Abstract
A principal barrier to the development of effective vaccines is the availability of adjuvants and formulations that can elicit both effector and long-lived memory CD4 and CD8 T cells. Cellular immunity is the presumptive immune correlate of protection against intracellular pathogens: a group composed of bacteria, viruses and protozoans that is responsible for a staggering level of morbidity and mortality on a global scale. T-cell immunity is also correlated with clinical benefit in cancer, and the development of therapeutic strategies to harness the immune system to treat diverse malignancies is currently undergoing a renaissance. Cyclic dinucleotides (CDNs) are ubiquitous small molecule second messengers synthesized by bacteria that regulate diverse processes and are a relatively new class of adjuvants that have been shown to increase vaccine potency. CDNs activate innate immunity by directly binding the endoplasmic reticulum-resident receptor STING (stimulator of interferon genes), activating a signaling pathway that induces the expression of interferon-β (IFN-β) and also nuclear factor-κB (NF-κB) dependent inflammatory cytokines. The STING signaling pathway has emerged as a central Toll-like receptor (TLR) independent mediator of host innate defense in response to sensing cytosolic nucleic acids, either through direct binding of CDNs secreted by bacteria, or, as shown recently, through binding of a structurally distinct CDN produced by a host cell receptor in response to binding cytosolic double-stranded (ds)DNA. Although this relatively new class of adjuvants has to date only been evaluated in mice, newly available CDN-STING cocrystal structures will likely intensify efforts in this field towards further development and evaluation in human trials both in preventive vaccine and immunotherapy settings.
Keywords: adjuvant, cellular immunity, cyclic dinucleotides, cytosolic nucleic acid sensing, formulation, innate immunity, stimulator of interferon genes, STING, vaccine
Introduction
How the innate immune system is engaged by targeted ligands shapes the development of an adaptive response and is central to vaccine design [Coffman et al. 2010; Dubensky and Reed, 2010; Kastenmuller et al. 2011; Reed et al. 2009]. In particular, despite a broadly held belief that an immune correlate of effective preventive or therapeutic vaccination against intracellular pathogens and cancer is cellular immunity, there is a lack of available adjuvants and formulations that initiate antigen-specific effector and long-lived memory CD4 and CD8 T-cell responses. Potent adjuvants should in addition promote humoral immunity, as functional antibodies play an essential role either by directly neutralizing extracellular pathogens, or killing pathogen-infected or malignant cells through antibody-dependent cell-mediated cytotoxicity (ADCC) mechanisms. Intracellular pathogens and cancer continue to have a major impact on morbidity and mortality at a global scale due in part to the continuing need for adjuvants that promote effective cellular and humoral immunity.
Adjuvants are compounds that serve to enhance the magnitude, breadth, quality and longevity of specific immune responses to antigens, but have minimal toxic side effects. Optimal adjuvants can address the vaccinologist’s dilemma, which in general is defined by a spectrum of vaccines ranging from those based on live-attenuated pathogens that are immunogenic but with potential safety risks, to sub-unit vaccines that, while safe, have weak potency. A potent adjuvant is characterized by affording dosage sparing (number of vaccinations required to elicit an effective immune response), dose sparing (reduction in the amount of antigen required to achieve an effective immune response), and also immune response broadening [Reed et al. 2009]. There continues to be a strong need for molecularly defined adjuvants that can be coformulated with antigens derived from intracellular pathogens or malignancies to initiate and/or activate effective cellular and humoral immune responses that are correlated with protection against intracellular pathogens and reduction of tumor burden [Rappuoli et al. 2011].
Compartmentalization of pattern recognition receptors and adjuvant design
The design and development of modern adjuvants are guided by a fundamental understanding that conserved microbial structures known as pathogen-associated molecular patterns (PAMPs) are sensed by germ-line encoded host cell pattern recognition receptors (PRRs), triggering a downstream signaling cascade resulting in the induction of cytokines and chemokines, and initiation of a specific adaptive immune response [Iwasaki and Medzhitov, 2010]. How the innate immune system is engaged by PAMPs presented from an infectious agent shapes the development of an adaptive response appropriate to combat the invading pathogen from causing disease. One objective of adjuvant design is to select defined PAMPs or synthetic molecules which activate designated PRRs and initiate a desired response. Adjuvants such as monophosphoryl lipid A (MPL A) and CpG are microbial-derived PAMPs recognized by Toll-like receptors (TLRs), a class of PRRs that signal through MyD88 and Toll-interleukin-1-resistance (TIR) domain-containing adaptor-inducing interferon-β (TRIF) adaptor molecules and mediate induction of nuclear factor-κB (NF-κB) dependent proinflammatory cytokines [Kawai and Akira, 2010]. MPL A (TLR-4 agonist) and CpG (TLR-9 agonist) are the most clinically advanced adjuvants, and are components of vaccines that are approved or pending approval by the US Food and Drug Administration (FDA) [Ahmed et al. 2011; Einstein et al. 2009]. While TLRs present on the cell surface (e.g. TLR-4) and endosomes (e.g. TLR-9) sense extracellular and vacuolar pathogens, the productive growth cycle of multiple pathogens including viruses and intracellular bacteria occurs in the cytosol. The compartmentalization of extracellular, vacuolar and cytosolic PRRs has led to the hypothesis that the innate immune system can sense productively replicating pathogenic microbes by monitoring the cytosol [Vance et al. 2009]. This provides a rationale for the use of agonists that activate PRRs comprising the cytosolic surveillance pathway (CSP) and may be an effective strategy for the design of effective vaccines for eliciting cellular immunity, a presumptive immune correlate of protection against intracellular pathogens.
The CSP is integral to immunity against intracellular pathogens
Nucleic acids from bacterial, viral, protozoan and fungal pathogens are sensed by several distinct cytosolic signaling pathways. When activated, these individual pathways induce a characteristic cytokine profile, which in turn shapes the antigen-specific immune response. For example, the nucleotide binding oligomerization domain (NOD)-like receptor (NLR) family, such as ‘absent in melanoma 2′ (AIM2), senses cytosolic double-stranded (ds)DNA, triggering activation of the inflammasome and caspase-1 dependent production of interleukin-1β (IL-1β) [Strowig et al. 2012]. The signaling cascade resulting from activation of the inflammasome stimulates priming of Th17-biased CD4+ T-cell immunity, associated with protection against diverse pathogens, such as Streptococcus pneumoniae [Olliver et al. 2011].
