
DOI has been shown to exert an effect on LSEC morphology through serotonin-mediated activation of a VEGF-dependent pathway (17). VEGF is a potent angiogenic factor that has been shown by us and others to induce fenestration formation (21–23). VEGF blockade during development leads to impaired LSEC fenestration development (24) and VEGF inhibition by bevacizumab, a VEGF antibody, has been shown to impair regeneration after partial hepatectomy (25,26).
In this study, DOI increased LSEC porosity in young but not old animals (Figure 2). Here, the increase in porosity in the 7-mo-old animals is mediated through a DOI induced increase in fenestration diameter (Figure 3), while DOI treatment did not induce a change in fenestration number in either age groups (Figure 4).
The results suggest that DOI is active not just on a VEGF-dependent pathway, but on another pathway that influences fenestrations. Previous work with serotonin and the LSEC has shown that serotonin influences fenestration diameter through calcium-dependent changes in the actin cytoskeleton of the cell (27). A possible explanation for the inactivity of DOI in the old LSEC is that age-related pseudocapillarization, in the absence of partial hepatectomy, may be mediated through a non-VEGF-dependent mechanism. Recently, we have shown that the composition of the plasma membrane, particularly lipid rafts, is highly relevant in fenestration formation (21) and changes in membrane composition with age are unknown. A further possible mechanism for reduced responsiveness to DOI by the old LSEC is that components of the VEGF pathway may undergo age-related impairment.
While the effect of age on 5-hydroxytryptamine receptors in the LSEC is unknown, another plausible mechanism for the age-related loss of responsiveness to DOI is reduced receptor number, binding, or activity with age. Reduced serotonin-dependent augmentation of respiration has been shown previously in older rats (28).
Despite these further unresolved questions, we have shown that DOI is a potent modulator of fenestration size in young mice and this alone suggests it is worth further research as a potential therapeutic agent for reversing pseudocapillarization.
Funding
This study was supported in part by the Intramural Research Program of the National Institutes of Aging, National Institutes of Health, and the Ageing and Alzheimer’s Research Fund of the Concord Hospital. National Medical Health and Research Council of Australia CJ Martin Early Career Fellowship (RGMS ID 2010-01671 to S.J.M.).
Conflict of Interest
None.
Acknowledgments
We are extremely grateful to Dawn Nines, Justine Lucas, and Dawn Phillips-Boyer for their excellent animal care.
References
- 1. Cogger VC, Le Couteur DG. Fenestrations in the liver sinusoidal endothelial cell. In: Arias IM, Boyer JL, Fausto N, et al., eds. The Liver: Biology and Pathobiology. Hoboken NJ: John Wiley & Sons; 2009: 387–404 [Google Scholar]
- 2. Fraser R, Cogger VC, Dobbs B, et al. The liver sieve and atherosclerosis. Pathology. 2012;44:181–186 [DOI] [PubMed] [Google Scholar]
- 3. Sorensen KK, McCourt P, Berg T, Crossley C, Le Couteur DG, Wake K, Smedsrod B. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol. 2012;303:217–230 [DOI] [PubMed] [Google Scholar]
- 4. Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42(6):1349–1354 [DOI] [PubMed] [Google Scholar]
- 5. Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001;33(5):1154–1165 [DOI] [PubMed] [Google Scholar]
- 6. Mitchell SJ, Huizer-Pajkos A, Cogger VC, et al. Age-related pseudocapillarization of the liver sinusoidal endothelium impairs the hepatic clearance of acetaminophen in rats. J Gerontol A Biol Sci Med Sci. 2011;66(4):400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fraser R, Le Couteur DG, Warren A, Cogger VC, Bertolino P, Smith M. The liver sieve and gene therapy. Blood. 2003;101(8):3338; author reply 3338–3339 [DOI] [PubMed] [Google Scholar]
