Abstract
Background
Invasive meningococcal disease (IMD) has been reported to be endemic in children from Tijuana, Mexico and the risk of an outbreak was always a threat.
Objectives
To describe all clinical, epidemiological and microbiological features of a meningococcal outbreak that occurred in Tijuana, Mexico.
Methods
All cases with IMD were admitted at different emergency departments within the city and diagnosed by culture and agglutination tests. Further restriction fragment length polymorphism pulse field gel electrophoresis (RFLP–PFGE) and multi locus sequence typing (MLST) were performed. All clinical and epidemiological characteristics and interventions were evaluated, as well as risk factors associated with mortality.
Results
From 30 January 2013 to 30 March 2013 there were 19 cases of IMD all caused by Neisseria meningitidis serogroup C. The median age was 16 years (2–47), with higher frequency among individuals at least 13 years old (73.7%). At admission, meningitis was the main clinical presentation (94.7%), followed by purpura (78.9%), septic shock (42.1%) and disseminated intravascular coagulation (DIC, 36.8%). Overall mortality was seven (36.8%). Variables associated with higher mortality were, at admission, presence of septic shock, DIC and thrombocytopenia less than 70,000. All 19 cases had no identifiable site or cluster as the source of the outbreak. RFLP-PFGE showed a discriminatory power for only one profile on all N. meningitidis strains analyzed and a clone ST-11 was identified in all strains. Public health interventions were continuous case reporting of all suspected cases of IMD, an increase in active surveillance in all hospitals, training of medical and laboratory personnel, massive and rapid chemoprophylaxis to all close contacts as indicated, and promotion of good health habits.
Conclusions
An outbreak with high mortality of IMD occurred in Tijuana, Mexico. This event and evidence of endemicity should encourage health authorities to evaluate meningococcal vaccination in the region.
Keywords: meningococcal disease, outbreak, neisseria meningitidis, neisseria meningitidis serogroup C, meningococcal clonal complex ST-11
Introduction
Neisseria meningitidis is currently the leading cause of bacterial meningitis (BM) in children in the USA [Chang et al. 2012; Cohn et al. 2010] and many countries where pneumococcal conjugated vaccine was introduced; it is also a significant cause of sepsis with or without clinical purpura fulminans in many developing countries, such as the sub-Saharan African belt, among others. Invasive meningococcal disease (IMD) has not only been associated with endemicity but also with outbreaks with high lethality and morbidity, and a subsequent effect on society [Chang et al. 2012; Cohn et al. 2010; Baccarini et al. 2012; Brayer and Humiston, 2011; WHO, 2011].
IMD in Mexico is being reported as an infrequent health problem, with total national cases as low as two per year [Almeida-Gonzalez et al. 2004; Safadi and Cintra, 2010], however surveillance for IMD in Mexico is passive and underreporting is an issue of concern.
Tijuana, Mexico is a city that borders with San Diego, California and is considered the highest transited frontier in the world. The city has five government hospitals and several other private hospitals. Active surveillance for IMD it is being done only at the General Hospital of Tijuana (GHT), which is the only one in the city with both human and material resources for proper isolation and identification of N. meningitidis from sterile samples. Accordingly, we have previously published reports and presented at international meetings that IMD in children under 16 years old is present in similar numbers in Tijuana as in San Diego and is locally the leading cause of BM in children [Chacon-Cruz et al. 2011a, 2011b, 2012b, 2013]. Furthermore, these studies led to national active surveillance in 10 Mexican hospitals, which confirms that IMD is endemic in children from Tijuana but infrequent in other hospitals within the country [Chacon-Cruz et al. 2012a]. On the basis of having enough evidence of endemicity in children (especially in young infants), the fear of having an outbreak was present.
In this report we present the main clinical/epidemiological/microbiological features of a serogroup C (clonal complex ST-11) meningococcal outbreak.
Materials and methods
During the outbreak, all blood/cerebrospinal (CSF) samples were sent to the Microbiology Laboratory at GHT. Following N. meningitidis isolation, serogroup identification was performed at GHT by standard latex well agglutination methods using Pastorex Meningitis kit (Alere Ltd, Stockport, UK).
