Abstract
Background.
Atrial fibrillation (AF) is associated with higher risk of dementia and Alzheimer’s disease. To better understand the mechanism, we examined neuropathologic changes seen with AF.
Methods.
We analyzed data from an autopsy series arising from a population-based, prospective cohort study set within Group Health, an integrated health care delivery system. Participants were people aged 65 and older, community-dwelling, and nondemented at study enrollment, who died during follow-up and underwent autopsy. AF was defined from medical records. Permanent AF was defined as having two or more electrocardiograms showing AF between 6 and 36 months apart with no evidence of sinus rhythm in between. The primary study outcomes were gross infarcts, neuritic plaques, and neurofibrillary tangles, ascertained using consensus guidelines. Adjusted relative risks and 95% CIs were calculated using modified Poisson regression, weighted to account for selection into the autopsy cohort.
Results.
Three hundred and twenty-eight participants underwent autopsy; 134 (41%) had AF. People with AF were more likely to have gross infarcts than those without AF (45% vs 31%; relative risk 1.82, 95% CI 1.23–2.71); in 30%, these infarcts were not clinically recognized before death. Alzheimer’s disease neuropathologic changes were not associated with ever having AF but were more common in people with permanent AF. Adjusted relative risks for frequent neuritic plaques and neurofibrillary tangles were 1.47 (0.96–2.28) and 1.40 (0.79–2.49), respectively, for people with permanent AF versus no AF.
Conclusions.
AF is associated with gross infarcts. Permanent AF may contribute to Alzheimer’s disease neuropathologic changes, but more study is needed.
Key Words: Alzheimer's, Cardiac arrhythmias, Stroke, Brain aging, Cardiovascular.
Dementia is common in older adults and imposes a heavy burden on individuals, their families, and society. Few modifiable risk factors have been identified (1). There is growing evidence that atrial fibrillation (AF) is associated with higher risk of dementia and Alzheimer’s disease (AD) (2–6), raising the possibility that interventions targeting AF might prevent or delay some cases of dementia.
A better understanding of the mechanism underlying the association between AF and dementia could guide future interventions. Possible mechanisms include embolic strokes (7,8), silent cerebral emboli (9), and cerebral hypoperfusion (10,11). It is not known whether AF contributes to AD-associated neuropathologic changes such as neurofibrillary tangles and neuritic plaques. The only study to examine this question found that AF was associated with large ischemic lesions but not amyloid beta load or neurofibrillary degeneration (12). However, this study included few people with AF and observed no overall association between AF and dementia, so their neuropathology results are difficult to interpret.
We investigated neuropathologic changes associated with AF using data from a large, population-based autopsy series (13). We hypothesized that AF would be associated with ischemic changes but not AD-associated changes and that greater exposure to AF would be associated with more severe neuropathologic changes.
Methods
Setting
The Adult Changes in Thought (ACT) study is a population-based prospective cohort study of dementia risk factors (14). It is set within Group Health (GH), an integrated health care delivery system in the Northwest United States. Participants gave written consent, and all procedures were approved by GH’s Human Subjects Review Committee.