Type I interferons (IFN-α, IFN-β) are the signature cytokines induced by two distinct TLR-independent cytosolic signaling pathways. In the first pathway, various forms of single-stranded (ss)RNA and dsRNA are sensed by RNA helicases, including retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5), and through the IFN-β promoter stimulator 1 (IPS-1) adaptor protein mediate phosphorylation of the interferon regulatory factor 3 (IRF-3) transcription factor, leading to induction of IFN-β [Ireton and Gale, 2011]. IPS-1-/- deficient mice have increased susceptibility to infection with RNA viruses. Sensors that signal through the IPS-1 pathway are directly targeted for inactivation by various viral proteins, demonstrating a requirement of this cytosolic host defense pathway to control productive virus infection. Synthetic dsRNA, such as polyinosinic:polycytidylic acid (poly(I:C)) and poly ICLC, an analog that is formulated with poly-l-lysine to resist RNase digestion, is an agonist for both TLR3 and MDA5 pathways, is a powerful inducer of IFN-β, and is currently being evaluated in several diverse clinical settings [Caskey et al. 2011].
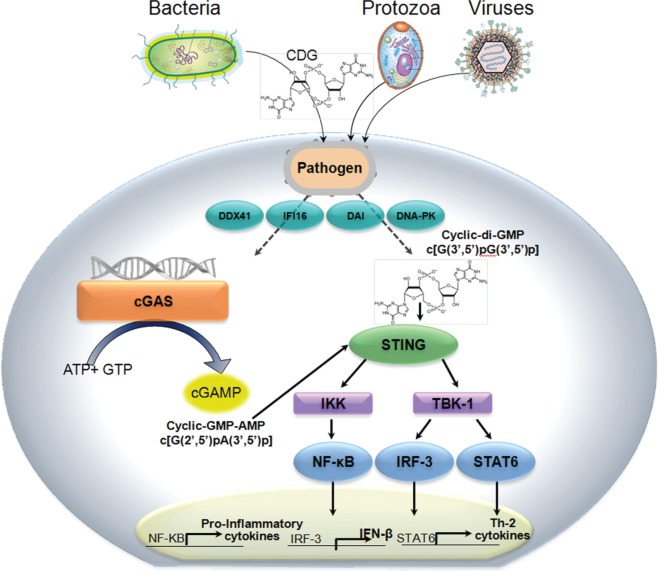
STING (stimulator of interferon genes) is the central mediator for the second cytosolic pathway that triggers type 1 interferon, in response to sensing cytosolic dsDNA from infectious pathogens or aberrant host cells (danger associated molecular patterns, DAMPS) [Barber, 2011]. Several recent landmark discoveries provide the rationale for the development of vaccine adjuvants which activate the STING signaling pathway. Alternatively known as TMEM173, MITA, ERIS and MPYS, STING was discovered by Glen Barber and colleagues using cDNA expression cloning methods as a MyD88-independent host cell defense factor expressed in macrophages, dendritic cells (DCs) and fibroblasts was found to induce expression of IFN-β and NF-κB dependent pro-inflammatory cytokines in response to sensing cytoplasmic DNA (Figure 1) [Ishikawa and Barber, 2008].
Figure 1.
STING (stimulator of interferon genes) is a central sensor of cytosolic nucleic acids. STING is a MyD88-independent host cell defense factor that senses cytosolic nucleic acids, and in response activates and TBK-1/IRF-3 signaling cascades, inducing the expression of pro-inflammatory cytokines and IFN-β. The activating ligands for STING are CDNs, second messengers that are produced by bacteria or by cellular cGAS in response to binding cytosolic dsDNA. CDNs produced by bacteria and cGAS are structurally distinct. Less well studied, activation of STING by CDNs has also been shown to induce the expression of STAT6-dependent Th2 cytokines.
AMP, adenosine monophosphate; CDN, cyclic dinucleotide; cGAS, cyclic GMP–AMP synthase; ds, double-stranded; GMP, guanosine monophosphate; IFN-β, interferon-β; IRF-3, interferon regulatory factor 3; NF-κB, nuclear factor κB; STING, stimulator of interferon genes; TBK-1, TANK-binding kinase 1.
STING-activating second messengers
Cyclic dinucleotides (CDNs) have long been studied as ubiquitous small molecule second messengers synthesized by bacteria which regulate diverse processes including motility and formation of biofilms [Romling et al. 2013]. Recently, Vance and colleagues discovered that CDNs are the principal ligand for STING [Burdette and Vance, 2011; McWhirter et al. 2009]. In response to binding CDNs, STING activates signaling through the TANK-binding kinase 1 (TBK-1)/IRF-3 axis and induces the expression of IFN-β [Burdette and Vance, 2013]. Portnoy and colleagues showed that cyclic di-adenosine monophosphate (c-di-AMP) is the critical signaling molecule secreted by multidrug resistance efflux pumps from the intracellular bacterium Listeria monocytogenes (Lm) into the cytosol of infected host antigen presenting cells, and is correlated with CD4 and CD8 T-cell mediated protection in the mouse listeriosis model [Crimmins et al. 2008; Woodward et al. 2010]. When cells are infected with Lm mutants that are confined to the vacuole, IFN-β is not induced and protective immunity is not induced [Bahjat et al. 2006; Crimmins et al. 2008]. Induction of IFN-β in Lm-infected macrophages is dependent upon activation of the STING signaling pathway, and the levels of type I IFN induced by c-di-AMP in macrophages from MyD88 -/- Trif -/- and C57BL/6 parental mice are indistinguishable [Leber et al. 2008; Witte et al. 2012]. In contrast, IFN-β is not induced by CDNs in macrophages derived from goldenticket (gt) mice encoding a nonfunctional mutant STING protein [Sauer et al. 2011]. Interestingly, the extracellular bacterium, Vibrio cholera, was shown by the John Mekalanos laboratory to produce a hybrid cyclic guanosine monophosphate (GMP-AMP (cGAMP) molecule, which also induces the STING pathway [Davies et al. 2012]. The activation of innate immunity with these ubiquitous second messengers suggests that sensing CDNs may be integral to host defense against bacterial infection.