- 8. Latta H, Fligiel S. Mesangial fenestrations, sieving, filtration, and flow. Lab Invest. 1985;52(6):591–598 [PubMed] [Google Scholar]
- 9. Mitchell SJ, Huizer-Pajkos A, Cogger VC, McLachlan AJ, Le Couteur DG, Hilmer SN. Poloxamer 407 increases the recovery of paracetamol in the isolated perfused rat liver. J Pharm Sci. 2011;100(1):334–340 [DOI] [PubMed] [Google Scholar]
- 10. Cogger VC, Hilmer SN, Sullivan D, Muller M, Fraser R, Le Couteur DG. Hyperlipidemia and surfactants: the liver sieve is a link. Atherosclerosis. 2006;189(2):273–281 [DOI] [PubMed] [Google Scholar]
- 11. Raines SM, Richards OC, Schneider LR, et al. Loss of PDGF-B activity increases hepatic vascular permeability and enhances insulin sensitivity. Am J Physiol Endocrinol Metab. 2011;301(3):E517–E526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Couteur DG, Cogger VC, Markus AM, et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33(3):537–543 [DOI] [PubMed] [Google Scholar]
- 13. Ito Y, Sørensen KK, Bethea NW, et al. Age-related changes in the hepatic microcirculation in mice. Exp Gerontol. 2007;42(8):789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stacchiotti A, Lavazza A, Ferroni M, et al. Effects of aluminium sulphate in the mouse liver: similarities to the aging process. Exp Gerontol. 2008;43(4):330–338 [DOI] [PubMed] [Google Scholar]
- 15. Mitchell SJ, Kane AE, Hilmer SN. Age-related changes in the hepatic pharmacology and toxicology of paracetamol. Curr Gerontol Geriatr Res. 2011;2011:624156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian Y, Graf R, El-Badry AM, et al. Activation of serotonin receptor-2B rescues small-for-size liver graft failure in mice. Hepatology. 2011;53(1):253–262 [DOI] [PubMed] [Google Scholar]
- 17. Furrer K, Rickenbacher A, Tian Y, et al. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci U S A. 2011;108(7):2945–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cogger VC, Hilmer SN, Sullivan D, Muller M, Fraser R, Le Couteur DG. Hyperlipidemia and surfactants: the liver sieve is a link. Atherosclerosis. 2006;189(2):273–281 [DOI] [PubMed] [Google Scholar]
- 19. Cogger VC, Muller M, Fraser R, McLean AJ, Khan J, Le Couteur DG. The effects of oxidative stress on the liver sieve. J Hepatol. 2004;41(3):370–376 [DOI] [PubMed] [Google Scholar]
- 20. Cogger VC, Warren A, Fraser R, Ngu M, McLean AJ, Le Couteur DG. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp Gerontol. 2003;38(10):1101–1107 [DOI] [PubMed] [Google Scholar]
- 21. Svistounov D, Warren A, McNerney GP, et al. The relationship between fenestrations, sieve plates and rafts in liver sinusoidal endothelial cells. PLoS One. 2012;7(9):e46134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cogger VC, Arias IM, Warren A, et al. The response of fenestrations, actin, and caveolin-1 to vascular endothelial growth factor in SK Hep1 cells. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G137–G145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yokomori H, Oda M, Yoshimura K, et al. Vascular endothelial growth factor increases fenestral permeability in hepatic sinusoidal endothelial cells. Liver Int. 2003;23(6):467–475 [DOI] [PubMed] [Google Scholar]
- 24. Carpenter B, Lin Y, Stoll S, Raffai RL, McCuskey R, Wang R. VEGF is crucial for the hepatic vascular development required for lipoprotein uptake. Development. 2005;132(14):3293–3303 [DOI] [PubMed] [Google Scholar]
- 25. Assy N, Spira G, Paizi M, et al. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol. 1999;30(5):911–915 [DOI] [PubMed] [Google Scholar]
- 26. Shimizu H, Miyazaki M, Wakabayashi Y, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001;34(5):683–689 [DOI] [PubMed] [Google Scholar]
- 27. Gatmaitan Z, Varticovski L, Ling L, Mikkelsen R, Steffan AM, Arias IM. Studies on fenestral contraction in rat liver endothelial cells in culture. Am J Pathol. 1996;148(6):2027–2041 [PMC free article] [PubMed] [Google Scholar]
- 28. Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001;531(2):509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]