Study Population
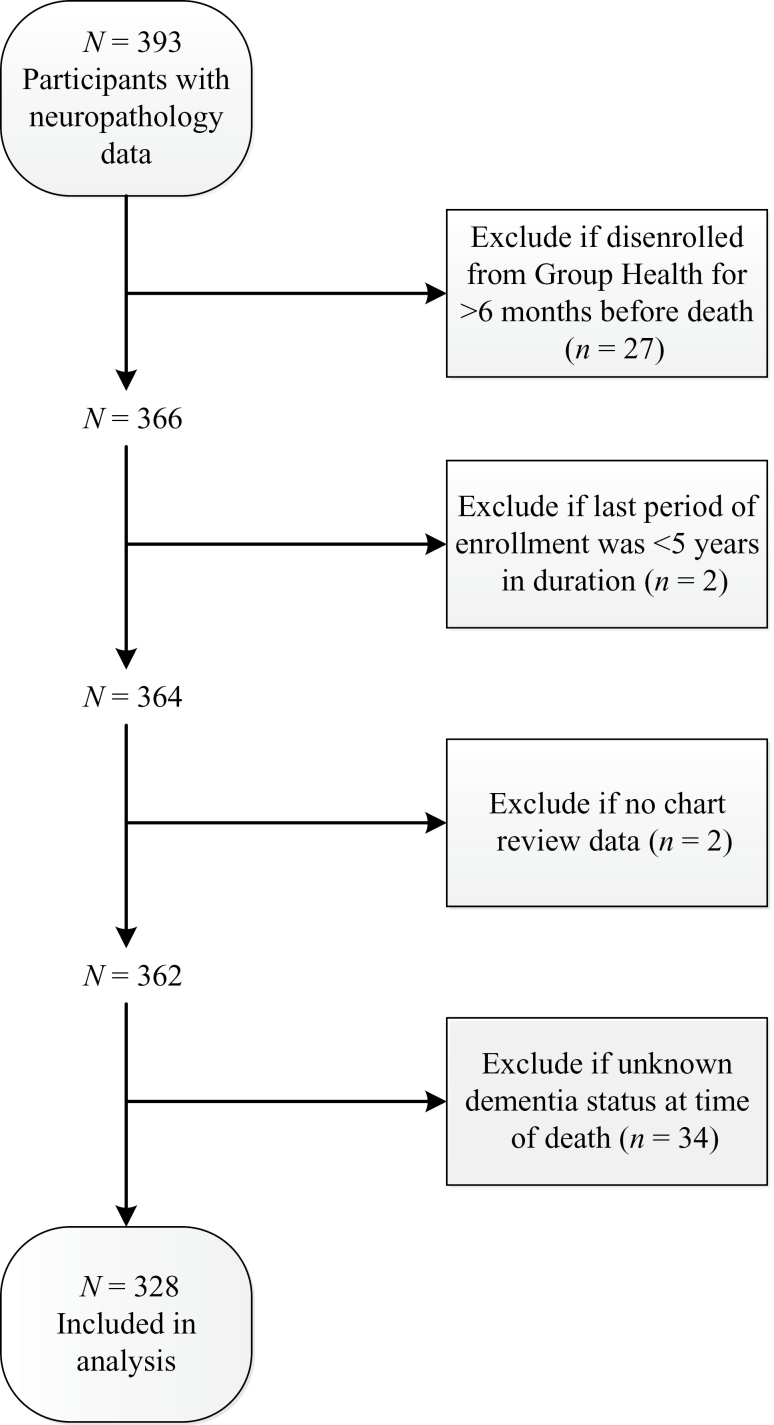
The ACT study recruits community-dwelling, nondemented adults aged 65 and older from among GH members living in or near Seattle, Washington. The original cohort (n = 2,581) was enrolled between 1994 and 1996 and an expansion cohort (n = 811) between 2000 and 2002. In 2004, the study began ongoing enrollment to replace people who die, develop dementia, or drop out. In all phases, the sample was randomly selected. Biennial study visits include an interview and cognitive screening to detect possible dementia. The current analyses include people who died and came to autopsy for whom medical record review has been completed. To ensure adequate data, we required at least 5 years of GH enrollment and excluded people who disenrolled from GH more than 6 months before death. We also excluded people with unknown dementia status at the time of death because this information is needed to account for selection into the autopsy cohort (see later). Figure 1 shows the flow of participants through the study.
Figure 1.
Flow of potential participants through study.
Study Measures
Exposure.—
Atrial fibrillation and flutter (henceforth abbreviated as AF) were assessed from GH medical records including automated electrocardiogram (EKG) data. Ninety-seven percent of participants had at least one EKG in the automated database. We defined AF as “permanent” when AF was observed on two or more EKGs from 6 to 36 months apart with no evidence of sinus rhythm in between, an approach we used in a prior study (15).