Once isolated, strains were also sent to the Microbiology Research Laboratory of Hospital Manuel Gea Gonzalez, Mexico City and Carlos III, Institute of Health, National Reference Laboratory for Meningococcus, Madrid, Spain. In the former location, serogroup identification was performed using the polymerase chain reaction multiplex test [Bennet and Cafferkey, 2006], as well as restriction fragment length polymorphism pulse field gel electrophoresis for all bacterial cultures using 20 U of Spe I for enzymatic restriction digestion, according to the method described by Bygavres and colleagues [Bygavres and Maiden, 1992]. In Spain, multilocus sequence typing for clonal identification was done according to the method described by Maiden and Feavers and colleagues [Maiden et al. 1998; Feavers et al. 1999]. Once all molecular information was obtained, the results were sent back to GHT for further analysis. Data analysis was mostly descriptive (Excel) and comparison of proportions was used when needed using the z-test (VassarStat).
Results
From 30 January 2013 to 30 March 2013, there were 19 confirmed cases of IMD in the city of Tijuana, all caused by N. meningitidis serogroup C. The median age was 16 years (2–47), with higher frequency among individuals at least 13 years old (73.7%). The median days of symptoms was 1 (1–4). Fever (100%), cephalalgia (94.7%) and altered state of consciousness (73.6%) were the leading symptoms. At admission, meningitis was the main clinical presentation (94.7%), followed by purpura (78.9%), septic shock (42.1%) and disseminated intravascular coagulation (DIC, 36.8%).
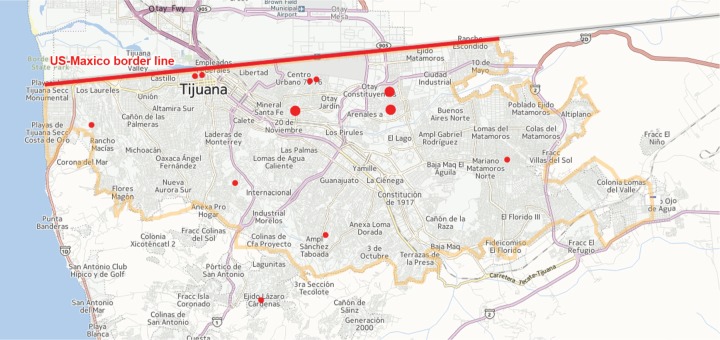
Overall mortality was seven (36.8%). Variables associated with higher mortality were, at admission, presence of septic shock (100% among deceased patients versus 5.2% among survivors, p < 0.002), presence of DIC (100% among deceased patients versus 0% among survivors, p < 0.001) and thrombocytopenia less than 70,000 (100% among deceased patients versus 8.3% among survivors, p < 0.002). From 12 survivors, 3 (25%) had sequelae at discharge (hypoacusia/vertigo, motor disorders and skin scars). Although 10 of 19 cases were admitted at GHT, all cases were confirmed at GTH. All 19 cases had no identifiable site or cluster as the source of the outbreak (see Figure 1), however in 7 patients (36.8%) the source was family related (Table 1). There were 11 cases (57.9%) during February followed by a decrease in the number of cases during March (Figure 2). The attack rate for the municipality of Tijuana was 1.070/100,000 population (95% confidence interval 1.055–1.085).
Figure 1.
Invasive meningococcal disease (red points) distribution in Tijuana, Mexico during the outbreak. Larger points indicate family-related cases (total cases = 19, family-related cases = 7).
Table 1.