Until recently, how STING sensed cytoplasmic DNA remained elusive. Unlike the AIM2 cytosolic sensor which directly binds dsDNA, STING lacks any obvious DNA-binding domains. The evidence that other candidate cytosolic DNA sensors such as DDX41, DNA-PK and DAI kinase were the primary mediators of dsDNA signaling through STING was inconclusive. This conundrum was solved with the discovery by Chen and colleagues of cyclic GMP-AMP synthase (cGAS), a host cell nucleotidyl transferase that directly binds dsDNA, and in response synthesizes a second messenger, cGAMP, which activates the STING pathway and induces IFN-β expression [Kranzusch et al. 2013; Sun et al. 2013; Wu et al. 2013]. Cells without a functional cGAS are unable to express IFN-β in response to stimulation with cytosolic DNA. Although the helicase DDX41was shown to sense both CDNs and cytosolic dsDNA, and activate IFN-β expression through the STING pathway [Parvatiyar et al. 2012], Chen and colleagues showed subsequently that induction of IFN-β in response to foreign DNA in cells expressing both cGAS and STING was not diminished by knockdown of helicase DDX41 expression [Sun et al. 2013]. Furthermore, the magnitude of induced IFN-β was orders of magnitude higher in cells transfected with cGAS as compared with DDX41, demonstrating that cGAS is the critical cytosolic DNA sensor.
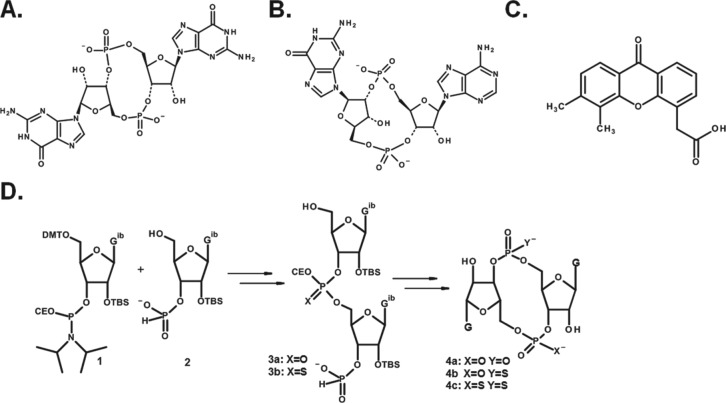
However, there was an additional twist of cGAS activity that remained to be discovered. Cells expressing a particular STING allele were nonresponsive to stimulation by CDNs, but responsive to stimulation with dsDNA in a cGAS-dependent and TLR9 (MyD88)-independent manner. This observation was incompatible with a mechanism defined by cGAS synthesizing STING-activating CDN ligands in response to sensing cytosolic dsDNA. This apparent paradox was resolved very recently by several independent investigators, including the laboratories of Patel, Vance, Hornung, Hopfner and Chen, who showed that cGAS produces a noncanonical CDN that activates STING alleles that are nonresponsive to canonical CDNs [Ablasser et al. 2013; Civril et al. 2013; Diner et al. 2013; Gao et al. 2013; Kranzusch et al. 2013; Zhang et al. 2013]. Unlike the CDN second messengers produced by bacteria, in which the two purine nucleosides are joined by a phosphate bridge with bis-(3′,5′) linkages, the internucleotide phosphate bridge in the cGAMP synthesized by cGAS is joined by noncanonical 2′,5′ and 3′,5′ linkages, represented as c[G(2′,5′)pA(3′,5′)p] (Figure 2).
Figure 2.
STING activating ligands and CDN synthesis: (A) ‘canonical’ cyclic-di-GMP (cyclic[G(3′,5′)pG(3′,5′)p]) produced by bacteria; (B) ‘noncanonical’ cyclic-GMP-AMP cyclic[G(2′,5′)pA(3′,5′)p] produced by cellular cGAS; (C) DMXAA, a small molecule structurally unrelated to CDNs; and (D) schematic representation for synthesis of dioxo- and dithio-CDNs using phosphoramidite and H-phosphonate methods for the dimerization and cyclization steps, respectively. Full sequence requires 10 steps from commercially available starting material.
AMP, adenosine monophosphate; CDN, cyclic dinucleotide; DMXAA, 5,6-dimethylxanthenone-4-acetic acid; cGAS, GMP–AMP synthase; GMP, guanosine monophosphate.
Human STING has known polymorphisms, including alleles encoding histidine at position 232, which are refractory to canonical CDNs, but not noncanonical CDNs [Diner et al. 2013; Jin et al. 2011]. Interestingly, 5,6-dimethylxanthenone-4-acetic acid (DMXAA) (Figure 2), a small molecule that is structurally unrelated to CDNs, stimulates STING signaling in mice, but binds without activating human STING [Conlon et al. 2013; Prantner et al. 2012; Roberts et al. 2007]. DMXAA has been shown to induce an innate immune-mediated antitumor response in mice [Fridlender et al. 2013], but ultimately failed when combined with chemotherapy in a large placebo-controlled phase III clinical efficacy trial in patients with nonsmall cell lung cancer [Lara et al. 2011]. It is tempting to speculate that the disappointing results of this clinical trial may have been due to the inability of DMXAA to activate the STING signaling cascade in humans, but how STING signaling participates in shaping the adaptive immune response in humans in response to infection with bacteria, which produce CDNs with canonical linkages, is a fundamental question that remains to be answered.
Immunologic potency of CDNs in mice
The immunostimulatory and vaccine adjuvant properties of CDNs were first published in 2007 [Karaolis et al. 2007a], long before STING was shown to be a direct sensor of c-di-GMP [Burdette et al. 2011]. The first report to demonstrate the presence of CDNs in eukaryotic cells showed that plants express a c-di-GMP dependent cellulose synthase similar to the bacterium Acetobacter xylinum [Amor et al. 1991; Ross et al. 1990]. Several years later, the observation that c-di-GMP increased CD4 T-cell receptor expression in Jurkat cells provided early insight that CDNs affect gene expression in immune cells [Steinberger et al. 1999].