Outcomes.—
We examined several neuropathologic outcomes associated with AD: neurofibrillary tangles (Braak staging criteria) (16,17), neuritic plaques (Consortium to Establish a Registry for Alzheimer’s Disease staging) (18,19), and amyloid angiopathy (20). We also examined vascular brain injury. Both sides of the brain were evaluated for cerebral infarcts observed grossly (including territorial and lacunar infarcts), and we also assessed the number of cerebral microinfarcts in standardized screening sections of brain as a measure of microvascular brain injury. We dichotomized outcomes using cutpoints associated with clinical dementia. For Braak stage, we compared stages V–VI versus 0–IV; for neuritic plaques, moderate or frequent versus none or sparse, and for amyloid angiopathy, grades 1–4 versus none (20). For cerebral microinfarcts, we compared individuals with three or more infarcts to those with zero to two infarcts, because this cutpoint was used in our prior study, which found higher dementia risk in people with three or more infarcts (13). As a descriptive analysis, we examined the number and type of pathologic changes present in subgroups of participants stratified by the presence of AF and dementia. For these analyses, we defined AD neuropathologic changes as present when NIA-Reagan criteria (21) for intermediate or high likelihood of AD were met. These descriptive analyses also examined presence of Lewy bodies in brainstem, amygdala, and/or association cortex as previously described (22).
Covariates.—
Dementia status was determined using standard ACT study procedures (14). Every 2 years, participants are screened with the Cognitive Abilities Screening Instrument (23). People scoring below 86 undergo detailed neurological and neuropsychological assessment. Results of recent cranial imaging are reviewed, if available. Cases are reviewed by a multidisciplinary consensus committee that uses Diagnostic and Statistical Manual of Mental Disorders (fourth edition) (24) criteria to identify dementia cases.
ACT study interviews provided information about self-reported race, education, and smoking history. Blood pressure was measured at the ACT baseline study visit. Participants provided blood samples for APOE genotyping (25,26). Medical records provided information about height and weight and history of stroke, transient ischemic attack, diabetes mellitus, congestive heart failure (CHF), hypertension, coronary artery bypass grafting (CABG), coronary angioplasty, myocardial infarction, and angina. From medical records, abstractors reviewed reports from cranial imaging performed as part of clinical care, including recording whether infarcts were noted. Information about warfarin use came from GH electronic pharmacy data.
We dichotomized race as white versus other race. Because more than 90% of participants were white, there were so few people belonging to any other racial group that we grouped all of these categories together. Body mass index was calculated as weight in kilograms divided by the square of height in meters, using the weight obtained closest to age 65 during routine clinical care. Obesity was defined as body mass index ≥ 30kg/m2 (27). We dichotomized genotyping results as the presence of one or two versus zero copies of the APOE ε4 allele. Coronary artery disease was defined as any history of myocardial infarction, CABG, coronary angioplasty, or angina.
Measures Used in Models Accounting for Selection Into the Autopsy Cohort.—
Statistical models used to account for selection bias (described later) included covariates from ACT study visits or GH electronic data. We could not use variables from medical record review because reviews were completed only for autopsied participants, and the selection models needed to include the entire cohort. At ACT interviews, participants were asked whether a physician had ever told them they had angina or a heart attack and whether they had ever had CABG or angioplasty. For the selection models, we defined AF as present if a person received an International Classification of Diseases, version 9 code for AF or had an EKG showing AF in the electronic database. Covariates were defined based on the information available at the time of the participant’s last study visit.
Statistical Analysis
The association between AF and neuropathologic outcomes was examined using modified Poisson regression models for binary data estimated via generalized estimating equations (28), weighted to account for selection into the autopsy cohort as described later. These models utilize a log link function to allow for estimation of relative risks. Analyses were conducted separately for each outcome and compared risk in people with a history of AF to those with no history of AF. Primary analyses adjusted for age at death (continuous), ACT cohort (original, expansion, or replacement), diabetes, hypertension, obesity, smoking (ever versus never), and coronary artery disease. Separate models were used to examine the impact of AF persistence, each comparing a subgroup (permanent AF or not permanent) to people without AF. Sensitivity analyses further adjusted for CHF and APOE status; these analyses excluded people missing APOE data (11%; see Table 1 footnote).