Cases by order of presentation in time, associated contacts and outcome.
| Case number | Age | Gender | Associated contacts | Outcome |
|---|---|---|---|---|
| 1 | 12 | Female | Yes, case 2 | Survived |
| 2 | 10 | Female | Yes, case 1 | Survived |
| 3 | 13 | Male | No | Deceased |
| 4 | 10 | Female | No | Survived |
| 5 | 14 | Male | No | Deceased |
| 6 | 2 | Male | Yes, case 9 | Survived |
| 7 | 13 | Female | No | Survived |
| 8 | 27 | Female | No | Deceased |
| 9 | 7 | Female | Yes, case 6 | Survived |
| 10 | 5 | Male | Yes, cases 12 and 13 | Survived |
| 11 | 35 | Male | No | Survived |
| 12 | 16 | Male | Yes, cases 10 and 13 | Survived |
| 13 | 33 | Female | Yes, cases 10 and 12 | Deceased (30 weeks pregnancy) |
| 14 | 16 | Female | No | Survived |
| 15 | 18 | Male | No | Deceased |
| 16 | 22 | Male | No | Survived |
| 17 | 38 | Male | No | Deceased |
| 18 | 22 | Male | No | Deceased |
| 19 | 47 | Male | No | Survived |
Figure 2.
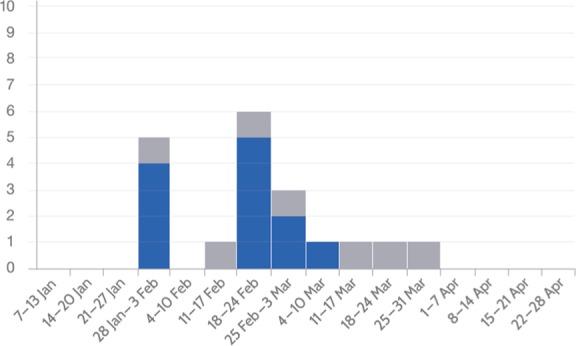
Epidemic curve of invasive meningococcal disease cases who survived (blue) and died (gray) in Tijuana, Mexico, 2013.
Public health interventions were continuous case reporting of all suspected cases of IMD, an increase in active surveillance in all private and public hospitals, training of medical and laboratory personnel, a total of 3929 questionnaires performed in all areas where cases were identified, rapid chemoprophylaxis in all close contacts as indicated (see below), and promotion of good health habits (e.g. not sharing drinks, no smoking, not attending crowded places).
Chemoprophylaxis was given to all household contacts, classroom contacts, all people who had suspected direct exposure to any confirmed case’s secretion through kissing, sharing eating/drinking utensils at any time during the 7 days before onset of illness. Ciprofloxacin was the antibiotic chosen to treat all nonpregnant women aged over 18 years, while rifampin was used for all those under 18 years old, and the number of antibiotic doses given was of 1430. Vaccination was not implemented during and after the outbreak.
Twelve of 19 isolates were sent to Mexico City and Madrid, Spain for molecular analysis. Accordingly, PFGE showed discriminatory power for the same profile on the 12 N. meningitidis strains analyzed (Figure 3), and a clonal complex ST-11 was identified in all strains. All isolates were susceptible to ceftriaxone, penicillin and ciprofloxacin.
Figure 3.
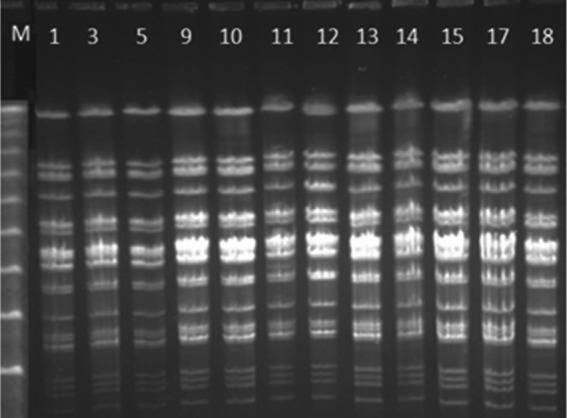
Electrophoretic patterns by restriction fragment length polymorphism pulse field gel electrophoresis of Neisseria meningitidis isolates. Line M: molecular weight marker λ ladder. Lines 1, 3, 5, 9, 10, 11, 12, 13, 14, 15, 17, 18: isolates, Tijuana outbreak, 2013.
Discussion and conclusion
Human infections caused by N. meningitidis remain a serious health problem, infecting 500,000 to 1.2 million people and killing between 50,000 and 135,000 per year worldwide [Chang et al. 2012].