First demonstration of immunostimulatory properties and use as vaccine adjuvant
Karaolis and colleagues were the first to demonstrate that c-di-GMP has immunostimulatory properties [Karaolis et al. 2007a]. Although this group initially explored the antimicrobial activity of CDNs, they later demonstrated that c-di-GMP significantly reduced colonization of Staphylococcus aureus [Karaolis et al. 2007a] in a mouse model of mastitis and provided protection against bacterial pneumonia [Karaolis et al. 2007b]. These investigations showed that c-di-GMP elicited a broad proinflammatory cytokine and chemokine profile, and also recruited and/or activated mouse and human immune effectors, including DCs. Intranasal pretreatment with c-di-GMP resulted in infiltration of activated innate [natural killer (NK) and natural killer T (NKT)] and adaptive (T- and B-cells) effectors into the lungs, and correlated with a significant reduction of lung colonization following intratracheal challenge with Klebsiella or Streptococcus pneumoniae species, and in the case of K. pneumoniae, extended survival [Karaolis et al. 2007b; Ogunniyi et al. 2008]. Although previous studies showed that TLR agonists, such as oligodeoxynucleotides (TLR9 agonist) or monophospohryl lipid A (TLR4 agonist), afforded short-term protection against pathogen challenge, these results nevertheless provided the first scientific rationale that targeting the STING pathway though the use of CDNs can be used to harness innate immunity to prevent or treat disease. In addition, the landmark 2007 paper showed that co-injection of c-di-GMP with S. aureus clumping factor A (ClfA) recombinant protein was required to elicit robust Th1-biased antigen-specific humoral immunity, and are the cornerstone results for CDN-adjuvanted vaccines. The Karaolis group showed in subsequent studies that c-di-GMP was more effective than alum-based adjuvants at inducing humoral immunity specific for co-injected protein antigens that correlated with protection against S. pneumoniae or S. aureus (MRSA) challenge [Hu et al. 2009].
Although the work of Karaolis and colleagues was groundbreaking, it also left unanswered several essential questions regarding the use of CDN-adjuvanted vaccines, including: (1) What is the capacity for promoting cellular immunity? (2) Does vaccination induce long-term memory? (3) Does coformulation of antigen with adjuvant (instead of co-injection) affect the quality of the CDN-induced response or the optimal adjuvant dose level? (4) Can the potency of CDNs be enhanced with targeted modifications? These questions have begun to be addressed by other groups and continue to be relevant issues for the eventual advancement of CDN-based adjuvants to human trials.
First demonstration of vaccine-induced cellular immunity and induction of mucosal immunity
The most extensive body of work to characterize the immunological profile of CDN-adjuvanted vaccines has been conducted by Guzman and colleagues [Ebensen et al. 2007, 2011; Libanova et al. 2010; Madhun et al. 2011; Pedersen et al. 2011]. The results from these studies provide a significant basis for the rationale for the continued development and advancement of this class of adjuvants to human trials. The Guzman group showed for the first time that CDNs promote priming of both cellular and humoral immunity [Ebensen et al. 2007]. Following three intranasal co-administration of c-di-GMP and antigen, significant ovalbumin (OVA) specific cytotoxic T lymphocytes (CTL) or β-galactosidase (β-gal)-specific CD4 T-cell responses could be detected in mice, also demonstrating that CDNs are an effective mucosal adjuvant. Intranasal co-administration of c-di-GMP and β-gal protein induced significant immunoglobulin A (IgA) responses specific for the model vaccine antigen in both local (lungs) and distal (vagina) tissues, and also robust systemic immunoglobulin G (IgG) antibody titers, with an isotype profile that was indicative of a Th1-biased response.
Although evidence supported both c-di-GMP and c-di-AMP as ubiquitous second messengers in diverse bacterial species, c-di-GMP was the only CDN molecule evaluated in prior vaccine studies. Based in part on the observation by Portnoy that the secretion of c-di-AMP by the intracellular bacterium L. monocytogenes triggered a host cytosolic innate immune response that was characterized by the induced expression of IFN-β, the Guzman group compared the adjuvant properties of c-di-AMP, cyclic-di-inosine monophosphate (c-di-IMP) and c-di-GMP [Libanova et al. 2010]. The results from these studies provided evidence that the immunostimulatory properties of individual CDN molecules may differ. However, while c-di-AMP was more potent than c-di-GMP in stimulating the activation of mouse macrophages and DCs, these differences measured in vitro did not translate to significant differences between these CDNs in promoting mucosal and systemic cellular and humoral immunity in vaccinated mice. Nevertheless, these investigations showed that intranasal co-administration of any of these three CDNs with soluble protein antigen elicited a balanced antigen-specific response, characterized by local and distal mucosal IgA immunity, systemic humoral immunity, and a balanced antigen-specific CD4 helper T-cell response. When restimulated with vaccine antigen, spleen cells from immunized mice expressed a diverse profile including IFN-γ and IL-2 (Th1), IL-4 (Th2) and IL-17 (Th17) cytokines, which was dependent upon co-administration of c-di-GMP, c-di-AMP or c-di-IMP.
An effective mucosal adjuvant would have obvious application to subunit influenza virus vaccines. A first proof-of-concept study with CDN-based adjuvants showed that mice vaccinated intranasal with plant-derived influenza A virus H5N1 (A/Anhui/1/05) antigen in combination with c-di-GMP generated significant cellular and humoral immunity in both mucosal and systemic compartments [Madhun et al. 2011]. Interestingly, the hemagglutination inhibition (HI) antibody titers that were observed in mice following two intranasal vaccinations with a dose that ranged between 1.5 and 15 µg of H5 protein were at least 10-fold higher than the HI titer ≥40 considered to be protective in humans. Since significant HI titers were not detected in the absence of adjuvant, these results suggested that the minimal antigen dose in combination with c-di-GMP required to induce significant HI titers was not reached. Disappointingly, while high HI titers against the homologous vaccine antigen were generated, significant cross-reactive serum HI titers against drifted H5 viruses were not observed. In contrast, significant cross-reactivity of IgA was observed against drifted H5N1 antigenic variant viruses, but not against an H3N2 heterosubtypic virus.
Intranasal vaccination in combination with c-di-GMP also induced a significantly higher frequency of poly-functional [IFN-γ/IL-2/ tumour necrosis factor-α (TNF-α)] Th1 CD4 T-cells in response to restimulation with either homologous vaccine hemagglutinin (HA) or with HA from drifted H5N1 virus strains compared with mice immunized by the intramuscular route (IM) In addition, splenocytes from intranasal vaccinated mice expressed significantly higher levels of Th1 (IFN-γ, IL-2), Th2 (IL-10) and Th17 (IL-17) cytokines, compared with intramuscular (IM) vaccinated mice, which expressed significantly lower levels of Th1 and Th17 cytokines but higher levels of Th2 cytokines (IL-4, IL-5) in response to antigen restimulation. CDN-dependent stimulation of a Th17 response is of interest, as this cytokine may play a significant role in shaping the immune response to mucosal pathogens. A second study utilized H5N1 virosomes containing both HA and neuraminidase (NA) proteins in a synthetic lipid membrane as a source of antigen [Pedersen et al. 2011]. Virosomes present antigens to the immune system in a conformation that more closely parallels the native state in infectious virus and are inherently more immunogenic than the soluble recombinant proteins. When combined with c-di-GMP, immunization with H5N1 virosomes induced significant systemic HI titers following IM vaccination, and also induced significant Th1 CD4 T-cell immunity specific for both the vaccine H5N1 antigen and an H1N1 heterosubtypic virus strain, demonstrating that CDNs indeed have the capacity to broaden the immune response under optimal formulation conditions. Overall, it is difficult to compare the potency of the CDN-adjuvanted flu vaccine candidates from these first investigations with other adjuvanted subunit vaccines being developed, since virus challenge experiments to measure protective immunity were not performed.