Table 1.
Characteristics of the Sample, Overall, and by AF Status at Death
| Total (N = 328) | Never AF (N = 194) | AF, never permanent* (N = 77) | Permanent AF* (N = 57) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Age at death, y, mean (SD) | 87.6 (6.8) | 87.3 (7.1) | 87.8 (6.5) | 88.2 (6.0) |
| Female | 181 (55.2) | 110 (56.7) | 40 (52.0) | 31 (54.4) |
| White race | 317 (96.7) | 186 (95.9) | 75 (97.4) | 56 (98.3) |
| Education | ||||
| <High school | 39 (11.9) | 23 (11.9) | 10 (13.0) | 6 (10.5) |
| Completed high school | 80 (24.4) | 48 (24.7) | 22 (28.6) | 10 (17.5) |
| Some college | 88 (26.8) | 52 (26.8) | 19 (24.7) | 17 (29.8) |
| Completed college | 121 (36.9) | 71 (36.6) | 26 (33.8) | 24 (42.1) |
| Smoking history | ||||
| Nonsmoker | 137 (41.8) | 79 (40.7) | 34 (44.2) | 24 (42.1) |
| 1–40 pack-years | 90 (27.5) | 56 (28.8) | 21 (27.3) | 13 (22.8) |
| >40 pack-years | 101 (30.8) | 59 (30.4) | 22 (28.6) | 20 (35.1) |
| BMI, kg/m2, mean (SD)† | 25.6 (4.3) | 25.5 (4.2) | 25.9 (3.9) | 25.5 (5.2) |
| Cerebrovascular disease‡ | 147 (44.8) | 77 (39.7) | 38 (49.4) | 32 (56.1) |
| Clinical stroke | 82 (25.0) | 36 (18.6) | 22 (28.6) | 24 (42.1) |
| Transient ischemic attack | 69 (21.0) | 36 (18.6) | 19 (24.7) | 14 (24.6) |
| Silent infarct on imaging | 73 (22.3) | 36 (18.6) | 23 (29.9) | 14 (24.6) |
| Diabetes | 67 (20.4) | 35 (18.0) | 21 (27.3) | 11 (19.3) |
| Congestive heart failure | 178 (54.3) | 77 (39.7) | 57 (74.0) | 44 (77.2) |
| Hypertension | 225 (68.6) | 130 (67.0) | 51 (66.2) | 44 (77.2) |
| Coronary artery disease | 196 (59.8) | 101 (52.1) | 53 (68.8) | 42 (73.7) |
| APOE ε4 allele present§ | 91 (31.3) | 52 (30.8) | 17 (25.0) | 22 (40.7) |
| Dementia|| | 143 (43.6) | 86 (44.3) | 30 (39.0) | 27 (47.4) |
| Any warfarin after AF diagnosis | — | — | 28 (36.4) | 46 (80.7) |
| ≥5 warfarin fills after AF diagnosis | — | — | 16 (20.8) | 42 (73.7) |
Notes: AF = atrial fibrillation or flutter; APOE = apolipoprotein E; BMI = body mass index; SD = standard deviation.
*Permanent AF was defined as AF on electrocardiogram on two occasions from 6 to 36 mo apart with no evidence of sinus rhythm in between.
†From medical records.
‡Clinically recognized stroke, transient ischemic attack, or silent infarct on cranial imaging.
§Overall, 11.3% were missing APOE data, including 12.9% among those who never had AF, 9.0% who ever had AF, 11.7% with AF that was never permanent, and 5.3% with permanent AF. No data were missing for other variables in this table.
||Based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (24).