IMD in Mexico has been reported as an infrequent infectious disease (even though it is a notifiable condition) based on Mexican epidemiologic reports, with rates as low as 0.06/100,000 [Almeida-Gonzalez et al. 2004]. Other reviews of IMD in Mexico suggest the same [Safadi and Cintra, 2010]. However, we published a binational study of Tijuana, Mexico and San Diego, California proving that IMD is present in Tijuana [Chacon-Cruz et al. 2011b], with higher incidence of serogroup C than in San Diego where serogroup B was the most frequent. This publication was the first that actually proves that IMD is present in Mexican territory to the same extent as in its counterpart in the USA (at least on the Mexico–California border). Furthermore, following 7 years of active surveillance, we have not only confirmed that IMD is endemic in this northwestern part of Mexico (contrary to what has been published in national reports), but have also produced significant clinical, epidemiological and microbiological information about the disease [Chacon-Cruz et al. 2013].
For Mexico, meningococcal meningitis (MM) and IMD are considered diseases of immediate notification, nevertheless this system is based on passive surveillance and national reports show very low rates for IMD in the country [Almeida-Gonzalez et al. 2004]. We believe that underreporting due to lack of awareness of IMD is the main reason for this problem.
Based on our findings in Tijuana, Mexico there is currently a national Mexican network based on active surveillance for meningitis in children from 10 hospitals: a 2-year advance of this network was presented at IDWeek in San Diego [Chacon-Cruz et al. 2012a]. Accordingly, these preliminary data showed that N. meningitidis and Streptococcus pneumoniae caused 30.4% and 28.6% of all culture-confirmed BM case (n = 56) respectively, with higher rates of MM in the northwest, while pneumococcal meningitis is more equally distributed in the country. In sub-Saharan countries of Africa, extending from Senegal in the west to Ethiopia in the east, known as the African meningitis belt, there have been large periodic epidemics of serogroup A meningococcal disease occurring every 8–10 years since 1905 [Harrison et al. 2009]. The patterns of this region are linked to environmental factors, such as climatic change (dry seasons, wind of the Hartmattan), coinfection, crowding and possible specific population susceptibility [Molesworth et al. 2003; Greenwood et al. 1985; Sultan et al. 2005]. In Tijuana, we have sufficient information proving that IMD is endemic, and in this study we describe the first confirmed meningococcal outbreak. However, we do speculate that good active surveillance is not the only reason (better and more accurate detection of cases); there could also be other influencing factors, such as the Santa Ana conditions (dry/windy seasons) and high colonization rates, among others, factors that will be analyzed in the future.
Meningococcal disease patterns and incidence can vary dramatically both geographically and over time in populations influenced by differences in invasive meningococcal serogroups and specific genotypes designated as sequence type (ST) clonal complexes. Serogroup A (ST-5, ST-7), B (ST-41/44, ST-32, ST-18, ST-269, ST-8, ST-35), C (ST-11), Y (ST-23, ST-167), W-135 (ST-11) and X (ST-181) meningococci currently cause almost all invasive diseases [Harrison et al. 2009]. The incidence of meningococcal disease is cyclical in nature, having peaks and troughs every 5–8 years in some epidemiological settings [Harrison et al. 2009]. However, disease patterns and incidence vary in populations geographically and over time among the different invasive meningococcal serogroups and ST complexes [Chang et al. 2012; Harrison et al. 2009].
In the USA, serogroup C disease is responsible for endemic disease as well as clusters of local outbreaks, accounting for approximately 30% of overall disease [Cohn et al. 2010; Harrison, 2010; Harrison et al. 2010]. Increases in serogroup C meningococcal disease were seen in the 1980s and 1990s worldwide, attributed to the spread of a hypervirulent ST-11 complex clone, and affecting not only children, but mostly outbreaks among adolescents and adults, with high mortality rates [Harrison et al. 2009]. Our outbreak affected predominantly adolescents and young adults, mortality was high (36.8%), and a meningococcal serogroup C, ST-11 clonal complex was identified in all strains analyzed.