A recent report using a synthetic TLR4 agonist known as GLA, when formulated with oil-in-water emulsion together with recombinant H5N1 protein and administered by the IM route, elicited significant HI sera titers against both the vaccine A/Vietnam/1203/04 (rH5VN) antigen and drifted H5N1 viruses at sub-microgram protein antigen doses [Clegg et al. 2012]. Although mucosal IgA responses were not evaluated in this study, the GLA-dependent immune response broadening correlated with protection against heterosubtypic virus challenge in mice and in ferrets. The HI titers generated by intranasal vaccination with c-di-GMP adjuvanted H5N1 virosomes were at least comparable with those protective responses induced by GLA-adjuvanted H5N1 protein formulated in oil-in-water emulsion. While this seems indeed promising for potency of the c-di-GMP-based vaccine, a successful outcome of virus challenge studies are needed for this approach to be validated and to warrant continued development towards evaluation in humans.
First demonstration of immunostimulatory properties of CDN synthetic derivatives
The laboratory of Yan, Chen and colleagues are the third group to make significant contributions that support the scientific rationale for continued development of CDN-adjuvanted vaccines [Chen et al. 2010a,b; Yan and Aguilar, 2007; Yan et al. 2008, 2009]. This group developed methods for CDN synthesis (discussed below), and expanded the previous work of the Karaolis and Guzman groups by showing that c-di-GMP was an effective mucosal adjuvant that conferred some protection against challenge with S. pneumoniae [Yan et al. 2009]. Most significantly, this was the first group to evaluate the immunostimulatory properties of phosphorothioate analogs of c-di-GMP [Yan et al. 2008]. The internucleotide phosphate bridge is susceptible to digestion by phosphodiesterases and substitution of the nonbridging oxygen with sulfur may increase the intracellular half-life of CDNs, and in turn increase the efficiency of STING signaling and activation of innate immunity. When administered intranasally, c-di-GMP-S1 or c-di-GMP-S2 derivative molecules with a sulfur substitution for one or both unbound hydroxyl groups in the CDN internucleotide phosphate bridge, respectively, stimulated the recruitment of neutrophils at equivalent levels to the lungs, although at levels which were about three-fold lower than with unmodified c-di-GMP. In addition, the dose–response and profile of a wide panel of proinflammatory cytokines measured in bronchoalveolar lavage (BAL) was indistinguishable between phosphorothioate and unmodified c-di-GMP molecules. Unfortunately, immunogenicity studies were not conducted, making it difficult to determine whether phosphorothioate-substituted CDN based adjuvants confer any potency or adjuvant dose sparing advantages compared with unmodified c-di-GMP.
CDN synthesis and design of compounds with increased potency
The availability of CDN-STING crystal structures along with recent results describing human STING allele-CDN dependent signaling relationships will facilitate structure-based studies to develop effective CDN compounds that activate innate immunity across genetically diverse human populations [Chin et al. 2013; Huang et al. 2012; Ouyang et al. 2012; Shang et al. 2012; Shu et al. 2012; Su et al. 2012]. Hydrogen bonding between CDN oxygen and phosphate atoms with amino acids at the interface of the STING homo-dimer binding cleft promotes its conformational transition and activate downstream signaling [Burdette and Vance, 2013]. The recent report from the Vance group showing that particular human STING alleles were refractory to CDNs with canonical 3′–5′ linkages suggests that noncanonical CDNs may be the preferred structure of compounds for advancement to human trials [Diner et al. 2013]. The design and screening of novel compounds will be facilitated by well-established processes for synthesizing CDNs. As shown in Figure 2, a wide diversity of ligands can bind to STING and activate signaling, including c-di-AMP, c-di-GMP, c-di-IMP and c-GAMP as well as DMXAA, a planar aromatic small molecule.
The synthesis of CDNs is achieved by the coupling of suitably protected and activated purine nucleosides to form a linear dimer followed by a ring closure reaction. A range of chemistries have been employed for the coupling and ring closure reactions including phosphate triester, H-phosphonate and phosphoramidite [Gaffney et al. 2010; Grajkowski et al. 2010; Hayakawa, 2003; Hyodo et al. 2006; Ross et al. 1990; Yan and Aguilar, 2007]. Figure 2 schematically depicts the conversion of a 3′-O-phosphoramidite (1) to linear dimer (3) followed by ring closure via the H-phosphonate to give cyclic dinucleotide (4). The phosphorous III intermediates generated at these two steps can either be oxidized or sulfurized (Gaffney et al. 2010). Both mono- and di-thio modified c-di-GMPs retain immunostimulatory capacity in vivo that is comparable with unmodified c-di-GMP [Yan et al. 2008]. After deprotection and purification, the product CDN is characterized by both one- and two-dimensional NMR spectroscopy in conjunction with liquid chromatography-mass spectrometry (LC/MS) and high resolution mass spectroscopy [Gao et al. 2013]. 1H–31P heteronuclear multiple bond correlation (HMBC) experiments can be especially useful in establishing the regiochemistry of the phosphodiester bond in noncanonical CDNs [Diner et al. 2013].
Next steps: prospects for advancement of CDN adjuvants to evaluation in humans
The rapidly advancing mechanistic understanding of the central role STING plays in host defense against cytosolic pathogens will likely intensify efforts to develop novel vaccine adjuvants which target this signaling pathway. Several hurdles, including selection and formulation of a molecule that can signal in a diverse human population, remain to be overcome prior to evaluation of CDN-based adjuvants in humans. While noncanonical linkages enable cGAMP to mediate signaling through particular STING alleles that are nonresponsive to canonical cGAMP, whether other CDNs such as c-di-GMP having a comparatively higher STING binding avidity also require 2′,5′-3′,5′internucleotide phosphate bridges to activate nonresponsive human alleles remains to be determined. The combined results from published studies evaluating the relative potency of c-di-AMP and c-di-GMP adjuvanted vaccines have been in general inconclusive. With the exception of the H5N1 virosomes, previous work evaluating the impact of CDNs on vaccine immunogenicity in mice relies on admixing adjuvant and soluble protein antigen prior to injection. How defined adjuvants are formulated with antigen and administered affects the profile of the induced immune response and is central to vaccine design [Baldwin et al. 2012; Moon et al. 2011; Reed et al. 2009; Wille-Reece et al. 2005]. Codelivery of antigen and immunostimulatory factors to antigen-presenting cells is a necessary step to elicit functional antigen-specific Th1 CD4 and CD8 T cells and protective immunity.