We used inverse probability weighting to account for selection into the autopsy cohort, because unweighted results will be biased if autopsied participants are not representative of the broader population (29,30). Whether a participant undergoes autopsy depends on whether he or she remains in the study, consents to autopsy, and dies. Each of these processes may be influenced by demographic, behavioral, and clinical characteristics. We developed weights from a logistic regression model characterizing the probability of selection into the autopsy sample and incorporated these weights into our primary analytic models. We did not model the three processes listed earlier separately; associations observed in the selection model could be due to any or all of these processes. Variables in the selection model were dementia status, age, sex, race, education, ACT cohort, AF, coronary artery disease, and smoking history. We estimated standard errors for parameters in our primary regression models accounting for uncertainty in selection weights using a bootstrap approach to compute bias-corrected and accelerated bootstrap CIs (31), as we have in prior publications (13,32,33).
As sensitivity analyses, we repeated the primary analyses excluding six people diagnosed with AF within 30 days prior to death and two who had AF only in the setting of CABG. In post hoc descriptive analyses, we stratified participants according to dementia and AF status and described for each subgroup the number and type of coexisting pathologic changes, considering the following pathologic changes: NIA-Reagan criteria for AD (21), stroke, cerebral microinfarcts, and presence of Lewy bodies.
Analyses were carried out using Stata 10 (StataCorp. 2007. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP) and R version 2.13.2 (34).
Results
Three hundred and twenty-eight individuals met inclusion criteria. The mean age at death was 87.6 years, 55% were women and 97% were white (Table 1). A large proportion had cardiovascular risk factors or diseases. One hundred and thirty-four participants (41%) were diagnosed with AF prior to death. Compared with people without AF, those with AF were more likely to have a history of stroke, diabetes, CHF, and coronary artery disease. Among those with AF, 43% (n = 57) met criteria for permanent AF. Compared with those who never had permanent AF, those with permanent AF were slightly more likely to have a history of stroke or hypertension, to have at least one APOE ε4 allele, and to have become demented. Many (74%) of those with permanent AF and a smaller proportion (21%) of those with nonpermanent AF used warfarin long term (five or more medication fills) after their AF diagnosis.
Table 2 shows the association between AF and neuropathologic changes. People with AF were more likely than those without AF to have gross infarcts (45% vs 31%, adjusted relative risk 1.82 [95% CI, 1.23–2.71]). Among 116 people with gross infarcts, 81 (70%) had a record of clinical stroke, transient ischemic attack, or silent infarct observed on cranial imaging done in the course of clinical care. Within subgroups, according to AF status, 45/58 (78%) of those with AF who had gross infarcts on autopsy had a record of clinical stroke, transient ischemic attack, or silent infarct on imaging, as did 36/58 (62%) of those without AF.
Table 2.
Association Between Atrial Fibrillation (AF) and Neuropathologic Findings
| Never AF (N = 194) | Ever AF (N = 134) | Adjusted and weighted relative risk (95% CI)* | |
|---|---|---|---|
| n (%) | n (%) | ||
| Neuritic plaques† | |||
| None or sparse | 107 (55.2) | 77 (57.5) | Referent |
| Intermediate or frequent | 87 (44.9) | 57 (42.5) | 1.06 (0.73, 1.56) |
| Neurofibrillary tangles | |||
| Braak stage 0–IV | 132 (68.0) | 104 (77.6) | Referent |
| Braak stage V–VI | 62 (32.0) | 30 (22.4) | 0.79 (0.48, 1.33) |
| Amyloid angiopathy | |||
| None | 137 (71.4) | 93 (69.9) | Referent |
| Any | 55 (28.7) | 40 (30.1) | 1.01 (0.63, 1.65) |
| Cerebral microinfarcts | |||
| 0–2 | 172 (89.1) | 110 (82.1) | Referent |
| 3 or more | 21 (10.9) | 24 (17.9) | 1.37 (0.61, 2.75) |
| Gross infarcts | |||
| 0 | 131 (69.3) | 72 (55.4) | Referent |
| ≥1 | 58 (30.7) | 58 (44.6) | 1.82 (1.23, 2.71) |
| Atherosclerotic disease | |||
| None or mild | 87 (46.5) | 50 (39.1) | Referent |
| Moderate or severe | 100 (53.5) | 78 (60.9) | 1.13 (0.86, 1.52) |
Notes: *Referent group is never AF. Weighting was used to account for selection into the autopsy cohort.