Serogroup C conjugated vaccines were introduced more than a decade ago, first in the UK in a mass vaccination campaign and now they are widely used [Harrison et al. 2009]. Tetravalent conjugated meningococcal vaccines containing serogroups A, C, Y and W-135 were first used in adolescents in the USA in 2005 and are now used in infants and young children [Chang et al. 2012; Harrison et al. 2009]. A new serogroup A conjugate vaccine has recently been introduced in sub-Saharan Africa [Chang et al. 2012]. Both monovalent C and tetravalent vaccines have shown effectiveness against diseases caused by the serogroups included in countries where these vaccines have been successfully given [Chang et al. 2012; Cohn et al. 2010; Harrison et al. 2009].
Decisions for vaccination either in a region or a country are mainly considered based on evidence of either high or intermediate endemicity (>10 cases/100,000 population and 2–10 cases/100,000 population, respectively), and the presence of outbreaks [WHO, 2011]. However, these recommendations from the World health Organization do not make it clear whether the denominator for the rates estimated are for age groups or for the whole population. Nevertheless, in our region, rates of 8.45/100,000 population in children less than 1 year old have been presented [Chacon-Cruz et al. 2012b], and the presence of an outbreak, especially when immunization against IMD has not yet been implemented, in addition to a circulating hypervirulent ST-11 clonal complex strain, is always a major concern.
In summary, in Tijuana, Mexico (on the Baja-California, Mexico–San Diego, California border), an outbreak of IMD by N. meningitidis serogroup C has occurred, affecting mostly adolescents and young adults, with a high fatality rate, and public health interventions based mostly on massive chemoprophylaxis have resulted in a dramatic reduction of cases. However, the facts of having a highly virulent strain (clonal complex ST-11) causing this outbreak, published and presented data showing IMD as an endemic problem in the region, and not yet implemented meningococcal vaccination in the region are of great concern for having continuous endemic cases and the reemergence of another outbreak.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
Contributor Information
Enrique Chacon-Cruz, Hospital General de Tijuana, Paseo Centario S/N, Zona del Rio, Tijuana, 22010, Mexico.
Luz Elena Espinosa-De Los Monteros, Hospital Dr Manuel Gea Gonzalez, Mexico City, Mexico.
Samuel Navarro-Alvarez, Hospital General de Tijuana, Tijuana, Mexico.
Jose Luis Aranda-Lozano, Tijuana’s Health Jurisdiction, Tijuana, Mexico.
Maria Luisa Volker-Soberanes, Hospital General de Tijuana, Tijuana, Mexico.
Rosa Maria Rivas-Landeros, Hospital General de Tijuana, Tijuana, Mexico.
Ariadna Annete Alvelais-Arzamendi, Hospital General de Tijuana, Tijuana, Mexico.
Julio Alberto Vazquez, Carlos III Institute of Health, Madrid, Spain.