Formulations that provide a sustained localized release of adjuvant and antigen sufficient to prime or boost effective immune responses without systemic exposure and induction of pro-inflammatory cytokines are an essential safety requirement for vaccine development. As the systemic cytokine profile was not evaluated in previous studies with CDN-based vaccines, the adjuvant dose-dependent safety profile determined in acceptable animal toxicology models remains a major unanswered question for this class of adjuvants that needs to be addressed prior to clinical evaluation. Since STING is a cytosolic PRR, it is likely that formulations which facilitate trafficking of vaccine compositions to this cellular compartment may increase the overall potency of CDN-based adjuvants.
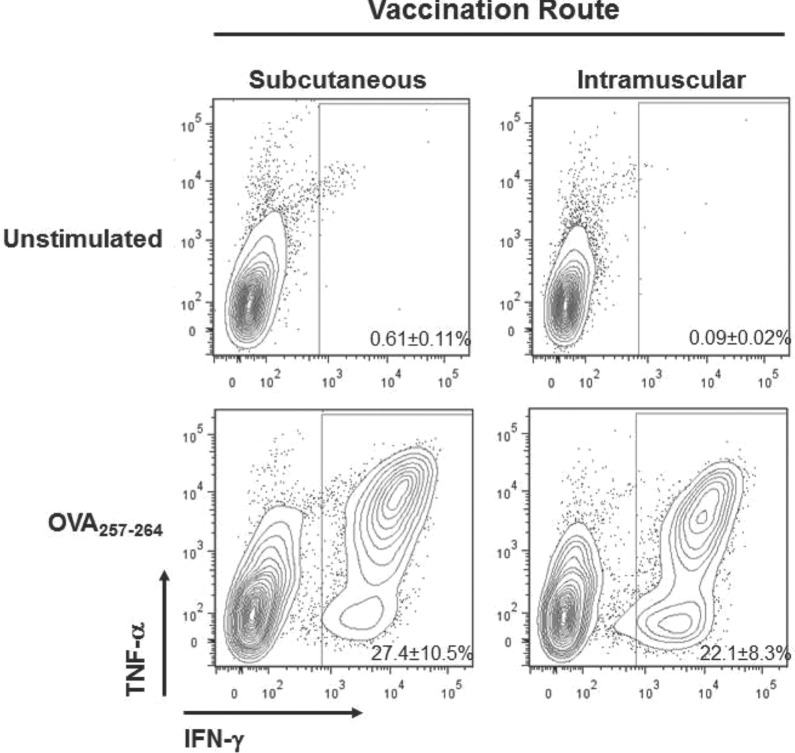
With the urgent need for vaccines that elicit protection against intracellular pathogens, we have focused our efforts on developing CDN compounds and formulations that promote priming of antigen-specific cellular immunity. When formulated together with antigen using Addavax (InvivoGen, San Diego, CA) a 2% squalene-in-water emulsion similar to MF-59, a component of Fluad© inactivated influenza virus vaccines marketed by Novartis [Seubert et al. 2011], we observed that c-di-GMP promoted priming of robust OVA-specific CD4+ and CD8+ T-cell immunity at levels that were substantially higher than those reported previously (Figure 3) [Ebensen et al. 2011]. We are exploring a variety of formulations and CDN compounds that have been modified to facilitate either coformulation/encapsulation efficiency with antigen or cellular uptake. Such an approach has been shown to increase the safety profile by limiting the systemic exposure of other adjuvants, including imidazoquinolines, a class of TLR7/8 agonists [Smirnov et al. 2011].
Figure 3.
CDNs promote priming of robust antigen-specific CD8+ T-cell immunity. C57BL/6 mice (n = 3) were vaccinated twice at a 3-week interval with 50 µg cyclic di-GMP coformulated with 10 µg OVA protein and 2% squalene-in water emulsion (Addavax). Mice were injected by subcutaneous (base of the tail) or intramuscular routes. OVA257-264-specific CD8+ T-cell responses were measured in the spleen at the peak of the secondary response at 6 days post boost by intracellular cytokine staining for IFN-γ and TNF-α cytokines. Shown are representative FACS plots, each containing the mean and standard deviation of responses for the group of mice.
CDN, cyclic dinucleotide; FACS, GMP, guanosine monophosphate; IFN-γ, interferon-γ; OVA, ovalbumin; TNF-α, tumour necrosis factor-α.
Conclusion
While the immunostimulatory properties of CDNs have been known since 2007, this class of adjuvants has not received extensive attention. However, with the multiple recent high visibility publications describing STING as a central player in a pathway to sense cytosolic nucleic acids, either indirectly from CDNs produced by cGAS in response to binding cytosolic DNA or directly from CDNs secreted by bacterial pathogens, this will more than likely change. Several steps in development will be required before the first vaccine candidates using CDN-based adjuvants are acceptable for evaluation in humans. These steps include selecting CDN molecules that can activate signaling across the diversity of human STING alleles, development of formulations that facilitate trafficking of vaccines to the cytosol, and conducting toxicology studies in animal models to define the CDN immunogenicity–toxicity therapeutic index to support eventual adjuvant dose levels for testing in humans. Since numerous intracellular pathogens still await the development of effective vaccines, there is both a strong scientific rationale and unmet medical need to develop CDN-based adjuvants for advancement to humans.
Acknowledgments
We thank Gina Forte for the preparation of figure graphics and for critical reading of this manuscript, along with Steven Bodovitz and Laura Hix Glickman.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: T.W.D., D.B.K. and M.L.L. are employees of Aduro BioTech, Inc., which owns intellectual property covering the compositions and methods of cyclic dinucleotide adjuvants. In addition, Aduro BioTech employees hold stock options in the company.
Contributor Information
Thomas W. Dubensky, Jr, Aduro BioTech, Inc., 626 Bancroft Way, 3C, Berkeley, CA, 94710, USA.