†Consortium to Establish a Registry for Alzheimer’s Disease scoring system.
No other neuropathologic findings were significantly associated with AF. Estimates showing the impact of adjustment and weighting are shown in Supplementary Table 1. Exploratory analyses showed that the greatest change in estimates between the main and sensitivity analyses came from adjusting for CHF, not APOE. Results were similar when we excluded people whose AF occurred only in the last 30 days of life or associated with CABG.
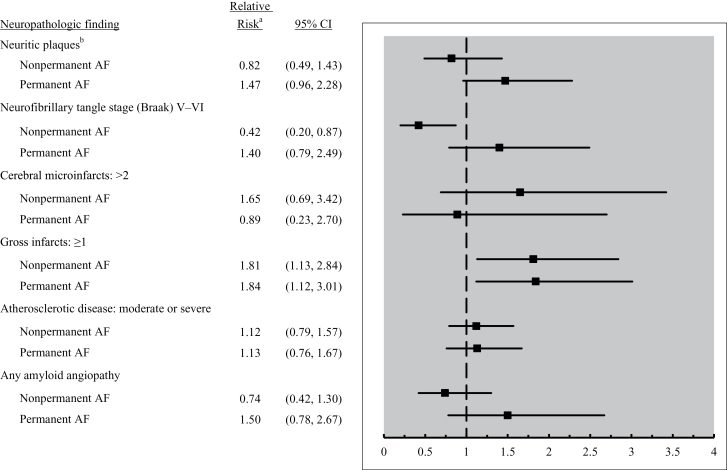
We examined neuropathologic findings according to AF persistence (Figure 2). People with permanent or nonpermanent AF were more likely to have gross infarcts on autopsy than people without AF, and the association was of similar magnitude regardless of AF persistence. Each of the three AD neuropathologic changes was 40%–50% more common in people with permanent AF than those without AF, but none of these associations were statistically significant. Supplementary Table 2 shows results from sensitivity analyses related to persistence of AF, with adjustment for APOE status and CHF. In these analyses, the relative risks for AD neuropathologic changes in relation to permanent AF were of greater magnitude than in the main analysis, and the association between permanent AF and Braak neurofibrillary tangle stage became statistically significant (relative risk 1.91, 95% CI 1.01–3.61).
Figure 2.
Neuropathologic findings in relation to atrial fibrillation (AF) persistence. aReferent is people without AF. Models include weighting and adjustment as detailed in Methods section. bConsortium to Establish a Registry for Alzheimer's Disease scoring system.
Supplementary Table 3 shows the co-occurrence of different brain diseases for subgroups of people stratified by AF and dementia status. Among people with both AF and dementia, 47% had pathologic evidence of two or more diseases, compared with 35% of those with dementia only, 17% of those with AF only, and 10% of those with neither condition. The most common combination was macroscopic infarct plus AD pathologic changes.
Discussion
In this population-based autopsy series, AF was associated with higher prevalence of gross infarcts, which in 30% of participants were not clinically recognized prior to death. Although AF overall was not associated with AD neuropathologic changes, people with permanent AF were 40%–50% more likely to have AD changes than people without AF, but these associations were not statistically significant.
Only one study has examined neuropathologic changes in people with AF. Rastas and coworkers (12) found that in people aged 85 and older, AF was associated with having multiple large ischemic lesions but not with amyloid beta load or Braak neurofibrillary tangle stage. They did not examine neuropathologic changes in relation to AF persistence nor did they account for selection bias. Compared with the prior study, ours includes many more AF cases who underwent autopsy (132 vs 55) and a younger population (mean age at baseline 73 vs 88 years). In addition, in their cohort as a whole, Rastas and coworkers did not observe an association between AF and dementia, whereas we did (2). This crucial difference makes their neuropathology results hard to interpret.