References
- Almeida-Gonzalez L., Franco-Paredes C., Perez L., Santos-Preciado J. (2004) Meningococcal disease caused by Neisseria meningitidis: epidemiological, clinical, and preventive perspectives. Sal Pub Mex 46: 438–450 [DOI] [PubMed] [Google Scholar]
- Baccarini C., Ternouth A., Wieffer H., Vyse A. (2012) The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vaccin Immunother 9: 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet D., Cafferkey M. (2006) Consecutive use of two multiplex two PCR-based assays for simultaneous identification and determination of capsular status of nine common Niesseria meningitidis serogroups associated with invasive disease. J Clin Microbiol 44: 1127–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayer A., Humiston S. (2011) Invasive meningococcal disease in childhood. Pediatr Rev 32: 152–161 [DOI] [PubMed] [Google Scholar]
- Bygraves J., Maiden M. (1992) Analysis of the clonal relationships between strains of Neisseria meningitidis by pulsed field gel electrophoresis. J Gen Microbiol 138: 523–531 [DOI] [PubMed] [Google Scholar]
- Chacon-Cruz E., Martinez–Longoria C., Llausas–Magana E., Luevanos-Velazquez A., Vazquez-Narvarte J., Beltran S., et al. (2012a) Neisseria meningitidis as the leading cause of bacterial meningitis in children: results from a 2 years National Active Surveillance Network in 10 Mexican Hospitals. IDWeek: A Joint Meeting of IDSA, SHEA, HIVMA AND PIDS, 17–21 October, San Diego, California: Abstract number 374. [Google Scholar]
- Chacon-Cruz E., Perez-Sanchez V., Santana-Ramirez Z., Hurtado-Montalvo J., Rivas-Landeros R., Volker-Soberanes M. (2011a) Invasive meningococcal disease in northern Mexico: an endemic, severe and preventable health problem. 29th Annual Meeting of the European Society for Paediatric Infectious Diseases, 7–11 June, The Hague, The Netherlands Abstract number 255. [Google Scholar]
- Chacon-Cruz E., Sanchez-Flores A., Rivas-Landeros R., Volker-Soberanes M. (2013) Neisseria meningitidis and Streptococcus pneumoniae serotype 19A as leading and emerging causes of bacterial meningitis in Northern Mexican children. 31st Annual Meeting of the European Society for Paediatric Infectious Diseases, 28 May–1 June, Milan, Italy Abstract number A-534–0012–00308. [Google Scholar]
- Chacon-Cruz E., Sanchez-Flores A., Volker-Soberanes M., Rivas-Landeros R. (2012b) Persistent endemicity of invasive meningococcal disease in northern Mexico: a severe, preventable and unresolved problem. 30th Annual Meeting of the European Society for Paediatric Infectious Diseases, 8–12 May, Thessaloniki, Greece Abstract number 329. [Google Scholar]
- Chacon-Cruz E., Sugerman D., Ginsberg M., Hopkins J., Hurtado-Montalvo J., Lopez-Viera J., et al. (2011b) Surveillance for invasive meningococcal disease in children, US–Mexico border, 2005–2008. Emerg Infect Dis 17:543–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Tzeng Y., Stephens D. (2012) Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol 4: 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn A., MacNeil J., Harrison L., Hatcher C., Theodore J., Schmidt M., et al. (2010) Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 50: 184–191 [DOI] [PubMed] [Google Scholar]
- Feavers I., Gray S., Urwin R., Russell J., Bygraves J., Kaczmarski E., et al. (1999) Multilocus Sequence Typing and Antigen Gene Sequencing in the Investigation of a Meningococcal Disease Outbreak. J Clin Microbiol 37: 3883–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B., Bradley A., Wall R. (1985) Meningococcal disease and season in sub-Saharan Africa. Lancet 2: 829–830 [DOI] [PubMed] [Google Scholar]
- Harrison L. (2010) Epidemiological profile of meningococcal disease in the United States. Clin Infect Dis 50(Suppl. 2): S37–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L., Shutt K., Schmink S., Marsh J., Harcourt B., Wang X., et al. (2010) Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era – United States, 2000–2005. J Infect Dis 201: 1208–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L., Trotter L., Ramsay M. (2009) Global epidemiology of meningococcal disease. Vaccine 27(Suppl. 2): B51–B63 [DOI] [PubMed] [Google Scholar]
- Maiden M., Bygraves J., Feil E., Morelli G., Russell J., Urwin R., et al. (1998) Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 95: 3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesworth A., Cuevas L., Connor S., Morse A., Thompson M. (2003) Environmental risk and meningitis epidemics in Africa. Emerg Infect Dis 9: 1287–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi M., Cintra O. (2010) Epidemiology of meningococcal disease in Latin America: current situation and opportunities for prevention. Neurol Res 32: 263–271 [DOI] [PubMed] [Google Scholar]
- Sultan B., Labadi K., Guegan J., Janicot S. (2005) Climate drives the meningitis epidemics onset in west Africa. PLos Med 2: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2011) Meningococcal vaccines: position paper, November 2011. Weekly Epidemiological Record 47: 521–539 [Google Scholar]