David B. Kanne, Aduro BioTech, Inc., Berkeley, CA, USA
Meredith L. Leong, Aduro BioTech, Inc., Berkeley, CA, USA
References
- Ablasser A., Goldeck M., Cavlar T., Deimling T., Witte G., Rohl I., et al. (2013) cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498: 380–384 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Plotkin S., Black S., Coffman R. (2011) Assessing the safety of adjuvanted vaccines. Sci Transl Med 3: 93rv92. [DOI] [PubMed] [Google Scholar]
- Amor Y., Mayer R., Benziman M., Delmer D. (1991) Evidence for a cyclic diguanylic acid-dependent cellulose synthase in plants. Plant Cell 3: 989–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahjat K., Liu W., Lemmens E., Schoenberger S., Portnoy D., Dubensky T., Jr, et al. (2006) Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect Immun 74: 6387–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin S., Bertholet S., Reese V., Ching L., Reed S., Coler R. (2012) The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol 188: 2189–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber G. (2011) Cytoplasmic DNA innate immune pathways. Immunol Rev 243: 99–108 [DOI] [PubMed] [Google Scholar]
- Burdette D., Monroe K., Sotelo-Troha K., Iwig J., Eckert B., Hyodo M., et al. (2011) STING is a direct innate immune sensor of cyclic di-GMP. Nature 478: 515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette D., Vance R. (2013) STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol 14: 19–26 [DOI] [PubMed] [Google Scholar]
- Caskey M., Lefebvre F., Filali-Mouhim A., Cameron M., Goulet J., Haddad E., et al. (2011) Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 208: 2357–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Kuolee R., Yan H. (2010a) The potential of 3′,5′-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine 28: 3080–3085 [DOI] [PubMed] [Google Scholar]
- Chen W., Patel G., Yan H., Zhang J. (2010b) Recent advances in the development of novel mucosal adjuvants and antigen delivery systems. Hum Vaccin 6: 706–714 [DOI] [PubMed] [Google Scholar]
- Chin K., Tu Z., Su Y., Yu Y., Chen H., Lo Y., et al. (2013) Novel c-di-GMP recognition modes of the mouse innate immune adaptor protein STING. Acta Crystallogr D Biol Crystallogr 69: 352–366 [DOI] [PubMed] [Google Scholar]
- Civril F., Deimling T., de Oliveira Mann C., Ablasser A., Moldt M., Witte G., et al. (2013) Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498: .332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C., Roque R., Van Hoeven N., Perrone L., Baldwin S., Rininger J., et al. (2012) Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci U S A 109: 17585–17590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R., Sher A., Seder R. (2010) Vaccine adjuvants: putting innate immunity to work. Immunity 33: 492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J., Burdette D., Sharma S., Bhat N., Thompson M., Jiang Z., et al. (2013) Mouse, but not Human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol 190: 5216–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins G., Herskovits A., Rehder K., Sivick K., Lauer P., Dubensky T., Jr, et al. (2008) Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proc Natl Acad Sci U S A 105: 10191–10196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Bogard R., Young T., Mekalanos J. (2012) Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149: 358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner E., Burdette D., Wilson S., Monroe K., Kellenberger C., Hyodo M., et al. (2013) The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3: 1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T., Jr, Reed S. (2010) Adjuvants for cancer vaccines. Semin Immunol 22: 155–161 [DOI] [PubMed] [Google Scholar]
- Ebensen T., Libanova R., Schulze K., Yevsa T., Morr M., Guzman C. (2011) Bis-(3′,5′)-cyclic dimeric adenosine monophosphate: strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine 29: 5210–5220 [DOI] [PubMed] [Google Scholar]
- Ebensen T., Schulze K., Riese P., Link C., Morr M., Guzman C. (2007) The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 25: 1464–1469 [DOI] [PubMed] [Google Scholar]
- Einstein M., Baron M., Levin M., Chatterjee A., Edwards R., Zepp F., et al. (2009) Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin 5: 705–719 [DOI] [PubMed] [Google Scholar]
- Fridlender Z., Jassar A., Mishalian I., Wang L., Kapoor V., Cheng G., et al. (2013) Using macrophage activation to augment immunotherapy of established tumours. Br J Cancer 108: 1288–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney B., Veliath E., Zhao J., Jones R. (2010) One-flask syntheses of c-di-GMP and the [Rp,Rp] and [Rp,Sp] thiophosphate analogues. Org Lett 12: 3269–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Ascano M., Wu Y., Barchet W., Gaffney B., Zillinger T., et al. (2013) Cyclic [G(20,50)pA(30,50)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajkowski A., Cieslak J., Gapeev A., Schindler C., Beaucage S. (2010) Convenient synthesis of a propargylated cyclic (3’-5’) diguanylic acid and its ‘click’ conjugation to a biotinylated azide. Bioconjug Chem 21: 2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y. (2003) A facile synthesis of cyclic bis(3′→5′)diguanylic acid. Tetrahedron 59: 6465–6471 [Google Scholar]
- Hu D., Narita K., Hyodo M., Hayakawa Y., Nakane A., Karaolis D. (2009) c-di-GMP as a vaccine adjuvant enhances protection against systemic methicillin-resistant Staphylococcus aureus (MRSA) infection. Vaccine 27: 4867–4873 [DOI] [PubMed] [Google Scholar]
- Huang Y., Liu X., Du X., Jiang Z., Su X. (2012) The structural basis for the sensing and binding of cyclic di-GMP by STING. Nat Struct Mol Biol 19: 728–730 [DOI] [PubMed] [Google Scholar]
- Hyodo M., Sato Y., Hayakawa Y. (2006) Synthesis of cyclic bis(3′-5′)diguanylic acid (c-di-GMP) analogs. Tetrahedron 62: 3089–3094 [Google Scholar]
- Ireton R., Gale M., Jr (2011) RIG-I like receptors in antiviral immunity and therapeutic applications. Viruses 3: 906–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Barber G. (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455: 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. (2010) Regulation of adaptive immunity by the innate immune system. Science 327: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Xu L., Yang I., Davidson E., Schwartz D., Wurfel M., et al. (2011) Identification and characterization of a loss-of-function human MPYS variant. Genes Immun 12: 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaolis D., Means T., Yang D., Takahashi M., Yoshimura T., Muraille E., et al. (2007a) Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol 178: 2171–2181 [DOI] [PubMed] [Google Scholar]
- Karaolis D., Newstead M., Zeng X., Hyodo M., Hayakawa Y., Bhan U., et al. (2007b) Cyclic di-GMP stimulates protective innate immunity in bacterial pneumonia. Infect Immun 75: 4942–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller K., Wille-Reece U., Lindsay R., Trager L., Darrah P., Flynn B., et al. (2011) Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest 121: 1782–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384 [DOI] [PubMed] [Google Scholar]