Our results regarding permanent AF and AD neuropathologic changes raise important questions. The magnitude of association (40%–50% higher prevalence of AD changes) would be clinically important, although it did not reach statistical significance in the primary analysis. A study including more people with permanent AF is needed to draw more definitive conclusions. An association between AF and AD neuropathologic changes is biologically plausible; some animal studies have found that hypoxia and ischemia increase beta-amyloid production and neuritic plaque formation (35,36), though not all studies agree (37). If ischemia were the predominant mechanism, then better anticoagulation could in theory decrease AD neuropathologic changes in people with AF. Alternatively, mechanisms such as neuroinflammation (38) or oxidative stress (39) could play an important role.
Strengths of this study include that participants were recruited from a well-defined, community-dwelling population. Statistical modeling was used to account for selection bias, a known limitation in autopsy studies, which is rarely addressed quantitatively. These features increase generalizability. Participants were followed over many years with repeated assessments using rigorous criteria, and medical records were reviewed to ascertain comorbid illnesses. The sample size is large relative to other community-based autopsy series. AF was defined from medical records, which are more accurate than self-report and likely more sensitive than a onetime EKG. The study also has limitations. We could only detect AF that came to clinical attention and thus we may have missed some transient or asymptomatic cases. If all AF subtypes are associated with neuropathologic changes, then this misclassification could bias results toward the null. Cranial imaging results in the ACT study came from imaging performed as part of clinical care, and thus results were not standardized or uniformly available. Despite our large sample size, we had relatively low power for analyses of permanent AF.
Our findings suggest that more research is needed. There is growing consensus that AF is associated with higher dementia risk (6). Because of the high and increasing prevalence of AF, this association raises concern for an impending epidemic of AF-related dementia. However, this association also provides opportunities. Greater awareness that dementia is a consequence of AF could result in more aggressive efforts to protect cognitive function in AF patients or perhaps to identify ways to prevent AF. Initiatives could include greater efforts to promote warfarin use, where appropriate, because warfarin has been proven to decrease stroke risk in AF but remains underutilized.
In conclusion, important questions remain about the neuropathologic outcomes of AF, including whether permanent AF may contribute to AD neuropathologic changes. Greater understanding of these processes could influence AF treatment and ultimately help prevent or delay many future dementia cases.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This study was supported by National Institute on Aging (U01AG006781, R01AG023801, and K23AG028954); the Barton Family Foundation; and the National Heart, Lung and Blood Institute (R01HL068986). Dr. Dublin’s Beeson (K23) Career Development Award was also supported by the American Federation for Aging Research and the Hartford and Starr Foundations and Atlantic Philanthropies.
Acknowledgement
This work does not necessarily reflect the views of the National Institute on Aging or National Institutes of Health.