- Kranzusch P., Lee A., Berger J., Doudna J. (2013) Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep 3: 1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara P., Jr, Douillard J., Nakagawa K., von Pawel J., McKeage M., Albert I., et al. (2011) Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol 29: 2965–2971 [DOI] [PubMed] [Google Scholar]
- Leber J., Crimmins G., Raghavan S., Meyer-Morse N., Cox J., Portnoy D. (2008) Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog 4: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libanova R., Ebensen T., Schulze K., Bruhn D., Norder M., Yevsa T., et al. (2010) The member of the cyclic di-nucleotide family bis-(3′, 5′)-cyclic dimeric inosine monophosphate exerts potent activity as mucosal adjuvant. Vaccine 28: 2249–2258 [DOI] [PubMed] [Google Scholar]
- Madhun A., Haaheim L., Nostbakken J., Ebensen T., Chichester J., Yusibov V., et al. (2011) Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 29: 4973–4982 [DOI] [PubMed] [Google Scholar]
- McWhirter S., Barbalat R., Monroe K., Fontana M., Hyodo M., Joncker N., et al. (2009) A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med 206: 1899–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J., Suh H., Bershteyn A., Stephan M., Liu H., Huang B., et al. (2011) Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater 10: 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunniyi A., Paton J., Kirby A., McCullers J., Cook J., Hyodo M., et al. (2008) c-di-GMP is an effective immunomodulator and vaccine adjuvant against pneumococcal infection. Vaccine 26: 4676–4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliver M., Hiew J., Mellroth P., Henriques-Normark B., Bergman P. (2011) Human monocytes promote Th1 and Th17 responses to Streptococcus pneumoniae. Infect Immun 79: 4210–4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S., Song X., Wang Y., Ru H., Shaw N., Jiang Y., et al. (2012) Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity 36: 1073–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatiyar K., Zhang Z., Teles R., Ouyang S., Jiang Y., Iyer S., et al. (2012) The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 13: 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G., Ebensen T., Gjeraker I., Svindland S., Bredholt G., Guzman C., et al. (2011) Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PLoS One 6: e26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prantner D., Perkins D., Lai W., Williams M., Sharma S., Fitzgerald K., et al. (2012) 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J Biol Chem 287: 39776–39788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R., Mandl C., Black S., De Gregorio E. (2011) Vaccines for the twenty-first century society. Nat Rev Immunol 11: 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S., Bertholet S., Coler R., Friede M. (2009) New horizons in adjuvants for vaccine development. Trends Immunol 30: 23–32 [DOI] [PubMed] [Google Scholar]
- Roberts Z., Goutagny N., Perera P., Kato H., Kumar H., Kawai T., et al. (2007) The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med 204: 1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U., Galperin M., Gomelsky M. (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77: 1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P., Mayer R., Weinhouse H., Amikam D., Huggirat Y., Benziman M., et al. (1990) The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J Biol Chem 265: 18933–18943 [PubMed] [Google Scholar]
- Sauer J., Sotelo-Troha K., von Moltke J., Monroe K., Rae C., Brubaker S., et al. (2011) The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of STING in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun 79: 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert A., Calabro S., Santini L., Galli B., Genovese A., Valentini S., et al. (2011) Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A 108: 11169–11174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G., Zhu D., Li N., Zhang J., Zhu C., Lu D., et al. (2012) Crystal structures of STING protein reveal basis for recognition of cyclic di-GMP. Nat Struct Mol Biol 19: 725–727 [DOI] [PubMed] [Google Scholar]
- Shu C., Yi G., Watts T., Kao C., Li P. (2012) Structure of STING bound to cyclic di-GMP reveals the mechanism of cyclic dinucleotide recognition by the immune system. Nat Struct Mol Biol 19: 722–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov D., Schmidt J., Capecchi J., Wightman P. (2011) Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine 29: 5434–5442 [DOI] [PubMed] [Google Scholar]
- Steinberger O., Lapidot Z., Ben-Ishai Z., Amikam D. (1999) Elevated expression of the CD4 receptor and cell cycle arrest are induced in Jurkat cells by treatment with the novel cyclic dinucleotide 3P,5P-cyclic diguanylic acid. FEBS Lett 444: 125–129 [DOI] [PubMed] [Google Scholar]
- Strowig T., Henao-Mejia J., Elinav E., Flavell R. (2012) Inflammasomes in health and disease. Nature 481: 278–286 [DOI] [PubMed] [Google Scholar]
- Su Y., Tu Z., Yang C., Chin K., Chuah M., Liang Z., et al. (2012) Crystallization studies of the murine c-di-GMP sensor protein STING. Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 906–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z. (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance R., Isberg R., Portnoy D. (2009) Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6: 10–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Reece U., Flynn B., Lore K., Koup R., Kedl R., Mattapallil J., et al. (2005) HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A 102: 15190–15194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte C., Archer K., Rae C., Sauer J., Woodward J., Portnoy D. (2012) Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity. Adv Immunol 113: 135–156 [DOI] [PubMed] [Google Scholar]
- Woodward J., Iavarone A., Portnoy D. (2010) c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328: 1703–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X., Du F., Shi H., Chen C., et al. (2013) Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339: 826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Aguilar A. (2007) Synthesis of 3′,5′-cyclic diguanylic acid (cdiGMP) using 1-(4-chlorophenyl)-4-ethoxypiperidin-4-yl as a protecting group for 2′-hydroxy functions of ribonucleosides. Nucleosides Nucleotides Nucleic Acids 26: 189–204 [DOI] [PubMed] [Google Scholar]
- Yan H., KuoLee R., Tram K., Qiu H., Zhang J., Patel G., et al. (2009) 3′,5′-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochem Biophys Res Commun 387: 581–584 [DOI] [PubMed] [Google Scholar]
- Yan H., Wang X., KuoLee R., Chen W. (2008) Synthesis and immunostimulatory properties of the phosphorothioate analogues of cdiGMP. Bioorg Med Chem Lett 18: 5631–5634 [DOI] [PubMed] [Google Scholar]
- Zhang X., Shi H., Wu J., Zhang X., Sun L., Chen C., et al. (2013) Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 51: 226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]