References
- 1. Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27(4):1–27. 10.7326/0003-4819-153-3-201008030-00260 [PubMed] [Google Scholar]
- 2. Dublin S, Anderson ML, Haneuse SJ, et al. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc. 2011;59(8):1369–1375. 10.1111/j.1532-5415.2011.03508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bunch TJ, Weiss JP, Crandall BG, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7(4):433–437. 10.1016/j.hrthm.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 4. Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment. Neurol Res. 2006;28(6): 625–629. 10.1179/016164106X130461 [DOI] [PubMed] [Google Scholar]
- 5. Tilvis RS, Kähönen-Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–274 [DOI] [PubMed] [Google Scholar]
- 6. Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76(10):914–922. 10.1212/WNL.0b013e31820f2e38 [DOI] [PubMed] [Google Scholar]
- 7. Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60(7):1082–1088 [DOI] [PubMed] [Google Scholar]
- 8. Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64(2):168–176. 10.1002/ana.21413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ezekowitz MD, James KE, Nazarian SM, et al. Silent cerebral infarction in patients with nonrheumatic atrial fibrillation. The Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. Circulation. 1995;92(8):2178–2182 [DOI] [PubMed] [Google Scholar]
- 10. Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke. 1980;11(1):35–38 [DOI] [PubMed] [Google Scholar]
- 11. Petersen P, Kastrup J, Videbaek R, Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. J Cereb Blood Flow Metab. 1989;9(3):422–425 [DOI] [PubMed] [Google Scholar]
- 12. Rastas S, Verkkoniemi A, Polvikoski T, et al. Atrial fibrillation, stroke, and cognition: a longitudinal population-based study of people aged 85 and older. Stroke. 2007;38(5):1454–1460. 10.1161/STROKEAHA.106.477299 [DOI] [PubMed] [Google Scholar]
- 13. Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62(4):406–413. 10.1002/ana.21208 [DOI] [PubMed] [Google Scholar]
- 14. Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746 [DOI] [PubMed] [Google Scholar]
- 15. Thacker EL, McKnight B, Psaty BM, et al. Association of body mass index, diabetes, hypertension, and blood pressure levels with risk of permanent atrial fibrillation. J Gen Intern Med. 2012;28(2):247–253. 10.1007/s11606-012-2220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer’s disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249(suppl 3):14–22 [DOI] [PubMed] [Google Scholar]
- 17. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259 [DOI] [PubMed] [Google Scholar]
- 18. Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18(4 suppl):S91–S94 [DOI] [PubMed] [Google Scholar]
- 19. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486 [DOI] [PubMed] [Google Scholar]
- 20. Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28(7):1418–1422 [DOI] [PubMed] [Google Scholar]
- 21. No authors listed. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(4 suppl):S1–S2 [PubMed] [Google Scholar]
- 22. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. 10.1016/S1474-4422(09)70238-8 [DOI] [PubMed] [Google Scholar]
- 23. Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross- cultural epidemiological studies of dementia. Int Psychogeriatr. 1994; 6(1):45–58 [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, D: C: American Psychiatric Association; 1994 [Google Scholar]
- 25. Emi M, Wu LL, Robertson MA, et al. Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics. 1988;3(4):373–379 [DOI] [PubMed] [Google Scholar]
- 26. Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548 [PubMed] [Google Scholar]
- 27. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253 [PubMed] [Google Scholar]
- 28. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706 [DOI] [PubMed] [Google Scholar]
- 29. Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E. Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology. 2009;32(3): 229–239. 10.1159/000197389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsuang D, Simpson KL, Li G, et al. Evaluation of selection bias in an incident-based dementia autopsy case series. Alzheimer Dis Assoc Disord. 2005;19(2):67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Monographs on Statistics & Applied Probability. London, UK: CRC Press; 1993 [Google Scholar]
- 32. Sonnen JA, Larson EB, Gray SL, et al. Free radical damage to cerebral cortex in Alzheimer’s disease, microvascular brain injury, and smoking. Ann Neurol. 2009;65(2):226–229. 10.1002/ana.21508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonnen JA, Larson EB, Walker RL, et al. Nonsteroidal anti-inflammatory drugs are associated with increased neuritic plaques. Neurology. 2010;75(13):1203–1210. 10.1212/WNL.0b013e3181f52db1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2011 [Google Scholar]
- 35. Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-{beta}. Am J Pathol. 2010;177(1):300–310. 10.2353/ajpath.2010.090750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2009;30(7):1091–1098. 10.1016/j.neurobiolaging.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 37. Koike MA, Garcia FG, Kitazawa M, Green KN, Laferla FM. Long term changes in phospho-APP and tau aggregation in the 3xTg-AD mice following cerebral ischemia. Neurosci Lett. 2011;495(1):55–59. 10.1016/j.neulet.2011.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. 10.1100/2012/756357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giasson BI, Ischiropoulos H, Lee VM, Trojanowski JQ. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radic Biol Med. 2002;32(12):1264–1275. 10.1016/S0891-5849(02)00804-3 [DOI] [PubMed] [Google Scholar]